Back to Journals » Cancer Management and Research » Volume 10
Altered epidermal fatty acid-binding protein expression in hepatocellular carcinoma predicts unfavorable outcomes
Authors Lu J, Cai S , Pan Y, Yun J
Received 26 July 2018
Accepted for publication 21 October 2018
Published 23 November 2018 Volume 2018:10 Pages 6275—6284
DOI https://doi.org/10.2147/CMAR.S181555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Rituraj Purohit
Jia-bin Lu,1,2,* Shao-hang Cai,1,3,* Ying-hua Pan,4,* Jing-ping Yun1,2
1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China; 2Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou 510060, China; 3Intensive Care Unit, Sun Yat-sen University Cancer Center, Guangzhou 510060, China; 4Department of Rheumatology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510060, China
*These authors contributed equally to this work
Objective: Hepatocellular carcinoma (HCC) is a rapidly proliferating malignancy that requires large amounts of fatty acids to synthesize cellular membranes and provide energy. Epidermal fatty acid-binding protein (EFABP) is uniquely expressed in epidermal cells, but its role and expression in HCC are not clear.
Subjects and methods: A total of 804 HCC specimens were collected to construct a tissue microarray (TMA) and for immunohistochemistry (IHC) analysis. The relationship between EFABP expression and clinical features of patients with HCC was analyzed.
Results: The EFABP IHC score for HCC tissue was 0.76±0.69, being significantly higher than that for matched nontumorous tissue (0.48±0.55; P<0.001). Using the median IHC score (ie, 0.8) in the tumorous tissue, a high level of EFABP expression was found in 57.3% (461/804) of the cases. Patients with HCC displaying high EFABP expression had poorer tumor differentiation (P=0.029), more vascular invasion (P=0.006), and a higher proportion of late TNM stage disease (P=0.042). Kaplan–Meier analysis revealed that the patients with high EFABP expression had significantly worse outcomes in terms of overall survival (P=0.003), worse disease-free survival (P=0.021), and a higher probability of recurrence (P=0.014). Multivariate analysis indicated that EFABP expression was an independent prognostic variable for overall survival (P=0.021) and disease-free survival (P=0.044). For HCC recurrence, only vascular invasion (P=0.020) and EFABP expression (P=0.026) were independent risk factors.
Conclusion: Our data revealed that EFABP expression was increased in HCC samples. High EFABP expression was correlated with shorter survival times in patients with HCC and served as an independent factor for worse outcomes. Our study therefore provides a promising biomarker for the prognostic prediction of HCC and a potential therapeutic target for the disease.
Keywords: epidermal fatty acid-binding protein, lipid metabolism, hepatocellular carcinoma, prognostic biomarker
Introduction
Hepatocellular carcinoma (HCC), the most common type of hepatic cancer,1 is best cured by surgical resection and transplantation.2 However, the rates of recurrence and metastasis are still high even after curative hepatectomy.3 The rate of HCC recurrence after curative surgical or regional therapy is 75% at the fifth year, whereas the rate of recurrence is 86.5% for intrahepatic metastasis and 13.5% for extrahepatic metastasis.4,5 At present, serum biomarkers, such as alpha-fetoprotein (AFP), and many clinicopathological factors are used as prognostic markers of HCC, but they are not adequate for predicting survival or recurrence after curative hepatectomy.6,7 Hence, new biomarkers that are effective for predicting the prognosis and recurrence of HCC are still greatly needed.
