Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Altered Functional Connectivity of Large-Scale Brain Networks in Psychogenic Erectile Dysfunction Associated with Cognitive Impairments
Authors Feng S , Dong L , Yan B, Zheng S , Feng Z, Li X, Li J, Sun N, Ning Y , Jia H
Received 16 June 2023
Accepted for publication 29 August 2023
Published 5 September 2023 Volume 2023:19 Pages 1925—1933
DOI https://doi.org/10.2147/NDT.S426213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Sitong Feng,1,2 Linrui Dong,1,2 Bin Yan,3 Sisi Zheng,1,2 Zhengtian Feng,1,2 Xue Li,1,2 Jiajia Li,1,2 Ning Sun,3 Yanzhe Ning,1,2 Hongxiao Jia1,2
1Beijing Key Laboratory of Mental Disorders, National Clinical Research Center for Mental Disorders & National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, People’s Republic of China; 3Department of Andrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China
Correspondence: Hongxiao Jia; Yanzhe Ning, Beijing Anding Hospital, Capital Medical University, No. 5 Ankang Hutong, Xicheng District, Beijing, 100088, People’s Republic of China, Tel +86 010-58303065, Email [email protected]; [email protected]
Purpose: Several studies have demonstrated that psychogenic erectile dysfunction (pED) patients potentially suffer from cognitive dysfunction. Despite that previous neuroimaging studies have reported abnormal functional connections of brain areas associated with cognitive function in pED, the underlying mechanisms of cognitive dysfunction in pED remain elusive. This study aims to investigate the underlying mechanisms of cognitive dysfunction by analyzing large-scale brain networks.
Patients and Methods: A total of 30 patients with pED and 30 matched healthy controls (HCs) were recruited in this study and scanned by resting-state functional magnetic resonance imaging. The Dosenbach Atlas was used to define large-scale networks across the brain. The resting-state functional connectivity (FC) within and between large-scale brain networks was calculated to compare pED patients with HCs. The relationship among cognitive performances and altered FC of large-scale brain networks was further explored in pED patients.
Results: Our results showed that the decreased FC within visual network, and between visual network and default mode network, visual network and frontoparietal network, and ventral attention and default mode network were found in pED patients. Furthermore, there was a positive correlation between immediate memory score and FC within visual network. The visuospatial score was negatively correlated with decreased FC between ventral attention network and default mode network.
Conclusion: Taken together, our findings revealed the relationship between cognitive impairments and altered FC between large-scale brain networks in pED patients, providing the new evidence about the neural mechanisms of cognitive dysfunction in pED patients.
Keywords: large-scale brain network, functional connectivity, cognitive impairment, psychogenic erectile dysfunction
Introduction
Psychogenic erectile dysfunction (pED), which is an erectile disorder related to mental health, seriously impacts on those who suffer from it and their partners in life.1,2 Patients with pED suffer from several psychogenic or interpersonal variables, such as anxiety, depression, and socioeconomic factors.3 When pED is not well treated, fear of failing sexual activity may prevent subsequent efforts, leading to the discontinuation of sexuality and greater distance between patients’ partners.4
It has been known that the brain is crucial to controlling male sexual behavior. The complex psychological and physiological responses are involved in sexual arousal.5 The brain responses of male sexual arousal are conceived into five-component model, which consists of cognitive, emotional, motivational, autonomic/neuroendocrine, and inhibitory processes.6 Functional magnetic resonance imaging (fMRI) has endeavored to map inter-regional interactions in pED patients to explore the neural mechanisms in sexual behavior.7 The aberrant FC of brain areas in pED has been reported in several neuroimaging studies.8–10 A fronto-insular network may play a pivotal role in pED, suggesting a potential function in inhibition and in hypervigilance.11 Other studies highlight the role of the insula in male sexual arousal processing, potentially involving salience network (SN).12 Furthermore, patients with pED display the decreased functional connectivity (FC) within default-mode network (DMN) and disrupted FC within SN compared to healthy controls (HCs).13 Notably, the connectivity of DMN and anti-correlated networks is considered to intervene the exchange of externally and internally oriented cognition.14 The FC between DMN and frontoparietal network (FPN) mediates trait mind wandering.15,16 Furthermore, functional activity patterns of spread brain regions which control relative functions in cognition are significantly changed in pED patients.17 A previous fMRI study has demonstrated that patients with pED show the activation in the bilateral temporoparietal junctions associated with cognitive empathy.18 Besides, the loss of cortical thickness in the left ventral premotor area and the bilateral inferior temporal cortex are associated with erectile function of pED patients, contributing to cognitive processes.19,20 Recently, researchers have found that the left precuneus is the important brain area for regulating attentional component in pED.8
Despite that several studies have shown pED patients potentially undergoing cognitive dysfunction, few studies report whether pED patients have cognitive impairment in neuropsychological state. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a psychometrically sound cognitive scanning tool in neuropsychological testing.21 Nevertheless, little is known about cognitive functions of pED patients in neuropsychological status. Moreover, large-scale brain networks consist of widely distributed brain regions that may provide a better understanding on the alterations of cognitive functions in pED patients.22 However, it still remains unclear in large-scale brain networks of pED patients in association with cognition. Here, we speculated that pED patients undergo cognitive dysfunction in association with altered FC between extensive brain networks. In this study, we aimed to explore whether pED patients have cognitive dysfunction and to explore the underlying mechanism by analyzing large-scale brain networks.
