Back to Journals » Journal of Inflammation Research » Volume 16
Altered Phenotypes of Colonic and Peripheral Blood Follicular Helper and Follicular Cytotoxic T Cells in Mice with DSS-Induced Colitis
Authors Long Y, Xia CS, Zeng X, Feng J, Ma Y, Liu C
Received 6 March 2023
Accepted for publication 12 May 2023
Published 10 July 2023 Volume 2023:16 Pages 2879—2892
DOI https://doi.org/10.2147/JIR.S411373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yan Long, Chang-Sheng Xia, Xingyue Zeng, Jinghong Feng, Yinting Ma, Chen Liu
Department of Clinical Laboratory, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Yan Long; Chen Liu, Department of Clinical Laboratory, Peking University People’s Hospital, 11 Xizhimen South Street, Beijing, 100044, People’s Republic of China, Email [email protected]; [email protected]
Background: Follicular helper T (Tfh), follicular regulatory T (Tfr), and follicular cytotoxic T (Tfc) cells play important roles in autoimmune diseases. Nevertheless, their changes of functional phenotypes in ulcerative colitis (UC), most importantly, their changes in colon tissue as the target-organ, have not been explored.
Methods: DSS-colitis was induced in Balb/c mice and lymphocytes were collected from spleen, mesenteric lymph nodes, peripheral blood and colon. Tfh, Tfr, and Tfc cells were analyzed using flow cytometry based on their CD4+CXCR5+FOXP3-Tfh, CD4+CXCR5+FOXP3+Tfr and CD8+CXCR5+Tfc expressions. Various functional characterization markers including CD44, CD62L, TIGIT, CD226, PD-1, ICOS, Helios, CTLA-4 and Bcl6 were analyzed in the T cell subsets of the organs.
Results: Tfh and Tfr cells in the colon were significantly increased in DSS-colitis mice. Additionally, the proportions of Tfr and Tfc cells in the peripheral blood were also increased, while Tfc cell proportions in the colon were decreased. The proportion of naïve cells in the Tfh, Tfr and Tfc cells in the colon and peripheral blood decreased, while the proportion of effector memory T cells increased. The TIGIT+CD226-Tfh and Tfc cells were upregulated in the colon of DSS-colitis mice. The PD-1+, ICOS+ and PD-1+ICOS+ Tfh cells were increased in both the colonic and peripheral blood Tfh and Tfc of DSS-colitis mice. The Bcl6+ proportions in the Tfh and Tfr were increased in the colon of DSS-colitis mice.
Conclusion: The colonic and peripheral blood Tfh and Tfc cells of DSS-colitis mice have a significantly activated T cell phenotype, which may play a significant role in the pathogenesis of UC.
Keywords: DSS-induced colitis, follicular helper T cells, follicular regulatory T cells, follicular cytotoxic T cells, PD-1, ICOS
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease caused by a loss of immune homeostasis, which mainly affects the colon and rectum. Its mucosal pathology is characterized by infiltration of a large number of neutrophils and lymphocytes in gut-associated lymphoid tissue.1 UC is characterized by alternating periods of active and remission, with a long-term risk of developing colitis-related cancers.1–3 There are currently no reliable biomarkers for monitoring active UC.4 Understanding the immune mechanisms in the pathogenesis of UC is crucial for understanding the disease and exploring biomarkers for monitoring disease activity.
The expansion of activated B cells and the production of disease-causing antibodies are believed to contribute to the pathogenesis of UC.5–8 Autoantibodies secreted by functional B cells have been implicated in both the development and exacerbation of UC.5,6,9,10 Patients with UC have a high number of activated B cells present in the lamina propria and an elevated expression of IgG in affected tissue.11 Additionally, circulating plasma cells in UC patients are significantly elevated and positively correlated with their Mayo score.12 Our research demonstrated that active UC was associated with a significant increase in new memory B cells and plasmablasts, which positively correlated with the severity of the disease.13 These findings suggest that abnormal immune activation through B cells plays a crucial role in both the pathogenesis and activity of UC.