An excellent biomarker should have unique characteristics, along with high sensitivity and specificity.8 In order to explore HCC-specific markers, it is practical to explore the unique biological characteristics of the HCC cells first, to subsequently better identify the related molecular markers. However, the extensive heterogeneity in tumor biology is an important aspect of HCC cells.9 The main characteristic of HCC is its rapid proliferation, which means that HCC cells must depend excessively on lipids.10,11 This is because lipids not only serve as a component for synthesizing the cell membrane (which is necessary for cell proliferation) but also provide the energy needed for rapid proliferation.9 However, the source of lipids in HCC cells is a question worth pondering. Previous studies have suggested that hyperactivation of lipid synthesis by HCC cells implies that these cells may convert glucose-derived carbon into lipids via the glycolytic pathway.12 However, increasingly more studies have suggested that HCC can absorb peripheral lipids for their own needs.10,13 As a result of tumor metabolic reprogramming, this may be one of the specific manifestations of HCC cell heterogeneity. For example,13 primary ovarian cancer cells undergo highly activated lipogenesis to supply the lipids required for uncontrolled cell proliferation. However, when ovarian cancer metastasizes to omental fat, which contains a microenvironment abundant in the adipocytes, the cancer cells are metabolically reprogrammed to favor lipid oxidation using the adipocyte-derived fatty acids.13 In patients with HCC, lipolysis of subcutaneous adipocytes occurs, and cachexia appears to be a proven clinical phenomenon.14 The fatty acid released into the circulatory system by the adipocytes is taken up by the HCC cells; this is also a well-known phenomenon.
Since HCC cells take up fatty acids in the peripheral system, it is unavoidable that an increase in fatty acid-binding proteins (FABPs) would be needed.15–17 The FABP family comprises proteins with a high affinity to fatty acids.18 Members of this family express tissue specificity. For example, liver FABP (LFABP) is expressed only in the hepatocytes. In the adipocytes, both adipocyte FABP and epidermal FABP (EFABP, FABP5) can be expressed. EFABP expression has been reported to be increased in various cancers.19,20 Ohata et al reported that FABP5 plays a significant role in HCC progression and metastasis through the induction of epithelial-to-mesenchymal transition.21 Wang et al reported that HSP 90-beta, FABP5, and alcohol dehydrogenase 4 are potential clinically used biomarkers for HCC.22 Similarly, Jeong et al found that FABP5 is significantly overexpressed in intrahepatic cholangiocarcinoma combined lymph node metastasis and is involved in cell proliferation and invasion in vitro.23 Interestingly, the expression of LFABP was reported to be decreased in HCC.24 Therefore, it remains to be elucidated whether ectopic EFABP expression occurs to transport fatty acid within HCC cells, and subsequently whether EFABP can be regarded as a marker of the peripheral lipid uptake ability of the HCC cells as well as a potential prognostic marker for the disease.
In this study, we investigated the role of EFABP in human HCC. We showed that EFABP expression is positively correlated with the overall survival of patients with HCC and, therefore, is a promising biomarker for the prognostic prediction of HCC and a potential therapeutic target for the clinical management of this disease.
Subjects and methods
Subjects
From January 2000 to December 2010, a total of 804 paraffin-embedded HCC specimens were collected from the Department of Pathology, Sun Yat-sen University Cancer Center. None of the patients from whom the samples were retrieved had received any chemotherapy or radiotherapy prior to surgery. The follow-up period was defined as the interval between the date of operation and the date of death or the last follow-up. This study was approved by the Medical Ethics Committee of Sun Yat-sen University Cancer Center. Because all specimens used were anonymous, the Medical Ethics Committee of Sun Yat-sen University Cancer Center waived the need for informed patient consent.
Tissue microarray (TMA) construction and immunohistochemistry (IHC)
The TMA slices comprised tumorous tissue and matched adjacent normal tissues from 804 cases of HCC. Using a tissue arrayer (MiniCore, Excilone, UK), each tissue core (diameter: 0.6 mm) was perforated and re-embedded from the marked area. All specimens were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hours and embedded in paraffin. The paraffin-embedded tissues were then sectioned into 4-µm sections and mounted on glass slides. After dewaxing, the slides were treated with 3% hydrogen peroxide in methanol and blocked with a biotin blocking kit (Dako Denmark A/S, Glostrup, Denmark). After blocking, the slides were incubated overnight with EFABP antibody (ab84028, 1:1,000; Abcam, Cambridge, MA, USA) in a humid chamber at 4°C, washed three times with PBS, incubated for 1 hour with biotinylated goat anti-mouse antibody, and then stained with 3,3′-diaminobenzidine tetrahydrochloride. Finally, the sections were stained with hematoxylin and observed under a microscope.