Patients and Methods
Participants
Thirty pED patients were enrolled at the male outpatient department in the Xiyuan Hospital of China Academy of Chinese Medical Science from March 2021 to August 2022. All patients firstly underwent thorough physical evaluation and sexual function examination (containing duplex Doppler ultrasonography, the International Index of Erectile Function-5 (IIEF-5), and RigiScan test) to disclose no differences between pED patients and HCs. And the recruited pED patients must meet the following criteria:3 (i) right-handed, aged from 18 to 45; (ii) no vasculogenic, organic, neurogenic, hormonal, or drug-induced erectile dysfunction; (iii) RigiScan was used to evaluate three consecutive nights of normal morning erection; (iv) evaluation of ED: IIEF5 <21; (v) detailed psychophysical status evaluation: Brief Psychiatric Rating Scale (BPRS) <35, Self-Rating Anxiety Scale (SAS) <50, Self-Rating Depression Scale (SDS) <50, the 17-item Hamilton Rating Scale for Depression (HAMD-17) <17, the 14-item Hamilton Rating Scale for Anxiety (HAMA-14) <14; (vi) no therapeutic medication in the last 2 weeks; (vii) no history of drug and alcohol addictions. Excluded criteria were as following: (i) evaluation of ED: IIEF5 >21; (ii) severe mental, neurological or other physical disorders; (iii) urological surgery; (iv) use of any psychotropic medicine and other drugs within the last 30 days that could impact on sexual function; (v) any MRI contraindications. Another 30 right-handed healthy males matching age and education without erectile dysfunction, absence of sexual experience, and history of sexual crimes were recruited as the control group.
Each participant had signed the informed consent. Furthermore, the present study was approved by the ethics committee of Beijing Anding Hospital, Capital Medical University. The clinical characteristics of pED patients and HCs in this research are illustrated in Table 1.
 |
Table 1 Demographic Characteristics of pED Patients and HCs |
Cognitive Assessments
The Chinese version of RBANS was utilized to assess the global cognitive function, with a good reliability and validity.23,24 There were 12 subtests of RBANS to evaluate five cognitive areas, including visuospatial capability (figure copy, line orientation), immediate memory (list learning, story memory), delayed memory (list recall, list recognition, story recall, figure recall), language (picture naming, semantic fluency), and attention (digital span, coding). Each domain has a raw score that is adjusted for sex and age using the RBANS table and turned to five cognitive domain scaled scores. The higher RBANS score meant the better cognitive function of subject. All participants were tested according to standardized procedures by a neuropsychologist. And the whole test was performed about 30 minutes.