The interactions between T and B cells play a critical role in transmitting signals that support the maturation and antibody production of B cells.14,15 Follicular helper T (Tfh) cells provide crucial signals that help to differentiate B cells into high-performing cells that produce isotype-specific antibodies.16–18 On the other hand, follicular regulatory T (Tfr) cells exert inhibitory effects on Tfh cells and germinal center B cells.17,19 An imbalance between Tfr and Tfh cells can cause diseases that result from abnormal B cell immunity.13,20,21 Tfh plays a pivotal role in advancing B cell recruitment and differentiation by secreting cytokines such as IL-4 and IL-21.15,22 Our research discovered that circulating Tfh cells were significantly increased in active UC, which was linked to the disease activity by stimulating B cell differentiation.13 The same mechanisms have been observed in the development of other autoimmune diseases.20,21,23 Follicular cytotoxic T (Tfc) cells, a subset of CD8+ T cells expressing CXC-chemokine receptor 5 (CXCR5), have been found in the vicinity of B cell follicles, which play a crucial role in treating malignant tumors derived from B cells.24 T cells can be categorized into naïve T cells, memory T cells, and other subsets, distinguished by their expression of CD44 and CD62L.24,25 Memory Tfh cells not only boost secondary responses to antigens upon rechallenge, but also travel to non-draining lymphoid tissues to differentiate into effector Tfh cells in response to systemic antigen or pathogen spread.26,27 Circulating CD62Llow memory Tfh cells are a valuable indicator for monitoring Tfh responses in autoimmune diseases, infection and vaccination.26,27
Several key transcription factors and surface marker molecules play a crucial role in differentiating various T cell subpopulations. Helios could strengthen the suppressor function of regulatory T cells by connecting to the FoxP3 promoter.28 The absence of Helios in Tfr cells results in a failure to regulate Tfh responses, leading to increased germinal center development.29,30 CTLA-4, an inhibitory co-stimulatory molecule, enhances B cell responses when absent on Tfh cells, while the deletion of CTLA-4 on Tfr cells causes defective suppression of antigen-specific antibody responses.31 TIGIT could bind to CD155 and CD112 ligands, leading to increased IL-10 secretion and decreased IL-12 secretion.32,33 Increased TIGIT could improve the stability and suppressor function of Tregs.33,34 CD226 could enhance the cytotoxic function of natural killer cells,35 and is negatively correlated with the inhibitory function of Tregs.33,36 CD226 and TIGIT bind to the same cell surface ligands, CD155 and CD112.37 PD-1 is a potent inhibitory receptor and is highly expressed in germinal center and peripheral blood Tfh cells.38,39 High expression levels of PD-1 on Tfh cells are critical for B cell responses and antibody production.40 ICOS is crucial for Tfh cells to support B cells.41 Deficiency of ICOS leads to severe depletion of memory B cells and completely prevents all antigen-specific IgG responses.42 Thus, the expression of ICOS and PD-1 is a hallmark of activated Tfh cells. Bcl-6, a key transcription factor of Tfh cells, could positively regulates Tfh cell differentiation.43 Additionally, Tfr cells expressing Foxp3 and Bcl-6 can suppress germinal center responses.44 In addition, Bcl6 can inhibit the expression of several microRNAs (miRNAs), which controls Tfh cell signaling by inhibiting the expression of CXCR5.45
To further understand the changes in T cell subsets during UC, the study utilized a mouse model of dextran sulfate sodium (DSS) -induced colitis to investigate changes in Tfh, Tfr, and Tfc subsets in peripheral immune organs such as the spleen, peripheral blood, and mesenteric lymph nodes, as well as the colon tissue. The study also explored changes in a range of functional T cell subsets in depth, aiming to gain a comprehensive understanding of the expression of T cell subsets in UC, which would provide insight into the immunological mechanism of UC.
Materials and Methods
Animal Model
In this study, 16 female BALB/c mice, 7 weeks of age, were obtained from Charles River Laboratories (Zhejiang, China) and housed in a specific pathogen-free environment with regulated temperature and humidity under a 12-hour light and 12-hour dark photoperiod. The mice were randomly divided into an experimental and a control group, each containing eight mice. The experimental group was induced to be with acute colitis by administering 3% DSS in their drinking water for 7 days. On the eighth day, the mice were supplied with water without DSS for two more days before being sacrificed. The colon tissue, peripheral blood, spleen, mesenteric lymph nodes and the entire colon were collected and preserved in ice-cold PBS within a maximum of 30 minutes. Part of the tissue with lesions was fixed in 4% paraformaldehyde and embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E) to evaluate the degree of inflammation and epithelial injury under a microscope. The results showed significant infiltration of inflammation and shortening of the colon, confirming the successful establishment of the colitis model. The animal experiments were approved by the Ethics Committee of Peking University People’s Hospital (approval number 2020PHE066). The mouse research was performed in accordance with the Laboratory Guidelines for Animal Use and Care.46
Mononuclear Cell Isolation
Lamina propria mononuclear cells (LPMCs) in the colon were isolated as follows. The intestine was removed from the mesenteric fat tissue and the Peyer’s patches were excised. The colon was then opened lengthwise and cut into approximately 1 cm sections, which were washed three times with ice-cold PBS. The intestine pieces were incubated with a pre-digestion solution containing Hanks’ balanced salt solution, EDTA, dithiothreitol, HEPES and fetal bovine serum at 37°C for 20 minutes while slowly rotating at 180 RPM. The intestine pieces were then vortexed and washed with ice-cold PBS three more times to remove the suspension of epithelial cells, villus cells, subepithelial cells and intraepithelial lymphocytes. The pieces were then incubated with a digestion solution containing collagenases and DNases at 37°C for 30 minutes under slow rotation at 180 RPM. After incubation, the cell solution was vortexed intensely for 20 seconds and passed through a 70-micron cell strainer. The suspension was subjected to Percoll gradient separation for 10 minutes at 500g. Peripheral blood was collected in heparin anticoagulant tubes, the spleen was obtained and ground into a cell suspension, and lymphocytes in peripheral blood and spleen cells were both isolated using Ficoll-Paque density gradient centrifugation. The mesenteric lymph nodes were ground into a cell suspension using a sieve.
