Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Altered Functional Activity and Functional Connectivity of Seed Regions Based on ALFF Following Acupuncture Treatment in Patients with Stroke Sequelae with Unilateral Limb Numbness
Authors Peng J, Su J, Song L, Lv Q, Gao Y, Chang J, Zhang H, Zou Y, Chen X
Received 28 September 2022
Accepted for publication 28 December 2022
Published 25 January 2023 Volume 2023:19 Pages 233—245
DOI https://doi.org/10.2147/NDT.S391616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Jing Peng,1,* Jiaming Su,2,* Lei Song,1 Qiuyi Lv,1 Ying Gao,1 Jingling Chang,1 Hua Zhang,1 Yihuai Zou,1 Xing Chen3
1Department of Encephalopathy, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Nephrology, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Department of Brain Function Examination, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xing Chen, Department of brain function examination, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, No. 05 Haiyuncang Road, Beijing, People’s Republic of China, Tel +86 18610044016, Email [email protected]
Objective: Limb numbness is a frequent symptom of post-stroke somatosensory dysfunction, which may be alleviated by non-invasive therapy such as acupuncture. However, the precise mechanism via acupuncture remains unknown. The goal of this study was to investigate how the amplitude of low-frequency fluctuations (ALFF) and functional connectivity (FC) changed between stroke patients with limb numbness and healthy people, as well as how acupuncture might work.
Methods: 24 stroke sequelae patients with unilateral limb numbness and 14 matched healthy controls were enrolled in the study. The patients with limb numbness received acupuncture therapy three days a week for four weeks. We mainly assessed the clinical outcomes via the visual analogue scale (VAS). In addition, fMRI data from patients with unilateral limb numbness at baseline and after treatment (4th week) were collected, as well as data from healthy controls at baseline.
Results: Compared with the healthy subjects, the patient group demonstrated significantly decreased ALFF in several brain regions, mainly associated with the sensorimotor network (SMN) and default mode network (DMN), including left superior frontal gyrus (SFG), right temporal fusiform cortex (TFC), right middle frontal gyrus (MFG), bilateral middle temporal gyrus (MTG), right putamen (PUT), right precentral gyrus (preCG), right planum polare (PP), and left supplementary motor area (SMA). These regions were chosen as the seeds for investigating the FC alteration induced by acupuncture. Several sensorimotor-related brain regions were activated by acupuncture, and the FC of the left supramarginal gyrus (SMG) with right MTG, as well as brain-stem, cerebellum vermis 9 with right MFG showed enhancement following acupuncture in the patient group, which had a significant correlation with clinical outcomes.
Conclusion: Acupuncture treatment may be used to stimulate brain areas associated with somatosensory processing and to strengthen the FC of sensorimotor and cognitive brain networks in order to achieve therapeutic effect.
Keywords: acupuncture, stroke, somatosensory dysfunction, functional magnetic resonance imaging, amplitude of low-frequency fluctuation, functional connectivity
Corrigendum for this paper has been published.
Introduction
Stroke is a common disease with a high occurrence rate and disability rate worldwide, with approximately 50% to 80% of stroke patients suffering from varied degrees of somatosensory dysfunction.1,2 Somatosensory dysfunction manifests itself in a variety of ways, with limb numbness being one of the most prevalent. However, during the convalescence period following a stroke, motor dysfunction is typically given more attention than somatosensory dysfunction. After motor function is restored, patients frequently focus their attention on somatosensory deficits. Since limb numbness is a conscious symptom that is seldom observed, and because the therapeutic impact is inadequate, the lingering state of numbness makes it easier to raise patients’ psychological and mental stress, resulting in sleep difficulties, anxiety, and depression.3 On the other hand, as somatosensory function is intrinsically linked to motor function, an intact somatosensory system has a beneficial effect on motor performance.4 Thus, it is critical to develop a therapy technique that is both safe and effective.
