Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Alpha-1 Antitrypsin Gene Variants in Patients without Severe Deficiency Diagnosed with Pulmonary Emphysema on Chest CT
Authors Laviña E, Lumbreras S , Bravo L, Soriano JB, Izquierdo JL , Rodríguez JM
Received 7 November 2023
Accepted for publication 14 January 2024
Published 3 February 2024 Volume 2024:19 Pages 353—361
DOI https://doi.org/10.2147/COPD.S448593
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Eduardo Laviña,1,2 Sara Lumbreras,3 Lara Bravo,4 Joan B Soriano,5 José Luis Izquierdo,1,6 Jose Miguel Rodríguez4,6
1Servicio de Neumología, Hospital Universitario de Guadalajara, Guadalajara, Spain; 2Escuela de Doctorado, Programa Doctoral en Ciencias de la Salud, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain; 3Departamento de Organización Industrial, Escuela Técnica Superior de Ingeniería (ICAI), Universidad Pontificia Comillas – IIT, Madrid, Spain; 4Servicio de Neumología, Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid, Spain; 5Servicio de Neumología, Hospital Universitario de la Princesa; Facultad de Medicina, Universidad Autónoma de Madrid; and Centro de Investigación Biomédica En Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III; All in Madrid, Madrid, Spain; 6Departamento de Medicina y Especialidades Médicas, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain
Correspondence: José Luis Izquierdo, Servicio de Neumología, Hospital Universitario de Guadalajara, Calle Donantes de Sangre, Guadalajara, SN, 19002, Spain, Email [email protected]
Introduction: Although pulmonary involvement due to alpha-1 antitrypsin (AAT) deficiency has been widely described, most studies focus on the genotypes causing severe deficiency (< 60 mg/dL).
Objective: The aim of this study was to analyze the prevalence of the different AAT gene variants that do not cause severe deficiency in patients with pulmonary emphysema diagnosed by thoracic computed tomography (CT). Furthermore, we assessed the risk associated with a non-severe decrease in AAT values in the pathogenesis of emphysema.
Methods: Case-control study design that included patients who had a CT scan available of the entire thorax. In total, 176 patients with emphysema (cases) and 100 control subjects without emphysema were analyzed.
Results: The prevalence of variants was higher among cases (25.6%; 45/176) than controls (22%; 22/100), although the difference was not statistically significant (P=0.504) when analyzed globally. In the control group, all the variants detected were MS. Excluding this variant, statistically significant differences were observed in the remaining variants (MZ, SS and SZ). Only 18% of the controls (all MS) presented values below our limit of normality, and all had values very close to the reference value (90 mg/dL). In contrast, 76% of patients with the other variants presented pathological levels. In a logistic regression model, both smoking and a non-severe reduction in AAT (60 to 90 mg/dL) increased the probability of emphysema.
Conclusion: Our study confirms an association between certain variants in the alpha-1 antitrypsin gene that do not cause severe deficiency and the presence of pulmonary emphysema. This association with variants that are associated with reductions in serum AAT values is statistically significant and independent of smoking habit.
Keywords: emphysema, alpha-1 antitrypsin, deficiency, variants
Introduction
Alpha-1 antitrypsin (AAT) deficiency is a condition derived from variants of the SERPINA1 (serine protease inhibitor) gene, which can manifest clinically as pulmonary emphysema, liver cirrhosis, neutrophilic panniculitis, systemic vasculitis, and possibly other diseases; however, more than one-third of cases present no symptoms during their lifetime.1,2 The laboratory diagnosis of AAT deficiency is based on quantitative AAT determination and identification of the phenotype in serum. Molecular analysis of the AAT gene, or genotype, is the gold standard for identifying rare allelic variants.
Pulmonary emphysema (PE) is a disease characterized by the destruction of pulmonary alveoli, causing impaired gas exchange. Although its pathogenesis is multifactorial in origin, the main genetic cause is the presence of variants in the SERPINA1 gene. The best diagnostic tool is computed tomography (CT), which also allows us to evaluate the severity and distribution of the emphysema.
Although lung involvement due to AAT deficiency has been widely analyzed, most studies that have examined this issue focus on the genotypes that cause severe deficiency (<60 mg/dL). However, there is little information on the role of other variants that do not cause severe deficiency. In these cases, the prevalence of variants has been evaluated in different respiratory pathologies, but no studies have thoroughly assessed their relationship with the presence of PE, its distribution, or its severity.3,4
The objective of this study is to analyze the risk of PE diagnosed by CT in patients with AAT gene variants that do not cause severe deficiency.