Semi-quantitative IHC was used to detect the protein expression levels of EFABP, according to the following standard scores: “0” (negative staining), “1” (weak staining), “2” (moderate staining), and “3” (strong staining). The final score was calculated as the percentage of positive expression multiplied by the intensity score. The score was independently determined by two pathologists. The median IHC score was used as the cutoff for judging high and low expression levels.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). A Student’s t-test and Pearson’s chi-squared test or Fisher’s exact test were chosen for examining the correlations between the EFABP expression level and the clinical and pathological variables. Survival curves were constructed using the Kaplan–Meier method (log-rank test). A multivariate Cox proportional hazards regression model was used to evaluate the independence of EFABP in predicting outcomes. Differences were defined as significant for P-values less than 0.05.
Results
Expression of EFABP in the HCC TMA
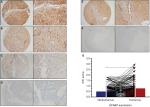
We used an HCC TMA (n=804) to detect EFABP expression. EFABP is expressed mainly in cytoplasm of HCC cells. The EFABP IHC score for HCC tissue was 0.76±0.69, which is significantly higher than that for matched nontumorous tissue (0.48±0.55; P<0.001) (Figure 1 and Figure S1). In addition, we also analyzed the difference in the expression levels of EFABP between normal liver tissue and cirrhotic tissue. The results showed no statistical difference between the two (normal liver vs cirrhosis, 0.79±0.71 vs 0.75±0.69, P=0.54).
Association of cytoplasmic EFABP with HCC clinical features
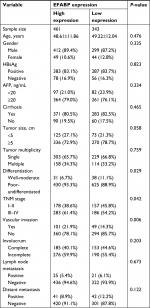
To determine the potential clinical significance of EFABP in HCC, the relationship between EFABP and the clinical features of patients with HCC was evaluated. Using the median IHC score (0.8) of the tumorous tissues, a high level of EFABP expression was found in 57.3% (461/804) of the cases. Patients with high levels of EFABP expression had poorer tumor differentiation (P=0.029), more vascular invasion (P=0.006), and a higher proportion of late TNM stage disease (P=0.042), as shown in Table 1. We also analyzed the level of EFABP expression in liver tissue with liver steatosis. The results showed no difference in EFABP expression levels in tissues with or without steatosis (non-steatosis vs steatosis, 0.69±0.65 vs 0.80±0.67, P=0.19).
Association of EFABP expression with clinical outcomes in patients with HCC
To determine the prognostic effect of EFABP expression on patients with HCC, we conducted a Kaplan–Meier survival analysis using data from the 804 patients enrolled in the study. For the patients with high EFABP expression, Kaplan–Meier analysis revealed that they had significantly worse outcomes in terms of overall survival (P=0.003). Similarly, compared with the patients with low EFABP expression, those with high EFABP expression had a significantly worse disease-free survival (P=0.021) and a higher probability of recurrence (P=0.014), as shown in Figure 2.
Univariate and multivariate analyses of prognostic variables in HCC
To evaluate whether EFABP expression was an independent risk factor for outcomes in HCC, both univariate and multivariate analyses were conducted. The serum AFP level, tumor size, tumor multiplicity, tumor differentiation, TNM stage, vascular invasion, involucrum, and EFABP expression were all shown to be prognostic variables for overall survival in patients with HCC. In the multivariate analysis, only tumor size (P=0.001), TNM stage (P<0.001), vascular invasion (P<0.001), involucrum (P=0.044), and EFABP expression (P=0.021) were found to be independent prognostic variables for overall survival (Table 2).