The World Health Organization classified acupuncture as stroke rehabilitation in 1998. Numerous clinical studies have demonstrated that acupuncture improves balancing function, decreases spasticity, increases muscular strength, and general health following stroke.5 However, there is a shortage of study on stroke-induced pure somatosensory disturbance. According to Traditional Chinese Medicine theory, the acupuncture therapy is effective for treating post-stroke limb numbness. Acupoints such as Baihui (GV20), Sishencong (EX-HN1), Shuigou (GV26), Quchi (LI11) and Zusanli (ST36) are included. Acupuncture at ST36 and LI11 has been shown to increase neurogenesis, anti-apoptosis, and cell proliferation in ischemic tissues, acupuncture at GV26 has been shown to induce neurogenesis, and acupuncture at GV20 has been shown to modulate neuroplasticity by reducing brain atrophy following ischemia, but the underlying neuroimaging mechanism remains unknown.6–10
Neuroimaging technology is a promising, noninvasive approach that is increasingly being utilized to diagnose and treat neuropsychiatric disorders. Among which, researches on the mechanisms underlying acupuncture utilizing functional magnetic resonance imaging (fMRI) has grown. The amplitude of low-frequency fluctuations (ALFF) could indicate the intensity of voxel spontaneous activity in terms of energy by calculating the mean values of all frequency amplitudes in the 0.01 ~ 0.08 HZ band.11 Functional connectivity (FC) is a term that refers to the level of functional connection between areas. It is calculated by analysing the correlation between one region and the rest of the brain.12 Somatosensory dysfunctions following stroke are related with a lack of activation of the primary somatosensory cortex and spontaneous slow oscillatory activity in postcentral areas, the study findings indicate. Additionally, researches indicate that acupuncture could activate somatosensory-related brain areas and improve somatosensory process following stroke, implying the feasibility of further research into the mechanism of acupuncture’s effect on somatosensory dysfunction following stroke.13,14
Evidence shows that there was spontaneous significant recovery in somatosensory deficit within 3 months of stroke onset, with only subtle additional improvements after 12 months.2 Thus, the study was designed to examine the consequences of steady spontaneous recovery in cerebral functional remodeling following stroke and to ensure that the stroke group has a stable baseline. We first used the ALFF analysis to evaluate changes in the brain regional activity of patients at baseline. The seed areas for FC analysis were selected from the brain regions of patients with aberrant ALFF. Then, we computed the FC of these seed locations in patients and healthy controls by applying a seed to voxel approach. Finally, we evaluated the ALFF activity and FC pattern between patients before and after acupuncture therapy to determine if acupuncture could modulate functional activity, thus providing evidence for acupuncture’s efficacy in promoting overall sensation and rehabilitation effect. We hypothesized that: there would be some variance in spontaneous activity and functional connectivity in patients with limb numbness following stroke; and the functional activity and resting-state functional connectivity would be regulated by acupuncture.
Materials and Methods
Subjects
A total of 24 ischemic stroke sequelae patients with unilateral limb numbness were recruited at Dongzhimen Hospital. All patients meet the following inclusion criteria: unilateral limb numbness following stroke; between 30 and 75 years old; right-handed; 6 months-5 years after the onset of stroke; lesion location indicated subcortical area confirmed by MRI; muscle strength of the affected limb is grade III or above; no mental disorders and no disturbance of consciousness; willing to accept acupuncture therapy for 4 weeks and sign informed consent. Patients were excluded if they met any of the criteria below: lesion location indicated cortical areas confirmed by MRI; history of epilepsy or other neurological disease and psychiatric disorder; patients with pre-existent peripheral neuropathy including diabetic neuropathy; serious cognitive deficits; those who are unable to comprehend and participate in the examination, or those who have a MRI contraindication, such as claustrophobia. 14 age- and gender-matched volunteers without cerebrovascular diseases were enrolled as healthy control subjects. The inclusion criteria were: right-handed; without central nervous system disease, psychosis and serious physical diseases; subjects had no history of psychiatric, neurological, or medical disease and no history of drug or alcohol dependence. This clinical research was approved by the Medical Ethics Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine (based on the Declaration of Helsinki (2021) Ethical Batch No: DZMEC-KY-2019-180). Risks and benefits were explained with all subjects, and informed consents were acquired.
Acupuncture Therapy and Clinical Evaluation
All stroke sequelae patients with unilateral limb numbness were examined before and after acupuncture therapy using a visual analogue scale (VAS): a score of 0 to 10 was used to describe the degree of numbness; 0 indicated no numbness, and 10 indicated intolerable acute numbness. Patients self-rated their level of numbness, with a higher score indicating a greater level of numbness.