Image Acquisition
The fMRI dataset was obtained by using the Prisma MRI scanner (3.0 T, Siemens AG, Erlangen, Germany) at Beijing Anding Hospital, Capital Medical University. Foam padding was utilized to reduce the head motion of participants in birdcage head coils. As previously described,25 a gradient-echo echo-planar imaging (EPI) sequence was used to acquire resting-state functional images. The imaging sequence of resting-state fMRI has the following parameters: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, matrix = 64 × 64, field of view = 200 mm × 200 mm, slice thickness = 3.5 mm, gap = 1 mm, 33 axial sections, and 240 volumes. The magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) images with T1 weighting were gained as well. The sequence of MPRAGE images with T1 weighting has the following parameters: repetition time = 2530 ms, echo time = 3.39 ms, slice thickness = 1.3 mm, voxel size = 1 × 1 × 1 mm3, field of view = 256 × 256 mm2, and 128 volumes. Before MRI scanning, all recruited subjects were instructed to rest for 30 minutes. All subjects were reminded to keep their eyes closed and stay awake during MRI scanning.
fMRI Data Preprocessing
All data preprocessing was performed using DPABISurf toolbox, which is a component of DPABI (http://rfmri.org/dpabi).26 The preprocessing pipeline of DPABISurf is based on SPM12 to acquire the data in surface and volume space. Specifically, DPABISurf’s default preprocessing pipeline was used to convert the user-specified data into brain imaging data structure, skull-stripping, spatial normalization, brain tissue segmentation, surface re-construction for T1-weighted images and slice-timing correction, realignment, head-motion estimation, spatial registration, and smooth for functional images. These details were illustrated in the previous literature.27 The quality control of brain images was screened via using all subjects’ head motion (mean FD_Jenkinson <2 mm).
Edge-Based FC and Large-Scale Network Analysis
The Dosenbach Atlas defines 160 brain region of interests (ROIs) throughout the brain.28 Among these ROIs, we excluded the cerebellum, which contained 18 ROIs from the analysis since it was not covered in all scans. BOLD signals were extracted from the remained 142 ROIs, which were matched with Yeo seven-network parcellation.29 There are seven networks including the visual network (VN, 22 ROIs), somatosensory‐motor network (SMN, 29 ROIs), dorsal attention network (DAN, 14 ROIs), ventral attention network (VAN, 14 ROIs), subcortical network (SCN, 7 ROIs), FPN (21 ROIs), and DMN (33 ROIs).29 After extracting the BOLD signals of each ROI, Pearson correlation coefficients were measured between the BOLD signals of any two ROIs, thereby generating a 142 × 142 FC matrix for each subjects. Then, the FC matrix was transformed to Fisher’s z-scores using the Fisher r-to-z transformation for the following analysis. Combined with the 142 × 142 FC matrix and the network parcellation, seven within-network connectivity and 21 between-network connectivity were calculated for each subject. Specially, the within- or between-network FC values were estimated by calculating the mean strength of the connection throughout all links within a network or between any two networks. The large-scale network FC between pED and HCs was analyzed by using independent two-sample t-test with false discovery rate (FDR) for correcting multiple comparisons (p < 0.05).
Correlation Analysis
We then explored the associations between cognitive function (RBANS scores) and large-scale network FC in pED patients via Spearman correlation analyses in pED group. The p value lower than 0.05 was considered as statistically significant.
Results
Reduced Cognitive Functions in pED Patients
Compared to HCs, pED patients had decreased cognitive functions evaluated by RBANS scale (p < 0.001), involving immediate memory (p = 0.003), visuospatial (p = 0.001), attention (p = 0.001), and delayed memory (p = 0.035). Nonetheless, we found that there was no statistically significant difference in language function between pED patients and HCs (shown in Table 2).
 |
Table 2 Reduced Cognitive Functions in pED Patients Compared to HCs |
Changed Large-Scale Network FC in pED Patients
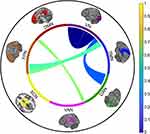
Reduced FC between four pairs of brain networks was found in pED patients, containing VN-DAN, VN-FPN, SMN-FPN, and VAN-DMN (Figures 1 and 2, FDR correction). Moreover, decreased FC within VN was observed in pED patients in contrast to HCs (Figures 1 and 2, FDR correction). In addition, there were 114 edges with decreased FC in pED patients compared to HCs (shown in Figure 2).
Correlation of Decreased Large-Scale Network FC and Reduced Cognitive Functions
Based on Spearman correlation analyses, we found that immediate memory score was positively related to FC within VN (p = 0.003, Figure 3A) and visuospatial score was negatively correlated with decreased FC of VAN-DMN (p = 0.033, Figure 3B).