Flow Cytometry
The isolated mononuclear cells were subjected to staining with specific monoclonal antibodies against various surface markers. The staining procedure was carried out as follows. The isolated cells were washed twice and then stained for 30 minutes with antibodies, including anti-CD4-APC-Cy7, anti-CXCR5-PE-Cy7, anti-CTLA4-PerCP-Cy5.5, anti-CXCR5-Alexa647, anti-CD226-PE-Cy7, anti-TIGIT-PE, anti-CD8-PerCP-Cy5.5, anti-CXCR5-FITC, anti-CD44-PE-Cy7, anti-PD1-APC-Cy7, anti-ICOS-FITC and anti-CD62L-PE. The cells were then washed in PBS and subjected to intracellular staining using the FoxP3 staining Buffer Kit from eBioscience (San Diego, CA, USA). The staining process involved incubating the cells for 30 minutes with antibodies, including anti-FoxP3-Alexa488, anti-FoxP3-Alexa647, anti-Helios-PE and anti-Bcl6-Alexa647. The samples were then washed twice and analyzed using the FACSCanto and Diva software from BD Biosciences (San Jose, CA, USA). All the antibodies were procured from BioLegend (San Diego, CA, USA).
Statistical Analysis
The results of the two groups were compared using a Student’s t-test. The analysis was performed using Prism software (GraphPad Software 8.0, San Diego, CA), and p-values less than 0.05 were considered significant.
Results
The Proportions of Tfh and Tfr Cells in Colonic Lymphocytes Were Significantly Increased in the Mice with DSS-Induced Colitis
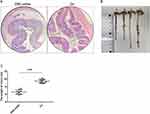
To better understand the changes in Tfh, Tfr and Tfc cells, the main T cell subpopulations in colon tissue and the periphery of UC patients, a DSS-induced ulcerative colitis mouse model was established according to the classical methods.45 We used HE staining to examine the colon tissues of DSS-induced colitis mice and found that they had significant inflammatory cell infiltration (Figure 1A). Further direct measurement of the colon length found that compared with healthy control mice, the colon length of DSS-induced colitis mice was significantly shortened, from about 10 cm to about 6 cm (Figure 1B and C). These results indicated that our establishment of a mouse model of UC was successful.
After the onset of the disease, the levels of these T cell subsets in the colon, peripheral blood, spleen, and mesenteric lymph nodes were compared. The results showed that the proportions of CD4+CXCR5+FoxP3+T cells (Tfr) and CD4+CXCR5+FoxP3-T cells (Tfh) in colon CD4+ lymphocytes significantly increased, but there were no significant changes in the spleen and mesenteric lymph nodes (Figure 2A and B). The proportion of Tfr in peripheral blood was significantly elevated in the DSS-induced colitis mice, but the proportion of Tfh was not significantly changed (Figure 2B). The Tfr/Tfh ratio was also significantly elevated in the DSS-induced colitis mice in both the colon and peripheral blood (Figure 2C). Additionally, the proportion of CD8+CXCR5+ Tfc cells in CD8+ T cells was decreased in peripheral blood but not significantly changed in the other three organs (Figure 2D).
There was a Decrease in the CD44lo/int CD62L+ Naïve Phenotype and an Increase in the CD62L- Effector Phenotype Within the Tfh, Tfr, and Tfc Cell Subsets in Mice with DSS-Induced Colitis
The expression of CD44 and CD62L in peripheral blood T cells allows for the classification of these cells into three subsets, including CD44low/intCD62L+ naïve T cells, CD44highCD62L+ memory T cells (or central memory T), and CD62L- effecter T cells. These subsets reflect different levels of T cell activation.25–27 In this study, we investigated the proportions of these subsets in Tfh, Tfr and Tfc cells of DSS-induced colitis mice (Figure 3). In the colon, we observed a significant decrease in the proportion of naïve T cells and a significant increase in the proportion of effecter T cells in all three cell subsets (Figure 3). Similarly, in peripheral blood, there was a decrease in the proportion of naïve T cells in Tfh, while an increase in effecter T cells was observed in Tfh cells only. No changes in the proportions of subpopulations were noted in Tfr cells. There were also no significant changes in T cell subsets in the spleen. In lymph nodes we found a significant increase in the proportion of naïve T cells and CD44highCD62L+ central memory T cells in Tfr, Tfh and Tfc in DSS-induced colitis mice (Figure 3).