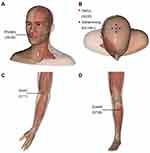
We applied acupuncture therapy to all of the stroke sequelae patients for 4 weeks, three times per week, and 20 mins each time. Acupoints were localized according to the name and location of acupoints (Chinese National Standards GB/T12346). Acupuncture was performed at the following acupoints (Figure 1A–D): Baihui (GV20, located in median line of head), Sishencong (EX-HN1, located around GV20), Shuigou (GV26, located in the upper part of philtrum), Quchi (LI11, located in lateral side of elbow striae)(affected side) and Zusanli (ST36, located in the lateral shinbone) (affected side). Acupuncture was performed by inserting a sterile, single-use silver needle (40mm in length and 0.30mm in diameter). At GV20 and EX-HN1, needles were inserted 15–30 degrees above to the scalp surface and 1.5 inches deep. The needle was placed towards the nose at a depth of 5–10 mm for GV26. At LI11 and ST36, acupuncture needles were inserted 20–30 mm obliquely downward toward the hand and foot. Following insertion, the needle sheath was twirled and rotated to induce the sensation known as Deqi (a sensation of soreness, numbness, distention, or radiating that is regarded to signify effective needling).15
fMRI Data Acquisition and Preprocessing
We acquired the fMRI data of stroke patients at baseline and after treatment (4th week) and from healthy controls at baseline. MRI data were collected using a 3.0 Tesla Siemens Magnetom Verio MRI System (Siemens Medical, Erlangen, Germany) in Dongzhimen hospital. All participants were advised to remain still, keep their eyes closed, and think nothing as custom-fit foam pads and earplugs were utilized to decrease head movements and scanner noise. The functional MR images were acquired utilizing a 6-minute gradient echo-planar imaging (EPI) sequence with interleaved acquisition and the following scan parameters: TR/TE = 2000 /30 ms, FA = 90°, FOV = 240 mm×240 mm, matrix = 128×128, slice thickness = 5 mm and slices = 31. The high resolution T1-weighted images comprised of a volumetric three-dimensional spoiled gradient recall sequence lasting 4 minutes and 26 seconds with the parameters: TR/ TE = 1900 / 3.93 ms, FOV = 240 mm × 240 mm, matrix: 256 × 256, FA = 15°, slice thickness = 1 mm and slices = 176.
Resting-state fMRI data were preprocessed using Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm) and NITRC Functional Connectivity (CONN, http://www.conn-toolbox.org). The DICOM data format was converted into NIFTI data format, and the initial 10 time points functional volumes were discarded to avoid the influence of unstable signal in the initial stage or the subject’s discomfort to the environment. Then, the rest of volumes were preprocessed by slice timing and realignment, the data of maximum head motion > 1.5 mm and rotation angle > 1.5° in each direction (x, y, z) were excluded from further analysis. Next, warping individual functional images to the standard Montreal Neurological Institute (MNI) space by applying the transformation matrix that can be derived by registering the T1 image (co-registered with functional images). The data after spatial normalization were smoothed with a 4-mm full width at half maximum (FWHM) Gaussian kernel. Nuisance signals were removed out from our data, including the six head motion parameters, white matter signal and cerebrospinal fluid (CSF) signal linear. Finally, linear drift and filtering were carried out to remove low frequency drift and high frequency breath and heartbeat noise. Moreover, ITK-SNAP (http://www.itksnap.org/pmwiki/pmwiki.php) software was used to manually delineate the abnormal T1-weighted image by layer, and then the patient’s lesion overlay map was obtained.
ALFF and Functional Connectivity Analysis
After the above preprocessing, the Amplitude of low-frequency fluctuation (ALFF) analysis was carried out in the CONN toolbox v18 (http://www.nitrc.org/projects/conn) The power spectrum was created by performing a Fast Fourier transform on the time series of each voxel to transform it to the frequency domain. The square roots of each frequency in the power spectrum were then obtained, then by the mean square root over a low-frequency range (0.01–0.08 Hz), which was referred to as the ALFF index. The ALFF values of all the patients and healthy controls will be compared by two-sample t-tests. According to the inter-group comparison of ALFF, the brain areas with abnormal ALFF were considered as the seed regions for further functional connectivity analysis. To explore resting-state properties in structurally affected brain areas, functional connectivity analysis was performed using the CONN functional connectivity toolbox v18 (http://www.nitrc.org/projects/conn).
In first-level analysis, the mean BOLD time series were extracted from seeds that were obtained by ALFF analysis of each subject under different conditions, including one rest condition in healthy subjects and two rest conditions before and after acupuncture treatment in patients. Pearson correlation analysis was used to determine the relationship between the mean time course of each seed and the time course of each voxel in the entire brain. Finally, Fisher’s r-to-z transformation is performed to standardize and improve the data’s normality in order to obtain the full brain’s z-score map for subsequent research.