We further explored the risk factors associated with disease-free survival (Table 3) and HCC recurrence (Table 4). Univariate analysis showed that age, serum AFP level, TNM stage, vascular invasion, and EFABP expression were risk factors associated with disease-free survival. In the multivariate analysis, vascular invasion (P=0.002) and EFABP expression (P=0.044) were independent risk factors associated with disease-free survival. For HCC recurrence, only vascular invasion (P=0.020) and EFABP expression (P=0.026) were the associated independent risk factors.
Subgroup analyses of the prognostic value of EFABP expression in the cytoplasm in HCC
A stratified survival analysis was conducted to further reveal the prognostic significance of EFABP expression among patients with HCC. Kaplan–Meier survival analysis showed that EFABP expression was associated with overall survival in both small and large HCCs (small HCCs: P=0.011; large HCCs: P=0.007), in serum hepatitis B virus surface antigen (HBsAg)-positive and -negative HCCs (HBsAg-negative HCCs: P=0.017; HBsAg-positive HCCs: P=0.030), in vascular invasion-positive and -negative HCCs and TNM stage III–IV HCCs (vascular invasion-positive HCCs: P=0.043; vascular invasion-negative HCCs: P=0.044), and in HCCs with and without cirrhosis (HCCs with cirrhosis: P=0.013; HCCs without cirrhosis: P=0.035), as shown in Figure 3.
Discussion
HCC is an end-stage liver disease. Chronic viral infection accounts for most of the global etiology of the disease, especially in Asia.25–28 Recently, nutritionally related liver diseases, such as non-alcoholic fatty liver disease (NAFLD), have been found to be associated with HCC, and energy metabolism reprogramming is also one of the markers of cancer.29–31 Cancer cells rely on non-glucose carbon sources and increased expression of enzymes involved in the synthesis of fatty acids to biosynthesize cell membrane, which promotes tumor aggressiveness by increasing cell proliferation.12,32 However, lipid absorption is another potential way for cancer cells to increase their lipid content for cell biosynthesis.11,17 EFABP has high affinity for fatty acids, and its content is directly proportional to the lipid content.17 Therefore, we evaluated the expression of EFABP in HCC and explored the relationship between EFABP and HCC prognosis.
EFABP, also known as psoriasis-associated FABP or skin FABP, is an isomer of FABPs that are small and soluble intracellular lipid-binding proteins that bind fatty acids.15,33 FABPs transport lipids to the cell compartment to be stored as lipid droplets, to the endoplasmic reticulum for membrane synthesis, and to the nucleus for lipid-mediated transcriptional regulation.34,35 The function of EFABP is to enhance the transcriptional activity of the nuclear receptors PPARβ/δ and promote cell migration, proliferation, and survival.36–38 EFABP is overexpressed in many human cancers, including prostate cancer,39,40 esophageal squamous cell carcinoma,41,42 and breast cancer.43,44 Previous studies have found that EFABP may play an important role in liver cancer. Ohata et al reported that FABP5 promotes HCC progression by epithelial-to-mesenchymal transition.21 Wang et al reported that FABP5 can be regarded as a potential clinically used biomarker for HCC.22 Similarly, Jeong et al found that FABP5 is significantly overexpressed in intrahepatic cholangiocarcinoma and is involved in cell proliferation and invasion in vitro.23 Interestingly, in our study, we found that patients with HCC displaying high EFABP expression had poorer tumor differentiation, more vascular invasion, and a higher proportion of late TNM stage disease. More importantly, the patients with high EFABP expression had significantly worse outcomes than those with low EFABP expression, with worse disease-free survival and a higher probability of recurrence. In addition, high EFABP expression was an independent prognostic variable for overall survival, disease-free survival, and HCC recurrence.