TIGIT and CD226 Expressions were Significantly Altered on Colonic Tfh, Tfr and Tfc Cells of DSS-Induced Colitis Mice
Further investigation was carried out on T cell subsets divided by molecules with critical characterization roles in T cells, including Helios, CTLA-4, CD226 and TIGIT.28–37 Results showed that the proportion of Helios+ in Tfh and Tfr cells of DSS-induced colitis mice was not significantly increased in any of the four organs, as demonstrated in Figure S1A and C. A significant increase in the proportion of CTLA-4+ in Tfh cells was found in the colon and peripheral blood of DSS-induced colitis mice, while the proportion of CTLA-4+ in peripheral blood Tfr was also significantly increased. However, no significant changes were detected in spleen and mesenteric lymph nodes, as illustrated in Figure S1B and D.
Based on CD226 and TIGIT, T cells can be sorted into CD226-TIGIT+, CD226+TIGIT+, CD226-TIGIT-, and CD226+TIGIT-subsets. Changes in the proportions of these four cell populations in Tfh, Tfr and Tfc between DSS-induced colitis mice and healthy control mice were examined and compared, as shown in Figure 4. Results showed that the proportions of CD226-TIGIT+, CD226+TIGIT+ and CD226+TIGIT- were increased in colon Tfh and Tfc of DSS-induced colitis mice, while the proportion of CD226-TIGIT- was decreased. In Tfr, the proportion of CD226+TIGIT+ was found to be increased, and the proportion of CD226-TIGIT- was decreased. Similar trends were observed in Tfh and Tfr cells in the spleen, but some subpopulations did not reach significant differences. The changes in total TIGIT+ and CD226+ percentages were also calculated and compared, and the results showed that TIGIT+ percentages were significantly increased in colon of Tfh, Tfr and Tfc of DSS-induced colitis mice. CD226+ percentages were all significantly increased in colon Tfh, Tfr and Tfc of DSS-induced colitis mice, as shown in Figure S2. Similar changes were exhibited in the spleen, but the differences were not significant in peripheral blood.
The Levels of PD-1+ and ICOS+ Tfh Cells as well as PD-1+ICOS+ Tfh Cells were Increased in Both Colon and Peripheral Blood Tfh and Tfc Cells of Mice with DSS-Induced Colitis
Additionally, we analyzed the expression of PD-1 and ICOS, two important functional molecules in Tfh and Tfr cells.16,17,38–42 The comparison of PD-1+ICOS+, PD-1+ICOS-, and PD-1-ICOS+ cell percentages in Tfh, Tfr, and Tfc cells between the DSS-induced colitis mice and control mice revealed that the proportion of PD1+ICOS+ and PD-1-ICOS+ cells in the colon Tfh and Tfr cells of DSS-induced colitis mice were significantly increased, while the proportions of PD-1+ICOS- cells in the colon Tfh and Tfr were significantly decreased (Figure 5). On the other hand, the percentage of PD-1-ICOS+ cells in Tfh in peripheral blood was significantly decreased, but the percentage of PD-1 +ICOS+ cells was increased and the percentage of PD-1+ICOS- cells did not change significantly (Figure 5). The analysis of PD-1 and ICOS in Tfc cells showed that the percentage of PD-1-ICOS+ and PD-1 +ICOS+ cells in colon and peripheral blood of DSS-induced colitis mice were significantly increased, while the percentage of PD-1 +ICOS- cells was increased in colon Tfc and decreased in peripheral blood Tfc (Figure 5C and F).
Furthermore, we analyzed and compared the total PD-1+ and total ICOS+ cell percentages. The results showed that both PD-1+ and ICOS+ percentages in colon and peripheral blood Tfh cells of DSS mice were significantly increased, and similar changes were found in Tfc cells of DSS mice (Figure S3). The PD-1+ percentages were increased in circulating Tfr cells of DSS-induced colitis mice, and ICOS+ percentages were increased in colon Tfr cells of DSS-colitis mice (Figure S3). Additionally, ICOS+ percentages in Tfr and Tfc of DSS-induced colitis mice were increased in mesenteric lymph nodes (Figure S3).
The Proportion of Bcl6-Positive Cells in Both Tfh and Tfr Was Found to Be Elevated in the Colon Tissue of Mice with DSS-Induced Colitis
Further analysis was conducted to examine the expression of the transcription factor Bcl6, which plays a crucial role in Tfh and Tfr cells. The Bcl6-positive cell percentages in Tfh and Tfr cells of mice with DSS-induced colitis were measured using flow cytometry.43,44 The results showed that the Bcl6-positive proportions in the colon of DSS mice were significantly increased in both Tfh and Tfr cells, Bcl6-positive percentages in circulating Tfh were increased but no significant difference was observed in the peripheral blood Tfr cells (Figure 6). Additionally, the Bcl6-positive proportions in Tfr and Tfh cells were also significantly increased in the mesenteric lymph nodes of DSS mice (Figure 6).