Statistical Analysis
Demographic data was compared by using two-sample t-test and Chi-square test. Clinical data before and after acupuncture treatment were compared using the paired-sample t-test. The significance threshold was set at P < 0.05 for all statistical analysis. To distinguish abnormal ALFF regions between patients and healthy subjects at baseline, a two-sample t-test was utilized. The abnormal regions were selected as seeds. Then, a two-sample t-test was utilized to investigate distinct patterns of FC between patients and healthy subjects based on seeds using CONN’s second-level analysis. The paired-samples t-test was used to assess the ALFF and FC changes before and after acupuncture. The significance thresholds for all statistical analysis were P<0.05 when the false discovery rate (FDR) was adjusted. Finally, Pearson correlation was used to evaluate the relation between fMRI data (t-values for ALFF, z-values for FC) and clinical characteristics of patients’ VAS scores, with age and gender regarded as covariates of no interest. P < 0.05 was set as the significance threshold.
Results
Demographic, Clinical Scale Results and Lesion Overlay Map
Patient characteristics and clinical scale are listed in Table 1 and a lesion overlay map is available in Figure 2. Twenty-four stroke sequelae patients with unilateral limb numbness and 14 matched healthy controls were enrolled in the study. One patient was excluded due to incomplete data. No significant differences in age and gender were found between patients and healthy controls groups. The visual analogue score (VAS) scale to measure numbness symptoms in patients groups before and after acupuncture therapy. According to Table 1, the numbness was significantly relieved according to VAS scores and the average VAS score was 4.38±0.71 before acupuncture therapy, and decreased to 1.42±1.41 (P<0.01).
 |
Table 1 Demographic and Clinical Scale |
ALFF Analysis Results
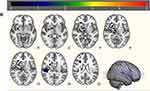
ALFF analysis identified decreased ALFF in left superior frontal gyrus (L_SFG), right temporal fusiform cortex (R_TFC), right middle frontal gyrus (R_MFG), left middle temporal gyrus (L_MTG), right putamen (R_PUT), right precentral gyrus (R_preCG), right planum polare (R_PP), left supplementary motor area (L_SMA) and right middle temporal gyrus (R_MTG) in stroke patients with unilateral limb numbness compared to healthy controls (Figure 3A and B and Table 2). The ALFF effect size comparison between two groups (Figure 4). In order to investigate the acupuncture effect in ALLF, a paired-samples t-test was conducted. There is significantly increased ALFF in left supramarginal gyrus (L_SMG), right thalamus, right postcentral gyrus (R_postCG), left precentral gyrus (L_preCG), and left parahippocampal gyrus (L_PHG) (Table 3).
 |
Table 2 Regions Showing Decreased ALFF in Patients with Numbness at Baseline |
 |
Table 3 Regions Showing Increased ALFF in Patients Before and After Acupuncture Treatment |
 |
Figure 3 ALFF difference maps (A) and (B). Cool color represents decreased ALFF brain area in numbness patients, and colorbar represents T value. |
Functional Connectivity Analysis Results
There were significantly abnormal FC patterns in stroke with numbness patients compared to healthy controls at baseline. Patients showed significantly decreased FC between L_SFG and sensorimotor area (especially right preCG and postCG), left inferior temporal gyrus (L_ITG), right parietal operculum cortex (R_POC). Meanwhile, patients had significantly increased FC between right putamen (R_PUT) and right precentral gyrus (R_preCG); R_MTG and right lateral occipital cortex (R_LOC) (Table 4).
 |
Table 4 Regions Showing Decreased or Increased FC in Stroke Patients with Numbness Comparing to Healthy Controls Based on Seed Regions of Abnormal ALFF |
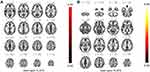
With regard to the FC alterations following acupuncture treatment, we noticed that increased FC in the left supramarginal gyrus (L_SMG) with R_MTG, increased FC in cerebellum vermis 9 with R_MFG, and increased FC in brain-stem with R_MFG (Figure 5A and B and Table 5).
 |
Table 5 Regions Indicating Increased FC After Acupuncture in Stroke Patients with Numbness Compared to Before Acupuncture |
Pearson Correlation Between fMRI Data and the Clinical Characteristics of Patients Results
In the numbness patients group, we found the t-values of ALFF in L_SMG (r = −0.401, P=0.007), L_preCG (r = −0.350, P=0.023), L_PHG (r = −0.570, P=0.000), R_postCG (r = −0.368, P=0.019) were negatively related to VAS; z-values of FC of R_MTG with L_ SMG was negatively related to VAS (r = −0.383, P=0.009); z-values of FC of R_MFG with cerebellum vermis 9 was negatively related to VAS (r = −0.528, P=0.000) (Figure 6A–F).