Discussion
We discovered the decreased cognitive functions and changed extensive brain networks’ FC in pED patients. Specifically, our results demonstrated the decreased FC within VN, and between VN-DMN, VN-FPN, SMN-FPN, and VAN-DMN. The FC within VN was positively correlated with the score of immediate memory, and the between-network FC in VAN-DMN was negatively correlated with visuospatial score in pED patients. These findings might shed light on the neural mechanisms of pED associated with cognitive dysfunction.
An erection that cannot be maintained or achieved due to psychological or interpersonal factors is considered as pED.30 The presence of pED is frequently associated with other sexual dysfunctions, particularly hypoactive sexual desire.31 In this study, we also found that pED patients accompanied by depression and anxiety compared to HCs. Previous literature has suggested that autonomic arousal and physiological anxiety are not fully correlated with erectile function.32 Cognitive responses may be of functional significance in sexual dysfunction if the correlation between autonomic arousal and other response systems is not complete.33 Generally, there is agreement on several cognitive activities affecting sexual arousal and impacting on the development of sexual dysfunction.34 In the five-component model of sexual arousal, the cognitive component works by assessing sexual stimuli, directing attention enhancement, and eliciting motor imagery.20 Our findings revealed the decreased cognitive function of pED patients involving in immediate memory, delayed memory, visuospatial, and attention in neuropsychological status. It is essential to explore the underlying mechanisms of relationships between cognitive dysfunction and decreased erectile function of pED in the future.
Notably, neuroscientists have focused on the nature of brain networks to understand the neural mechanisms of cognitive functions of human.35 Large-scale brain organization is essential to understanding how the human brain produces cognition. We observed decreased FC within VN and decreased FC between networks of VN-DAN, VN-FPN, SMN-FPN, and VAN-DMN in pED patients. Our findings revealed the decreased FC between relatively stationary networks (ie, VN and DMN) and “control systems”-related networks (ie, FPN, DAN, VAN, and SMN) in pED patients that might be useful to understand the neural mechanism underlying cognitive dysfunction in pED. Specifically, VN and DMN, which are relatively stationary, have the high similarity of node connections within somatosensory-motor cortex.36 Our findings further indicated that the FC within VN network was positively correlated with the function of immediate memory, suggesting that the decreased FC across the visual regions might reflect pED in relation to lower ability of immediate memory. Since DMN is firstly identified with resting states, it is thought that DMN functionality is associated with mind wandering and stimulus-independent responses.37 Importantly, previous studies have indicated that DMN plays an increased role during enhanced attentional demands.38 The balance between DMN and other networks which control attentional and executive functions is crucial in determining the subject’s level of impulsive behaviors.39 From the cognitive control perspective, DMN serves more as a ‘processing system’ in contrast with FPN.40 Neuroimaging studies have indicated that trait mind wandering is mediated by connectivity between the DMN and FPN.15,16 By evaluating the sensory representation in visual cortex, FPN, DAN, and VAN may have dynamic connections to promote visual attention.41,42 In the present study, we found that the FC between VAN and DMN networks was negatively correlated with the visuospatial ability, indicating that the unbalance between VAN and DMN might disturb the visuospatial ability in pED.
However, there still remained some limitations in interpreting the results of the present study. Firstly, recruited pED patients and HCs were not examined for hormonal levels in this study, which might contribute to mediation effects. The lower cognitive performance of pED patients was affected by several variables, which should be considered in future researches to investigate the median effect on functional brain networks. Secondly, this study was to evaluate the data at a single time point. Future researches are needed to explore whether the intervention on cognitive function can improve the erectile function. Thirdly, the relationship between cognitive functions and large-scale brain networks’ FC was needed to be verified. Hence, future researches with larger samples are required in exploring pED.
Conclusion
Conclusively, our findings suggested that aberrant FC in large-scale brain networks might underlie cognitive dysfunction in pED, which provided the new evidence concerning cognitive dysfunction accompanied by aberrant FC of large-scale brain networks in pED. Further understanding of pED associated with cognitive dysfunction might be obtained by studying the changed FC between large-scale brain networks.