Discussion
In the present study, we conducted a systematic investigation of the changes in levels of functional T cell subsets, including Tfh, Tfr, and Tfc, in various tissues of mice with colitis induced by DSS. To distinguish the changes in these T cell subsets, we selected key marker molecules. Our findings show that the proportions of Tfh and Tfr cells in the colonic T cells of DSS-colitis mice were significantly increased, as were those of Tfr cells in the peripheral blood. The proportion of naïve cells in the colon and peripheral blood Tfh cells of DSS-colitis mice decreased, while the number of effector memory T cells increased. TIGIT+CD226-Tfh and Tfc cells were upregulated in the colon of DSS-colitis mice, and PD-1+, ICOS+, and PD-1+ICOS+ Tfh cells all increased in both the colon and peripheral blood Tfh and Tfc cells of DSS-colitis mice. The proportion of Bcl6+ in both Tfh and Tfr was also increased in the colon of DSS-colitis mice. These results suggest that DSS-colitis mice have a pronounced activation of T cell phenotype in their colon and peripheral blood Tfh and Tfc cells, which may play a crucial role in the pathogenesis of UC.
The role of TFH in the pathogenesis of UC has attracted more and more attention. The study by Zhang, et al found that IRF8-regulated TFH can function as a B-cell-independent pathogenic mediator in colitis, suggesting that targeted TFH may be effective in the treatment of IBD.47 However, so far, there is still a lack of in-depth research on TFH and TFR-related subsets in the colon in the pathogenesis of UC, resulting in a lack of panoramic understanding of the imbalance between TFH and TFR-related subsets in organs such as the colon. Although some previous studies have reported changes in TFH and TFR in the peripheral blood of UC patients,13 the situation in peripheral blood can only reflect the changes in the cell subsets in the colon to a certain extent, and the changes in the subsets of TFH and TFR in the colon are still unclear, which is the main focus of this study. In addition, we further investigated the situation of Tfc in vivo.
According to our results, the proportions of Tfh and Tfr in the colonic CD4+ lymphocytes of DSS-colitis mice were significantly increased, indicating significant changes in the T cell subsets in the colon. The upregulation of CXCR5, an important chemokine receptor, was found to play a role in these changes, as it allows T cells to migrate to the lymph nodes and the B cell zones.48 Despite these changes, there were no significant alterations observed in the peripheral T cells of the spleen and mesenteric lymph nodes, suggesting that the changes were more pronounced at the site of UC. The proportion of Tfr in peripheral blood of DSS-colitis mice was elevated, but there was no significant change in the proportion of Tfh. In contrast to these results, studies in UC patients have shown down-regulated Tfr cells and elevated Tfh in active UC patients.13 The reason for this discrepancy may be attributed to limitations in the mouse model and the selection of cell marker molecules. The Tfr/Tfh ratio was elevated in both the colon and peripheral blood of DSS-colitis mice, but changes in the immune balance cannot be solely determined by the ratio changes and should be reflected by changes in related functional subsets. Tfc cells within germinal centers are thought to expand during late progression of autoimmune diseases, and CD8 Tfc cells are transcriptionally and phenotypically similar to CD4 T follicular helper (Tfh) cells in multiple models of spontaneous autoantibody-mediated diseases.49 The level of Tfc did not change in the colon, but showed a downward trend in peripheral blood, indicating that further analysis of its functional subset composition is needed to understand its role.
We used CD44 and CD62L to detect changes in the proportion of naïve, memory and effector cells in T cell subsets, and our results showed a decrease in naïve cells and an increase in effector cells in T cell subsets in colon. This suggests that colonic T cells play an important role in the activation and progression of UC. Additionally, we found that the ratio of Helios+ did not change significantly, but the proportion of CTLA-4+ cells was found to be increased in colonic Tfh cells and in Tfr cells in both the colon and peripheral blood. The loss of CTLA-4 on Tfh cells leads to heightened B cell responses, whereas the deletion of CTLA-4 on Tfr cells weakens the suppression of antigen-specific antibody responses.31 These changes in CTLA-4 levels suggest that the function of colonic Tfh cells has been weakened and the function of Tfr cells has been relatively enhanced. These results suggested CTLA-4 are implicated in regulating humoral immunity thus participate in pathogenesis of UC.