Discussion
Aberrant ALFF and FC in Patients with Limb Numbness
The study investigated the pattern of spontaneous brain activity and functional connectivity in stroke patients with limb numbness, as well as the potential modulatory effect of acupuncture therapy using rs-fMRI technology. The results validated previous hypothesis that: firstly, there exists aberrant functional activity in patients with limb numbness compared to healthy subjects. Mainly decreased ALFF in the SFG, TFC, MFG, MTG, PUT, preCG, PP, and SMA. Decreased L_SFG functional connectivity with R_preCG, R_postCG, L_ITG and R_POC, and increased PUT functional connectivity with R_preCG, increased R_MTG functional connectivity with R_LOC; secondly, acupuncture could increase the ALFF in the SMG, postCG, preCG, thalamus, PHG, and enhance the functional connectivity in R_MFG with brain-stem, cerebellum vermis 9; the functional connectivity in R_MTG with L_SMG, which showed strongly negative connection with VAS score, it may be the effective mechanism to improve the clinical symptoms of stroke patients with limb numbness.
The majority of the decreased ALFF brain regions in patients group are associated with sensorimotor processing. The SFG, preCG, and SMA are critical components of the sensorimotor network (SMN). Previous research has reported that the main symptoms of somatosensory network dysfunction is limb numbness, and fMRI has revealed reduced activation of related brain areas.16 Additionally, MTG is a key node in the default mode network (DMN), while MFG participates in the executive control network (ECN), and the PUT is comprised in the basal ganglia network (BGN), indicating that patients with abnormal functional activity regions following stroke were mainly involved in the SMN but also included other critical brain networks.17 Numerous studies demonstrate that the SMN is the most vulnerable network to stroke. On the other hand, advanced cognitive control networks related to sensorimotor function, such as the frontoparietal executive control network, will also experience functional impairments or compensation, a process referred to as functional reorganization.18
Additionally, FC reflects the connection across brain regions, allowing for the exploration of further functional brain networks. The decreased FC indicates significant damage to the neural network caused by ischaemia in stroke patients with numbness. The enhanced FC shows that the relevant brain areas are involved in the spontaneous reconfiguration in a significant way. The FC within the SMN was found to be attenuated in patients (L_SFG with R_preCG and R_postCG). Several studies on this topic have reported impaired connection between the interhemispheric SMN in stroke patients.19,20 Meanwhile, the FC of L_SFG combined with L_ITG and L_SFG combined with R_POC decreased. ITG is the visual cortex, and study found that the visual cortex is highly dependent on somatosensory stimulation, implying that the somatosensory cortex and the visual cortex have extensive functional connections.21 These findings imply that somatosensory dysfunction may alter the functional connections of the visual-related brain. Apart from the decreased FC activity in the L_SFG, we noticed enhanced FC in R_preCG with PUT and in R_LOC with R_MTG. As we all know, the LOC is a brain region responsible for scene and object perception and categorization.22 The PUT is a primary cortical input location to the basal ganglia loops and is associated with sensory processing.23 It is the brain area most usually affected in persons with sensory distinction.24 Increased FC between the putamen and precentral gyrus was associated with sensory pain and motor dysfunction in a prior investigation, which is congruent with our findings. Previous research has established that functional reconfiguration occurs following stroke.25,26 We hypothesize that PUT and MTG enhance their FC activity to compensate for sensory disturbances associated with stroke numbness.
Regulation Effects on the Functional Activity and FC of Seed Regions Based on ALFF Induced by Acupuncture
In addition to the abnormal ALFF reported previously in our work, we obtained several significant data indicating acupuncture’s regulative effect. After acupuncture, we observed a significant increase in ALFF in the L_SMG, R_postCG, L_preCG, right thalamus, and L_PHG. The postCG and preCG are key nodes in the SMN, which is responsible for human motor execution, sensory processing, and motor-sensory integration.27 The thalamus is the highest sensory region beneath the cortex, and all sensory information except for olfaction passes through it before reaching the cortex.28 Additionally, the thalamus regulates the activation in other brain regions via so-called “corticothalamocortical circuits”. With the development of research on limb numbness, it has been found that damage to the sensory pathway from the cortex to the contact fiber results in numbness, and so do changes in brain activity in the “corticothalamocortical circuits” and the SMN, which are considered to be the critical links in limb numbness following stroke.29,30 After acupuncture therapy, the ALFF of the thalamus and SMN increased, which may be one of the mechanisms of acupuncture. It’s important to mention that there was no evidence of a significantly decreased ALFF in the thalamus at baseline, this could be due to the location of the lesion in our patients. However, the ALFF increased significantly after acupuncture, we speculated that this was because we had always emphasized Deqi (sensation of soreness, numbness, distention, or radiating that is regarded to signify effective needling) throughout treatment, which is believed to be crucial for acupuncture’s therapeutic success.31 Naturally, the thalamus is the principal brain structure responsible for mediating the effects of acupuncture.32 Acupuncture on the left or right Yanglinquan (GB34) of a healthy population activates the thalamus physiologically, and the thalamus is assumed to be implicated in some of the pathological disorders treated with acupuncture.33,34 We inferred that acupuncture could activate sensorimotor-related brain regions in stroke patients with numbness based on increased spontaneous activity in the above brain regions following treatment and the strongly negative connection between the ALLF and VAS score.