Previous reports have shown the association of high EFABP expression with tumor metastasis and poor prognosis in various types of cancer.20,45 EFABP overexpression was reported to promote tumor metastasis by matrix metalloproteinase 9 upregulation, tumorigenesis of proteolytic enzymes to promote tumor metastasis, and increased expression of vascular endothelial growth factor, a protein in tumor angiogenesis.19 According to the results of our study, the patients with a high expression level of EFABP had a higher incidence of vascular invasion. This may indicate the molecular mechanism of EFABP in promoting HCC progression, in that the protein may facilitate epithelial–mesenchymal transition in HCC cells. Fatty acids are peroxisome proliferator-activated receptor-alpha (PPARα) ligands.46 Whether there is a correlation of EFABP and PPAR expression is an interesting question that is waiting for an answer. In addition, according to our study, EFABP expression is very low in nontumor liver tissues. A previous study suggested that there is a variation in FABPs.47 Whether or not that there is another variation of EFABP expressed in nontumor liver tissue requires further study to confirm.
Most HCCs occur in patients with chronic liver diseases, such as chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, and alcohol abuse.48–51 However, as a result of worldwide HBV vaccine immunization and antiviral therapy for HBV and HCV, NAFLD and non-alcoholic steatohepatitis have become the higher risk factors for HCC.29 Recent studies have reported obesity, metabolic syndrome, and type 2 diabetes as important risk factors for HCC.30 Other recent studies have reported the accumulation of lipids in NAFLD, HCV-related liver steatosis, and HBV-related HCC.52 Since the role of FABP is in binding fatty acids, the inhibition of EFABP may be a potential way to prevent metabolic liver disease progression to HCC. In addition, EFABP may be regarded as a metabolic-related target in HCC treatment. However, further studies are needed to confirm the potential clinical application of EFABP. In addition, EFABP can be measured in serum.53 It would be interesting to clarify that EFABP in serum is derived from HCC cells. However, EFABP in serum is affected by a variety of factors, including ethnicity, metabolic disorders, and cardiovascular disease.53–55 Further studies with strict enrollment criterion are needed to clarify whether there is a correlation between EFABP in HCC tissue and EFABP in serum.
Conclusion
In summary, our study results demonstrate a role for EFABP in the development of HCC. Our data revealed that EFABP expression was increased in HCC samples, and such an increase was significantly correlated with poorer tumor differentiation and more vascular invasion. High EFABP expression was also correlated with shorter survival times in patients with HCC and served as an independent factor for worse outcomes. Collectively, our data suggest that EFABP is a promising biomarker for the prognosis of patients with HCC and a potential metabolic-related target in HCC treatment.
Acknowledgments
We thank Xia Yang for her contribution to this study. The study was supported by grants from the National Key R&D Program of China (No. 2017YFC1309000), National Natural Science Foundation of China (Nos. 81572405, 81572406, 81502079, and 81602135), and Science and Technology Program of Guangzhou (No. 201707020038).
Disclosure
The authors report no conflicts of interest in this work.
References
Tsochatzis EA, Meyer T, Burroughs AK. Hepatocellular carcinoma. N Engl J Med. 2012;366(1):92–93. | ||
Sangiovanni A, Triolo M, Iavarone M, et al. Multimodality treatment of hepatocellular carcinoma: how field practice complies with international recommendations. Liver Int. 2018;38(9):1624–1634. | ||
Yin Z, Jin H, Ma T, Zhou Y, Yu M, Jian Z. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma ≤2 cm (BCLC very early stage. Int J Surg. 2018;56:61-67:61–67. | ||