We further found that the proportion of TIGIT+CD226- in colonic Tfh, Tfr and Tfc was significantly increased, with the proportion of TIGIT+ cells being higher in colonic Tfr and Tfc in DSS-colitis mice. TIGIT expression in Tfh cells has been shown to enhance B-cell help functions,50 while TIGIT plays an important role in Treg suppression of Th1 and Th17 cells.51 These results suggest that the functions of Tfh and Tfr have been strengthened. CD226 signals could promote the early phase of Tfh cell differentiation in humans.52 CD226 is predominantly expressed on Tregs, has a crucial role in diminishing Treg function.53 CD226-TIGIT+Treg cells are upregulated in patients with chronic hepatitis B and play a role in suppressing antiviral immunity.54 CD226+ Tfr cells have also been reported to be involved in microscopic polyangiitis, primary anti-phospholipid syndrome patients and rheumatoid arthritis.55–57 The role of CD226 in Tfc is still not clear. We observed an upregulation of CD226+ percentages in Tfh, Tfr and Tfc cells. The consistent trend of changes in TIGIT and CD226 on Tfh cell suggest that the functions of Tfh and Tfr have been strengthened. The interpretation of CD226 changes in Tfr and Tfc needs to be completed on the premise of further improving its related functions in the future.
We found that PD-1+ICOS+ proportions in the colon and peripheral blood Tfh, Tfr and Tfc of DSS-colitis mice were significantly increased. Meanwhile, the ICOS+ percentages were significantly upregulated in both colon and peripheral blood Tfh and Tfc. The expression of ICOS and PD-1 reflects the activation of Tfh cells, which play a crucial role in supporting B cell differentiation and antibody production.39–44 These results indicate the functional activation state of Tfh and Tfc in UC. Increased Bcl6+ proportions were observed in both Tfh and Tfr cells in the colon of mice with ulcerative colitis (UC). As a crucial transcription factor in Tfh cells, Bcl-6 is known to enhance the functions of Tfh and Tfr cells.43,44 Expression of Bcl-6 mRNA was reported to be upregulated in UC patients compared with that in healthy controls.58 This increase in Bcl6+ expression was only observed in the colon and similarly in the mesenteric lymph nodes, but not in peripheral spleen or blood, indicating a localized effect. The results suggest that the functions of colonic Tfh and Tfr cells were enhanced.
Our study has several limitations that must be acknowledged. Firstly, we utilized a mouse model to observe alterations in T cell subsets in the colon, draining lymph nodes, and spleen, as it is not ethical to obtain colon, mesenteric lymph node, and spleen tissues from UC patients. However, it is important to note that the mouse model may not fully replicate the human condition because of species differences. Additionally, we did not conduct in vitro functional assays. Although we had the opportunity to use mesenteric lymph node and spleen Tfh and Tfr cells for functional assays, T cell subsets in these tissues did not undergo significant changes. Hence, further functional tests were deemed unnecessary. Isolating sufficient numbers of colonic Tfh, Tfr and Tfc cells for in vitro experiments was challenging. We were able to partially achieve our goal by measuring a series of function-related markers to reflect the functional changes of these cells. We have not yet delved deeply into the specific mechanisms behind the alterations of these T cell subsets. Our focus so far has been on identifying the changes in these subsets during UC, and future studies will aim to uncover the specific mechanisms behind these changes.
Conclusion
To sum up, the presence of activated T follicular helper (TFH) and T follicular regulatory (TFC) cells in both the colon and peripheral blood of individuals with ulcerative colitis (UC) suggests a crucial role in the disease’s development. These T lymphocyte cells are known to be involved in the immune system’s response to various stimuli. Their activation in UC patients indicates an ongoing inflammatory process, contributing to the disease’s chronic nature. Further research is needed to fully understand the role of TFH and TFC cells in UC, but these findings highlight the significance of the immune system in UC’s pathogenesis and suggest potential targets for new treatments.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (82002214, 82271755), Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2021PY007), Peking University People’s Hospital Scientific Research Development Funds (RDY 2019-15) and the Fundamental Research Funds for the Central Universities.
Author Contributions
All authors made significant contributions to this work, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing this article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of this work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114(6):874–883. doi:10.14309/ajg.0000000000000183
2. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345–1353.e4. doi:10.1053/j.gastro.2019.01.002
3. Herszényi L, Barabás L, Miheller P, Tulassay Z. Colorectal cancer in patients with inflammatory bowel disease: the true impact of the risk. Digest Dis. 2015;33(1):52–57. doi:10.1159/000368447
4. Lee H, Schneider Y, Lichtenstein GR. Going third class: treatment of steroid-dependent ulcerative colitis. Dig Dis Sci. 2019;64(5):1138–1141. doi:10.1007/s10620-019-05610-w
5. Ebert EC, Geng X, Bajpai M, Pan Z, Tatar E, Das KM. Antibody to tropomyosin isoform 5 and complement induce the lysis of colonocytes in ulcerative colitis. Am J Gastroenterol. 2009;104(12):2996–3003. doi:10.1038/ajg.2009.455
6. Uo M, Hisamatsu T, Miyoshi J, et al. Mucosal CXCR4+ IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcγR-mediated CD14 macrophage activation. Gut. 2013;62(12):1734–1744. doi:10.1136/gutjnl-2012-303063
7. Pararasa C, Zhang N, Tull TJ, et al. Reduced CD27(-)IgD(-) B cells in blood and raised CD27(-)IgD(-) B cells in gut-associated lymphoid tissue in inflammatory bowel disease. Front Immunol. 2019;10:361. doi:10.3389/fimmu.2019.00361
8. Onuma EK, Amenta PS, Ramaswamy K, Lin JJ, Das KM. Autoimmunity in ulcerative colitis (UC): a predominant colonic mucosal B cell response against human tropomyosin isoform 5. Clin Exp Immunol. 2000;121(3):466–471. doi:10.1046/j.1365-2249.2000.01330.x
9. Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut. 1993;34(5):650–657. doi:10.1136/gut.34.5.650
10. Hibi T, Aiso S, Ishikawa M, et al. Circulating antibodies to the surface antigens on colon epithelial cells in ulcerative colitis. Clin Exp Immunol. 1983;54(1):163–168.