Ethical Conduct of Research
This study complies with the Declaration of Helsinki and was conducted on the base of guidelines by the ethics committee of Beijing Anding Hospital, Capital Medical University.
Acknowledgments
This study is supported by National Natural Science Foundation (Grant no. 81873398, 82174311), Beijing Hospitals Authority Clinical Medicine Development of Special Funding (Grant no. ZYLX202129), and Beijing Hospitals Authority’s Ascent Plan (Grant no. DFL20191901).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Georgiadis JR, Kringelbach ML, Pfaus JG. Sex for fun: a synthesis of human and animal neurobiology. Nat Rev Urol. 2012;9(9):486–498. doi:10.1038/nrurol.2012.151
2. Zou Z, Lin H, Zhang Y, et al. The role of nocturnal penile tumescence and rigidity (NPTR) monitoring in the diagnosis of psychogenic erectile dysfunction: a review. Sex Med Rev. 2019;7(3):442–454. doi:10.1016/j.sxmr.2018.10.005
3. Glina S, Cohen DJ, Vieira M. Diagnosis of erectile dysfunction. Curr Opin Psychiatry. 2014;27(6):394–399. doi:10.1097/YCO.0000000000000097
4. Sivalingam S, Hashim H, Schwaibold H. An overview of the diagnosis and treatment of erectile dysfunction. Drugs. 2006;66(18):2339–2355. doi:10.2165/00003495-200666180-00006
5. Calabrò RS, Cacciola A, Bruschetta D, et al. Neuroanatomy and function of human sexual behavior: a neglected or unknown issue? Brain Behav. 2019;9(12):e01389. doi:10.1002/brb3.1389
6. Stoléru S. Reading the Freudian theory of sexual drives from a functional neuroimaging perspective. Front Hum Neurosci. 2014;8:157. doi:10.3389/fnhum.2014.00157
7. Hagemann JH, Berding G, Bergh S, et al. Effects of visual sexual stimuli and apomorphine SL on cerebral activity in men with erectile dysfunction. Eur Urol. 2003;43(4):412–420. doi:10.1016/S0302-2838(03)00002-2
8. Zhang X, Guan M, Chen X, et al. Identifying neuroimaging biomarkers for psychogenic erectile dysfunction by fusing multi-level brain information: a resting-state functional magnetic resonance imaging study. Andrology. 2022;10(7):1398–1410. doi:10.1111/andr.13238
9. Zhao L, Guan M, Zhu X, et al. Aberrant topological patterns of structural cortical networks in psychogenic erectile dysfunction. Front Hum Neurosci. 2015;9:675. doi:10.3389/fnhum.2015.00675
10. Banner LL, Anderson RU. Integrated sildenafil and cognitive-behavior sex therapy for psychogenic erectile dysfunction: a pilot study. J Sex Med. 2007;4(4 Pt 2):1117–1125. doi:10.1111/j.1743-6109.2007.00535.x
11. Monteiro J, Castelhano J, Pignatelli D, et al. Do psychogenic erectile dysfunction and premature ejaculation share a neural circuit? Evidence from a fMRI systematic review and meta-analysis. Appl Sci. 2022;12(21):11249. doi:10.3390/app122111249
12. Cera N, Castelhano J, Oliveira C, et al. The role of anterior and posterior insula in male genital response and in visual attention: an exploratory multimodal fMRI study. Sci Rep. 2020;10(1):18463. doi:10.1038/s41598-020-74681-x
13. Cera N, Di Pierro ED, Ferretti A, et al. Brain networks during free viewing of complex erotic movie: new insights on psychogenic erectile dysfunction. PLoS One. 2014;9(8):e105336. doi:10.1371/journal.pone.0105336
14. Zhou Y, Friston KJ, Zeidman P, et al. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex. 2018;28(2):726–737. doi:10.1093/cercor/bhx307
15. Godwin CA, Hunter MA, Bezdek MA, et al. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia. 2017;103:140–153. doi:10.1016/j.neuropsychologia.2017.07.006
16. Golchert J, Smallwood J, Jefferies E, et al. Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. Neuroimage. 2017;146:226–235. doi:10.1016/j.neuroimage.2016.11.025
17. Jin C, Guan M, Dong M, et al. Aberrant baseline brain activity in psychogenic erectile dysfunction patients: a resting state fMRI study. Brain Imaging Behav. 2018;12(5):1393–1404. doi:10.1007/s11682-017-9805-9