Ikeda K, Arase Y, Kobayashi M, et al. Significance of multicentric cancer recurrence after potentially curative ablation of hepatocellular carcinoma: a longterm cohort study of 892 patients with viral cirrhosis. J Gastroenterol. 2003;38(9):865–876. | ||
Taketomi A, Toshima T, Kitagawa D, et al. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17(10):2740–2746. | ||
Shimada M, Takenaka K, Fujiwara Y, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein positive status as a new prognostic indicator after hepatic resection for hepatocellular carcinoma. Cancer. 1996;78(10):2094–2100. | ||
Eguchi S, Takatsuki M, Hidaka M, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34(5):1034–1038. | ||
Cai SH, Lu SX, Liu LL, Zhang CZ, Yun JP. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol. 2017;10(10):761–771. | ||
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. | ||
Cao D, Song X, Che L, et al. Both de novo synthetized and exogenous fatty acids support the growth of hepatocellular carcinoma cells. Liver Int. 2017;37(1):80–89. | ||
Li Z, Kang Y. Lipid metabolism fuels cancer’s spread. Cell Metab. 2017;25(2):228–230. | ||
Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140(3):1071–1083. | ||
Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–1503. | ||
O’Keefe SJ, Ogden J, Ramjee G, Rund J. Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Cancer Res. 1990;50(4):1226–1230. | ||
Furuhashi M, Ogura M, Matsumoto M, et al. Serum FABP5 concentration is a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. Sci Rep. 2017;7(1):217. | ||
Hoekstra M, Stitzinger M, van Wanrooij EJ, et al. Microarray analysis indicates an important role for FABP5 and putative novel FABPs on a Western-type diet. J Lipid Res. 2006;47(10):2198–2207. | ||
Guaita-Esteruelas S, Gumà J, Masana L, Borràs J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol Cell Endocrinol. 2018;462(Pt B):107–118. | ||
Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285(43):32679–32683. | ||
Fang LY, Wong TY, Chiang WF, Chen YL. Fatty-acid-binding protein 5 promotes cell proliferation and invasion in oral squamous cell carcinoma. J Oral Pathol Med. 2010;39(4):342–348. | ||
Wang W, Chu HJ, Liang YC, et al. FABP5 correlates with poor prognosis and promotes tumor cell growth and metastasis in cervical cancer. Tumour Biol. 2016;37(11):14873–14883. | ||
Ohata T, Yokoo H, Kamiyama T, et al. Fatty acid-binding protein 5 function in hepatocellular carcinoma through induction of epithelial-mesenchymal transition. Cancer Med. 2017;6(5):1049–1061. | ||
Wang S, Gao H, Zhang J, Ye X. Stable isotope labeling and parallel reaction monitoring-based proteomic quantification for biomarker screening and validation of hepatocellular carcinoma. Se Pu. 2017;35(9):934–940. | ||
Jeong CY, Hah YS, Cho BI, et al. Fatty acid-binding protein 5 promotes cell proliferation and invasion in human intrahepatic cholangiocarcinoma. Oncol Rep. 2012;28(4):1283–1292. | ||
Inoue M, Takahashi Y, Fujii T, Kitagawa M, Fukusato T. Significance of downregulation of liver fatty acid-binding protein in hepatocellular carcinoma. World J Gastroenterol. 2014;20(46):17541–17551. | ||
Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85. | ||
Chen SL, Liu LL, Lu SX, et al. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11(9):1225-1240–1240. | ||
Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J. 2016;13:64. | ||
Xue X, Cai S, Ou H, Zheng C, Wu X. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence. 2017;11:85–93. | ||
Cai S, Ou Z, Liu D, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J. 2018;6(4):558–566. | ||
Ou H, Cai S, Liu Y, Xia M, Peng J. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2017;10(2):207–217. | ||
Zeng J, Cai S, Liu J, Xue X, Wu X, Zheng C. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med. 2017;36(2):261–268. | ||
Kuemmerle NB, Rysman E, Lombardo PS, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10(3):427–436. | ||
Zendzian-Piotrowska M, Górski J. Fatty acid binding protein (FABP). Postepy Hig Med Dosw. 1994;48(6):753–761. | ||