11. Jinno Y, Ohtani H, Nakamura S, et al. Infiltration of CD19+ plasma cells with frequent labeling of Ki-67 in corticosteroid-resistant active ulcerative colitis. Virchows Archiv. 2006;448(4):412–421. doi:10.1007/s00428-005-0136-7
12. Wang X, Jiang Y, Zhu Y, et al. Circulating memory B cells and plasmablasts are associated with the levels of serum immunoglobulin in patients with ulcerative colitis. J Cell Mol Med. 2016;20(5):804–814. doi:10.1111/jcmm.12728
13. Long Y, Xia C, Xu L, et al. The imbalance of circulating follicular helper T cells and follicular regulatory T cells is associated with disease activity in patients with ulcerative colitis. Front Immunol. 2020;11:104. doi:10.3389/fimmu.2020.00104
14. Bsat M, Chapuy L, Rubio M, et al. Differential pathogenic Th17 profile in mesenteric lymph nodes of crohn’s disease and ulcerative colitis patients. Front Immunol. 2019;10:1177. doi:10.3389/fimmu.2019.01177
15. Xue G, Zhong Y, Hua L, et al. Aberrant alteration of follicular T helper cells in ulcerative colitis patients and its correlations with interleukin-21 and B cell subsets. Medicine. 2019;98(10):e14757. doi:10.1097/md.0000000000014757
16. Kräutler NJ, Suan D, Butt D, et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med. 2017;214(5):1259–1267. doi:10.1084/jem.20161533
17. Stebegg M, Kumar SD, Silva-Cayetano A, Fonseca VR, Linterman MA, Graca L. Regulation of the germinal center response. Front Immunol. 2018;9:2469. doi:10.3389/fimmu.2018.02469
18. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. 2016;34:335–368. doi:10.1146/annurev-immunol-041015-055605
19. Xie MM, Dent AL. Unexpected help: follicular regulatory T cells in the germinal center. Front Immunol. 2018;9:1536. doi:10.3389/fimmu.2018.01536
20. Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H. Increased circulating CD4(+)CXCR5(+)FoxP3(+) follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol. 2018;56:261–268. doi:10.1016/j.intimp.2018.01.038
21. Liu C, Wang D, Lu S, et al. Increased circulating follicular treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol. 2018;70(5):711–721. doi:10.1002/art.40430
22. Zhang H, Hu Q, Zhang M, et al. Bach2 deficiency leads to spontaneous expansion of IL-4-producing T follicular helper cells and autoimmunity. Front Immunol. 2019;10:2050. doi:10.3389/fimmu.2019.02050
23. Xu Y, Xu H, Zhen Y, et al. Imbalance of circulatory T follicular helper and T follicular regulatory cells in patients with ANCA-associated vasculitis. Mediators Inflamm. 2019;2019:8421479. doi:10.1155/2019/8421479
24. Leong YA, Chen Y, Ong HS, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187–1196. doi:10.1038/ni.3543
25. Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161(10):5338–5346. doi:10.4049/jimmunol.161.10.5338
26. Tsai LM, Yu D. Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunol Cell Biol. 2014;92(1):57–63. doi:10.1038/icb.2013.68
27. Fazilleau N, Eisenbraun MD, Malherbe L, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8(7):753–761. doi:10.1038/ni1472
28. Takatori H, Kawashima H, Matsuki A, et al. Helios enhances treg cell function in cooperation with FoxP3. Arthritis Rheumatol. 2015;67(6):1491–1502. doi:10.1002/art.39091
29. Kim HJ, Barnitz RA, Kreslavsky T, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350(6258):334–339. doi:10.1126/science.aad0616
30. Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios controls a limited subset of regulatory T cell functions. J Immunol. 2016;196(1):144–155. doi:10.4049/jimmunol.1501704
31. Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–1039. doi:10.1016/j.immuni.2014.12.005
32. Fuhrman CA, Yeh WI, Seay HR, et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol. 2015;195(1):145–155. doi:10.4049/jimmunol.1402381
33. Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869–3875. doi:10.4049/jimmunol.1103627
34. Kurtulus S, Sakuishi K, Ngiow SF, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053–4062. doi:10.1172/jci81187