18. Cera N, Carvalho J, Quinta-Gomes A, et al. Brain dynamics of the inhibition of genital response in psychogenic erectile dysfunction. J Sex Med. 2017;14(Supplement_4b):e241–e241. doi:10.1016/j.jsxm.2017.04.207
19. Zhao L, Guan M, Zhang X, et al. Structural insights into aberrant cortical morphometry and network organization in psychogenic erectile dysfunction. Hum Brain Mapp. 2015;36(11):4469–4482. doi:10.1002/hbm.22925
20. Stoléru S, Fonteille V, Cornélis C, et al. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012;36(6):1481–1509. doi:10.1016/j.neubiorev.2012.03.006
21. Finney GR, Minagar A, Heilman KM. Assessment of mental status. Neurol Clin. 2016;34(1):1–16. doi:10.1016/j.ncl.2015.08.001
22. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi:10.1016/j.tics.2011.08.003
23. Cheng Y, Wu W, Wang J, et al. Reliability and validity of the repeatable battery for the assessment of neuropsychological status in community-dwelling elderly. Arch Med Sci. 2011;7(5):850–857. doi:10.5114/aoms.2011.25561
24. Zhang BH, Tan YL, Zhang WF, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in Chinese: reliability and validity. Chin Ment Health J. 2008;22:865–869.
25. Feng S, Zheng S, Zou H, et al. Altered functional connectivity of cerebellar networks in first-episode schizophrenia. Front Cell Neurosci. 2022;16:1024192. doi:10.3389/fncel.2022.1024192
26. Wu P, Zhang A, Sun N, et al. Cortical thickness predicts response following 2 weeks of SSRI regimen in first-episode, drug-naive major depressive disorder: an MRI study. Front Psychiatry. 2021;12:751756. doi:10.3389/fpsyt.2021.751756
27. Yan CG, Wang XD, Lu B. DPABISurf: data processing & analysis for brain imaging on surface. Sci Bull. 2021;66(24):2453–2455. doi:10.1016/j.scib.2021.09.016
28. Dosenbach NU, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi:10.1126/science.1194144
29. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi:10.1152/jn.00338.2011
30. MacDonald SM, Burnett AL. Physiology of erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2021;48(4):513–525. doi:10.1016/j.ucl.2021.06.009
31. Chinchilla Alfaro K, van Hunsel F, Ekhart C. Persistent sexual dysfunction after SSRI withdrawal: a scoping review and presentation of 86 cases from the Netherlands. Expert Opin Drug Saf. 2022;21(4):553–561. doi:10.1080/14740338.2022.2007883
32. Gombert M, Ballester P, Segura A, et al. Introducing sexual dysfunction in mental care. Expert Opin Drug Saf. 2021;20(1):69–79. doi:10.1080/14740338.2020.1849135
33. Dusenbury W, Palm Johansen P, Mosack V, et al. Determinants of sexual function and dysfunction in men and women with stroke: a systematic review. Int J Clin Pract. 2017;71(7):e12969. doi:10.1111/ijcp.12969
34. Rossi V, Galizia R, Tripodi F, et al. Endometriosis and sexual functioning: how much do cognitive and psycho-emotional factors matter? Int J Environ Res Public Health. 2022;19(9):5319. doi:10.3390/ijerph19095319
35. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi:10.1016/j.tics.2010.04.004
36. Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49(4):3132–3148. doi:10.1016/j.neuroimage.2009.11.001
37. Andrews-Hanna JR, Reidler JS, Huang C, et al. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104(1):322–335. doi:10.1152/jn.00830.2009
38. Popa D, Popescu AT, Paré D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29(4):1191–1201. doi:10.1523/JNEUROSCI.4867-08.2009
39. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi:10.1146/annurev-neuro-071013-014030
40. Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–258. doi:10.1073/pnas.0135058100
41. Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10(1):38–45. doi:10.1016/j.tics.2005.11.008
42. Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16(2):129–135. doi:10.1016/j.tics.2011.11.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.