Further, we investigated the influence of acupuncture on the FC of nine seed regions acquired in ALFF analysis in comparison to each other region within the total brain between the pre- and post-acupuncture stages. Our research revealed an interesting result: increased FC between R_MTG and L_SMG. Notably, this study found that the functional activity of the L_SMG is significantly increased, regardless of whether ALFF or FC analysis is applied. The SMG is a somatosensory-associated cortex that is capable of interpreting sensory information and is involved in spatial and limb position perception.35 The left SMG is crucial for the integration of sensory and cognitive processes, such as selection and the control of tool-directed actions.36 MTG and SMG are both component nodes of the DMN, implying that acupuncture therapy enhanced FC within the DMN. There is evidence that suggests that abnormalities in the functional connectivity of the DMN may contribute to the development of post-stroke depression and anxiety.37 The rehabilitation of cognitive ability and sensorimotor function may be the mechanism by which acupuncture affects the DMN of stroke patients, which confirms our results.38
Our research also found that acupuncture can enhance the FC between the brain-stem and R_MFG, cerebellum vermis 9 and R_MFG. The FC of periaqueductal gray (PAG) within brain-stem is related to the sensory of pain, and acupuncture could modulate the FC of PAG to treat pain-related disease in previous study.39,40 Furthermore, the cerebellum has been regarded as a critical component of the sensorimotor system.41 More and more research suggest that the cerebellum can affect the higher cognitive function of the brain through the neural circuits between the cerebellum and the brain.42 Especially, vermis as an essential part of bilateral cerebellar hemisphere for structural connection and information transmission, it is not only related to sensorimotor dysfunction, but also impacts cognitive functions such as working memory and emotional regulation.43,44 Previous studies have demonstrated that acupuncture improves motor coordination and motor learning in stroke patients during stable recovery by improving the FC between the cerebellum and the primary sensorimotor cortex.45 In our study, MFG is one of the essential node of ECN, which is widely attributed to a set of cognitive processes.46 The enhanced FC between cerebellum vermis and ECN after acupuncture therapy once again revealed that acupuncture might exert its therapeutic effect through improving cognitive ability and sensorimotor function.
There are still some limitations in our research. Firstly, one of which is that the sample size is on the lower end. The difficulty in reproducing the results of the study is a significant barrier that must be overcome before the findings of contemporary neuroimaging research can be implemented successfully in therapeutic settings. As a result of the possibility that increasing the sample size is an efficient method for improving the consistency and reliability of neuroimaging data, we are collecting more patients in order to validate the findings that have already been obtained. Secondly, the great majority of studies have focused on single acupoint in order to demonstrate the specificity of each acupoint. However, this group of acupoints is widely used in clinics, and the mechanisms are more complex. As a result, we can only assume that group acupoints are responsible for the modulatory effects, whereas the mechanism of a single acupoint remains unknown. Last but not least, given that limb numbness is a conscious experience, we only employed a subjective VAS to evaluate the numbness degree, therefore the outcome measure of numbness using VAS may have a high placebo effect. Additionally, because there were no sham controls, it is difficult to determine how much of the improvement in numbness and fMRI performance was attributable to the placebo effect.
Conclusion
Our findings suggest that acupuncture therapy may increase the spontaneous activity of sensorimotor-related brain regions, as well as the functional connectivity of sensorimotor- and cognitive-associated networks, in stroke sequelae patients with unilateral limb numbness. This will aid in understanding the mechanisms of acupuncture and providing an effective method of clinical treatment in patients suffering from limb numbness following a stroke.