35. Shibuya A, Campbell D, Hannum C, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi:10.1016/s1074-7613(00)70060-4
36. Qiu ZX, Zhang K, Qiu XS, Zhou M, Li WM. CD226 Gly307Ser association with multiple autoimmune diseases: a meta-analysis. Hum Immunol. 2013;74(2):249–255. doi:10.1016/j.humimm.2012.10.009
37. Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi:10.1084/jem.20030788
38. Yusuf I, Kageyama R, Monticelli L, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol. 2010;185(1):190–202. doi:10.4049/jimmunol.0903505
39. Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802–810. doi:10.1097/01.pas.0000209855.28282.ce
40. Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. doi:10.1038/ni.1877
41. Gigoux M, Lovato A, Leconte J, Leung J, Sonenberg N, Suh WK. Inducible costimulator facilitates T-dependent B cell activation by augmenting IL-4 translation. Mol Immunol. 2014;59(1):46–54. doi:10.1016/j.molimm.2014.01.008
42. Warnatz K, Bossaller L, Salzer U, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107(8):3045–3052. doi:10.1182/blood-2005-07-2955
43. Kassiotis G, O’Garra A. Establishing the follicular helper identity. Immunity. 2009;31(3):450–452. doi:10.1016/j.immuni.2009.08.017
44. Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi:10.1038/nm.2426
45. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protocols Immunol. 2014;104:15.25.1–15.25.14. doi:10.1002/0471142735.im1525s104
46. Couto M, Cates C. Laboratory guidelines for animal care. Methods Mol Biol. 2019;1920:407–430. doi:10.1007/978-1-4939-9009-2_25
47. Zhang R, Qi CF, Hu Y, et al. T follicular helper cells restricted by IRF8 contribute to T cell-mediated inflammation. J Autoimmun. 2019;96:113–122. doi:10.1016/j.jaut.2018.09.001
48. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–1562. doi:10.1084/jem.192.11.1553
49. Valentine KM, Davini D, Lawrence TJ, et al. CD8 follicular T cells promote B cell antibody class switch in autoimmune disease. J Immunol. 2018;201(1):31–40. doi:10.4049/jimmunol.1701079
50. Godefroy E, Zhong H, Pham P, Friedman D, Yazdanbakhsh K. TIGIT-positive circulating follicular helper T cells display robust B-cell help functions: potential role in sickle cell alloimmunization. Haematologica. 2015;100(11):1415–1425. doi:10.3324/haematol.2015.132738
51. Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi:10.1016/j.immuni.2014.02.012
52. Yasutomi M, Christiaansen AF, Imai N, et al. CD226 and TIGIT cooperate in the differentiation and maturation of human Tfh cells. Front Immunol. 2022;13:840457. doi:10.3389/fimmu.2022.840457
53. Wang N, Liang S, Jin J, et al. CD226 attenuates Treg suppressive capacity via CTLA-4 and TIGIT during EAE. Immunol Res. 2019;67(6):486–496. doi:10.1007/s12026-019-09112-9
54. Liu C, Xu L, Xia C, et al. Increased proportion of functional subpopulations in circulating regulatory T cells in patients with chronic hepatitis B. Hepatol Res. 2020;50(4):439–452. doi:10.1111/hepr.13472
55. Long Y, Feng J, Ma Y, et al. Altered follicular regulatory T (Tfr)- and helper T (Tfh)-cell subsets are associated with autoantibody levels in microscopic polyangiitis patients. Eur J Immunol. 2021;51(7):1809–1823. doi:10.1002/eji.202049093
56. Long Y, Li W, Feng J, et al. Follicular helper and follicular regulatory T cell subset imbalance is associated with higher activated B cells and abnormal autoantibody production in primary anti-phospholipid syndrome patients. Clin Exp Immunol. 2021;206(2):141–152. doi:10.1111/cei.13647
57. Zeng X, Lu S, Li M, et al. Inflammatory cytokine-neutralizing antibody treatment prevented increases in follicular helper T cells and follicular regulatory T cells in a mouse model of arthritis. J Inflamm Res. 2022;15:3997–4011. doi:10.2147/jir.S355720
58. Yang Y, Lv X, Zhan L, et al. Case report: IL-21 and Bcl-6 regulate the proliferation and secretion of Tfh and Tfr cells in the intestinal germinal center of patients with inflammatory bowel disease. Front Pharmacol. 2020;11:587445. doi:10.3389/fphar.2020.587445
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.