Owada Y. Fatty acid binding protein: localization and functional significance in the brain. Tohoku J Exp Med. 2008;214(3):213–220. | ||
Wang YT, Liu CH, Zhu HL. Fatty acid binding protein (FABP) inhibitors: a patent review (2012–2015). Expert Opin Ther Pat. 2016;26(7):767–776. | ||
Wang D, Fu L, Ning W, et al. Peroxisome proliferator-activated receptor δ promotes colonic inflammation and tumor growth. Proc Natl Acad Sci U S A. 2014;111(19):7084–7089. | ||
Alshalalfa M, Bismar TA, Alhajj R. Detecting cancer outlier genes with potential rearrangement using gene expression data and biological networks. Adv Bioinformatics. 2012;2012:373506–13. | ||
Toth PM, Lieber S, Scheer FM, et al. Design and synthesis of highly active peroxisome proliferator-activated receptor (PPAR) β/δ inverse agonists with prolonged cellular activity. Chem Med Chem. 2016;11(5):488–496. | ||
Morgan EA, Forootan SS, Adamson J, et al. Expression of cutaneous fatty acid-binding protein (C-FABP) in prostate cancer: potential prognostic marker and target for tumourigenicity-suppression. Int J Oncol. 2008;32(4):767–775. | ||
Morgan E, Kannan-Thulasiraman P, Noy N. Involvement of Fatty Acid Binding Protein 5 and PPARβ/δ in Prostate Cancer Cell Growth. PPAR Res. 2010;2010. | ||
Arai M, Imazeki F, Sakai Y, et al. Analysis of the methylation status of genes up-regulated by the demethylating agent, 5-aza-2’-deoxycytidine, in esophageal squamous cell carcinoma. Oncol Rep. 2008;20(2):405–412. | ||
Ogawa R, Ishiguro H, Kuwabara Y, et al. Identification of candidate genes involved in the radiosensitivity of esophageal cancer cells by microarray analysis. Dis Esophagus. 2008;21(4):288–297. | ||
Schug TT, Berry DC, Toshkov IA, Cheng L, Nikitin AY, Noy N. Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARbeta/delta to RAR. Proc Natl Acad Sci U S A. 2008;105(21):7546–7551. | ||
Levi L, Lobo G, Doud MK, et al. Genetic ablation of the fatty acid-binding protein FABP5 suppresses HER2-induced mammary tumorigenesis. Cancer Res. 2013;73(15):4770–4780. | ||
Uma RS, Naresh KN, D’Cruz AK, Mulherkar R, Borges AM. Metastasis of squamous cell carcinoma of the oral tongue is associated with down-regulation of epidermal fatty acid binding protein (E-FABP). Oral Oncol. 2007;43(1):27–32. | ||
Xiao YB, Cai SH, Liu LL, Yang X, Yun JP. Decreased expression of peroxisome proliferator-activated receptor alpha indicates unfavorable outcomes in hepatocellular carcinoma. Cancer Manag Res. 2018;10:1781–1789. | ||
Gerbens F, Rettenberger G, Lenstra JA, Veerkamp JH, Te Pas MF. Characterization, chromosomal localization, and genetic variation of the porcine heart fatty acid-binding protein gene. Mamm Genome. 1997;8(5):328–332. | ||
Cai S, Li Z, Yu T, Xia M, Peng J. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist. 2018;11:469–477. | ||
Xue X, Cai S. Comment on “Assessment of liver stiffness in pediatric fontan patients using transient elastography”. Can J Gastroenterol Hepatol. 2016;2016:9343960. | ||
Cai S, Cao J, Yu T, Xia M, Peng J. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine. 2017;96(22):e7021. | ||
Cai S, Yu T, Jiang Y, Zhang Y, Lv F, Peng J. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med. 2016;16(3):429–436. | ||
Mandair DS, Rossi RE, Pericleous M, Whyand T, Caplin M. The impact of diet and nutrition in the prevention and progression of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2014;8(4):369–382. | ||
Furuhashi M, Ogura M, Matsumoto M, et al. Serum FABP5 concentration is a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. Sci Rep. 2017;7(1):217. | ||
Hong J, Gu W, Zhang Y, et al. Different association of circulating levels of adipocyte and epidermal fatty acid-binding proteins with metabolic syndrome and coronary atherosclerosis in Chinese adults. Atherosclerosis. 2011;217(1):194–200. | ||
Ishimura S, Furuhashi M, Watanabe Y, et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 2013;8(11):e81318. |
Supplementary material
  | Figure S1 The EFABP expression in HCC tissues and adjacent non-tumor tissue. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.