Acknowledgments
The current study was supported by the National Natural Science Foundation of China (No. 81904285).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi:10.1016/S0140-6736(11)60325-5
2. Kessner SS, Schlemm E, Cheng B, et al. Somatosensory deficits after ischemic stroke. Stroke. 2019;50:1116–1123. doi:10.1161/STROKEAHA.118.023750
3. Sullivan JE, Hedman LD. Sensory dysfunction following stroke: incidence, significance, examination, and intervention. Top Stroke Rehabil. 2008;15:200–217. doi:10.1310/tsr1503-200
4. De Bruyn N, Saenen L, Thijs L, et al. Sensorimotor vs. motor upper limb therapy for patients with motor and somatosensory deficits: a randomized controlled trial in the early rehabilitation phase after stroke. Front Neurol. 2020;11:597666. doi:10.3389/fneur.2020.597666
5. Chavez LM, Huang SS, MacDonald I, et al. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. Int J Mol Sci. 2017;18:2270. doi:10.3390/ijms18112270
6. Hong J, Wu G, Zou Y, et al. Electroacupuncture promotes neurological functional recovery via the retinoic acid signaling pathway in rats following cerebral ischemia-reperfusion injury. Int J Mol Med. 2013;31:225–231. doi:10.3892/ijmm.2012.1166
7. Xue X, You Y, Tao J, et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14–19. doi:10.1016/j.neulet.2013.10.029
8. Chen B, Tao J, Lin Y, et al. Electro-acupuncture exerts beneficial effects against cerebral ischemia and promotes the proliferation of neural progenitor cells in the cortical peri-infarct area through the Wnt/β-catenin signaling pathway. Int J Mol Med. 2015;36:1215–1222. doi:10.3892/ijmm.2015.2334
9. Luo D, Fan X, Ma C, et al. A study on the effect of neurogenesis and regulation of GSK3β/PP2A expression in acupuncture treatment of neural functional damage caused by focal ischemia in MCAO rats. Evid Based Complement Alternat Med. 2014;2014:962343. doi:10.1155/2014/962343
10. Chuang CM, Hsieh CL, Li TC, et al. Acupuncture stimulation at Baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injured Sprague-Dawley rats. Am J Chin Med. 2007;35:779–791. doi:10.1142/S0192415X07005260
11. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi:10.1038/nrn2201
12. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi:10.1016/j.euroneuro.2010.03.008
13. Castillo EM, Boake C, Breier JI, et al. Aberrant cortical functionality and somatosensory deficits after stroke. J Clin Neurophysiol. 2008;25:132–138. doi:10.1097/WNP.0b013e318176c0d4
14. Li G, Jack CR Jr, Yang ES. An fMRI study of somatosensory-implicated acupuncture points in stable somatosensory stroke patients. J Magn Reson Imaging. 2006;24:1018–1024. doi:10.1002/jmri.20702
15. Zhao L, Li D, Zheng H, et al. Acupuncture as adjunctive therapy for chronic stable angina: a randomized clinical trial. JAMA Intern Med. 2019;179:1388–1397. doi:10.1001/jamainternmed.2019.2407
16. Casas-Torremocha D, Clascá F, Núñez Á. Posterior thalamic nucleus modulation of tactile stimuli processing in rat motor and primary somatosensory cortices. Front Neural Circuits. 2017;11:69. doi:10.3389/fncir.2017.00069
17. Skandalakis GP, Komaitis S, Kalyvas A, et al. Dissecting the default mode network: direct structural evidence on the morphology and axonal connectivity of the fifth component of the cingulum bundle. J Neurosurg. 2020;134:1334–1345. doi:10.3171/2020.2.JNS193177
18. Wang C, Qin W, Zhang J, et al. Altered functional organization within and between resting-state networks in chronic subcortical infarction. J Cereb Blood Flow Metab. 2014;34:597–605. doi:10.1038/jcbfm.2013.238
19. Larivière S, Ward NS, Boudrias MH. Disrupted functional network integrity and flexibility after stroke: relation to motor impairments. Neuroimage Clin. 2018;19:883–891. doi:10.1016/j.nicl.2018.06.010
20. Thiel A, Vahdat S. Structural and resting-state brain connectivity of motor networks after stroke. Stroke. 2015;46:296–301. doi:10.1161/STROKEAHA.114.006307
21. Dinse HR, Krüger K, Akhavan AC, et al. Low-frequency oscillations of visual, auditory and somatosensory cortical neurons evoked by sensory stimulation. Int J Psychophysiol. 1997;26:205–227. doi:10.1016/s0167-8760(97)00765-4
22. Preusser S, Thiel SD, Rook C, et al. The perception of touch and the ventral somatosensory pathway. Brain. 2015;138:540–548. doi:10.1093/brain/awu370
23. Shafer AT, Matveychuk D, Penney T, et al. Processing of emotional distraction is both automatic and modulated by attention: evidence from an event-related fMRI investigation. J Cogn Neurosci. 2012;24:1233–1252. doi:10.1162/jocn_a_00206
24. Starr CJ, Sawaki L, Wittenberg GF, et al. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi:10.1093/brain/awr117
25. Kamtchum-Tatuene J, Allali G, Saj A, et al. An exploratory cohort study of sensory extinction in acute stroke: prevalence, risk factors, and time course. J Neural Transm. 2017;124:483–494. doi:10.1007/s00702-016-1663-x
26. Azqueta-Gavaldon M, Youssef AM, Storz C, et al. Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain. 2020;161:595–608. doi:10.1097/j.pain.0000000000001745
27. Guggisberg AG, Koch PJ, Hummel FC, et al. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol. 2019;130:1098–1124. doi:10.1016/j.clinph.2019.04.004
28. Caspers J, Rubbert C, Eickhoff SB, et al. Within- and across-network alterations of the sensorimotor network in Parkinson’s disease. Neuroradiology. 2021;63:2073–2085. doi:10.1007/s00234-021-02731-w
29. Basso MA, Uhlrich D, Bickford ME. Cortical function: a view from the thalamus. Neuron. 2005;45:485–488. doi:10.1016/j.neuron.2005.01.035
30. Ahissar E, Oram T. Thalamic relay or cortico-thalamic processing? Old question, new answers. Cereb Cortex. 2015;25:845–848. doi:10.1093/cercor/bht296
31. Yamamoto J, Ikeda A, Matsuhashi M, et al. Seizures arising from the inferior parietal lobule can show ictal semiology of the second sensory seizure (SII seizure). J Neurol Neurosurg Psychiatry. 2003;74:367–369. doi:10.1136/jnnp.74.3.367
32. Park J, Park H, Lee H, et al. Deqi sensation between the acupuncture-experienced and the naïve: a Korean study II. Am J Chin Med. 2005;33:329–337. doi:10.1142/S0192415X0500293X
33. Chen S, Wang S, Rong P, et al. Acupuncture for refractory epilepsy: role of thalamus. Evid Based Complement Alternat Med. 2014;2:950631. doi:10.1155/2014/950631
34. Yeo S, van den Noort M, Bosch P, et al. Ipsilateral putamen and insula activation by both left and right GB34 acupuncture stimulation: an fMRI study on healthy participants. Evid Based Complement Alternat Med. 2016;5:4173185. doi:10.1155/2016/4173185
35. Yu SW, Lin SH, Tsai CC, et al. Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Front Neurol. 2019;10:1114. doi:10.3389/fneur.2019.01114
36. Potok W, Maskiewicz A, Króliczak G, et al. The temporal involvement of the left supramarginal gyrus in planning functional grasps: a neuronavigated TMS study. Cortex. 2019;111:16–34. doi:10.1016/j.cortex.2018.10.010
37. Lassalle-Lagadec S, Sibon I, Dilharreguy B, et al. Subacute default mode network dysfunction in the prediction of post-stroke depression severity. Radiology. 2012;264:218–224. doi:10.1148/radiol.12111718
38. Zhang Y, Li K, Ren Y, et al. Acupuncture modulates the functional connectivity of the default mode network in stroke patients. Evid Based Complement Alternat Med. 2014;2014:765413. doi:10.1155/2014/765413
39. Mills EP, Alshelh Z, Kosanovic D, et al. Altered brainstem pain-modulation circuitry connectivity during spontaneous pain intensity fluctuations. J Pain Res. 2020;13:2223–2235. doi:10.2147/JPR.S252594
40. Yu S, Ortiz A, Gollub RL, et al. Acupuncture treatment modulates the connectivity of key regions of the descending pain modulation and reward systems in patients with chronic low back pain. J Clin Med. 2020;9:1719. doi:10.3390/jcm9061719
41. O’Reilly JX, Beckmann CF, Tomassini V, et al. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi:10.1093/cercor/bhp157
42. Van Overwalle F, Van de Steen F, van Dun K, et al. Connectivity between the cerebrum and cerebellum during social and non-social sequencing using dynamic causal modelling. Neuroimage. 2020;206:116326. doi:10.1016/j.neuroimage.2019.116326
43. Seese RR. Working memory impairments in cerebellar disorders of childhood. Pediatr Neurol. 2020;107:16–23. doi:10.1016/j.pediatrneurol.2020.02.005
44. Sans A, Boix C, Colomé R, et al. The contribution of the cerebellum to cognitive function in childhood. Rev Neurol. 2002;35:235–237. PMID: 12235585.
45. Xie Z, Cui F, Zou Y, et al. Acupuncture enhances effective connectivity between cerebellum and primary sensorimotor cortex in patients with stable recovery stroke. Evid Based Complement Alternat Med. 2014;3:603909. doi:10.1155/2014/603909
46. Smolker HR, Depue BE, Reineberg AE, et al. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct. 2015;220:1291–1306. doi:10.1007/s00429-014-0723-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.