Methods
Study population
We have conducted a case–control study from November 2018 to May 2023. Patients were included with the sole condition that a CT scan had been done of the entire thorax. Patients with pulmonary emphysema on CT were selected for study, and subjects without emphysema on CT were selected as controls, at a 2:1 ratio, respectively. Patients with severe AAT deficiency, or any condition that interfered with serum AAT values (infectious, autoimmune or inflammatory, liver disease, or kidney disease [ClCRT <30 mL/min]) were excluded. Figure 1 is a flowchart representing patient inclusion. We followed the STROBE guidelines for observational research.5
 |
Figure 1 STROBE flowchart of the study population. |
Diagnostic tests
Demographic and anthropometric variables were collected for all participants, and blood samples were obtained to determine inflammatory markers (high-sensitivity C-reactive protein [CRP] and fibrinogen), complete blood count, and AAT quantification by nephelometry. Only patients with AAT values above 60 mg/dL were included for study. Standard spirometry was performed after administering 400 μg of salbutamol and 80 μg of ipratropium bromide and conducted in accordance with current international quality standards with a Masterlab spirometer (Jaeger, Hoechberg Germany). Lastly, the study subjects completed a standardized questionnaire regarding clinical data, smoking habit, drug treatments, and comorbidities. For the cases, the presence, distribution and type of emphysema were analyzed jointly by a radiologist and a pulmonologist using standardized criteria.6
The genotype was determined by AAT gene sequencing (Progenika Biopharm SA, Spain). The AAT genotyping test uses the polymerase-chain reaction (PCR) technique to amplify sequences from the SERPINA1 gene that encode the AAT protein. This test uses allele-specific probes attached to colored microspheres that hybridize specifically with the PCR products. A subsequent stage of fluorescent labeling allows for detection and quantification of the hybridization. The test is capable of carrying out multiple reactions in parallel, thereby making the process more efficient. The test is designed for use with genomic DNA extracted from human blood or saliva samples.
Ethical considerations
All participants gave their informed consent for inclusion in the study and for genetic testing, complying with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Hospital Universitario de Guadalajara (P27/17; November 28, 2017).
Statistical Analysis
Sample size
The sample size was calculated for a case–control study comparing patients diagnosed with emphysema on CT (cases) versus those with no emphysema on CT (controls). To calculate the sample size, we considered a prevalence of mutated alleles of less than 4% in the general population without emphysema and 25% or more in those with emphysema, with an alpha error of 5% and a statistical power of 80%; therefore, 41 participants were required in each group (total: 82 participants) to detect significant differences. If the prevalence of AAT alleles is greater than 25% in cases or less than 4% in controls, the statistical power will be sufficient for most comparisons by subgroups (age, sex, emphysema severity, etc.). Calculations assuming higher prevalence of emphysema in controls or lower prevalence in cases would result in more powerful statistics.
Continuous data are presented as means and standard deviation (SD) or with 95% confidence intervals (95% CI). Differences between groups of continuous variables were analyzed with the Student’s t-test for independent samples or the chi-squared test for dichotomous variables.
In addition, to predict the probability of PE, a logistic regression analysis was carried out using low AAT concentration (<90 mg/dL), smoking and age as independent variables in a data set comprised 276 observations. To carry out this analysis, Python programming language was used, specifically the StatsModels library, which provides classes and functions to estimate statistical models.
In all cases, a P value <0.05 was considered significant.
Results
In the end, 176 cases and 100 controls were analyzed. Patient characteristics are described in Table 1.
 |
Table 1 Characteristics of the Study Population |
In the conducted study, there was a higher prevalence of variants observed in the cases (25.6%; 45 out of 176) compared to the controls (22%; 22 out of 100). However, this difference was not statistically significant in an overall analysis, as indicated in Figure 2A. It is noteworthy that in the control group, all identified variants were MS.
 |
Figure 2 Prevalence of variants in cases and controls. (A) Prevalence of variants in 2 two groups, including MS. (B) Prevalence of variants in the 2 groups, excluding MS. |
Excluding these variants, a statistically significant difference was observed with the remaining variants. In the emphysema group, 9 patients had the MZ variant, 3 SS and 5 SZ. None of the control subjects presented any of these variants (Figure 2B).
Table 2 shows the serum AAT values according to the variants observed. Only 18% of MS (22% in the emphysema group, and 13% in the control group) presented values below our limit of normality, and all had values that were very close to the reference value (90 mg/dL). In contrast, 76% of patients with the other variants presented pathological levels.
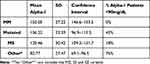 |
Table 2 Serum Levels of Alpha-1 Antitrypsin According to the Alleles Present; % of Patients with Serum Values <90mg/dL in Each Group |
Table 3 analyzes the emphysema characteristics based on type (centrilobular, panlobular, paraseptal or mixed) and distribution (unilateral/bilateral and distribution by lobes). The vast majority presented bilateral centrilobular emphysema, with no clear predominance by variant (Table 4) or normal versus pathological levels of AAT (Table 5).
 |
Table 3 Distribution of Emphysema Among the Cases |
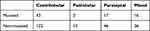 |
Table 4 Distribution of Emphysema by Variants |
 |
Table 5 Distribution of Emphysema According to Serum Alpha-1 Antitrypsin Levels |
To analyze the risk of PE associated with a decrease in AAT levels, a logistic regression model was adjusted, which showed an accuracy of 64.49%. According to the results obtained from the confusion matrix, 163 of the 276 observations were correctly classified as class 1 and 15 as class 0, resulting in a total of 98 erroneous classifications. The final model reached a precision rate of 65.73% and a recall of 92.61%.
Regarding the coefficients of the model, we found that the variable that identifies patients with low AAT concentration (LowC) had a coefficient of 1.6799, with an odds ratio (OR) of 5.3651. The 95% confidence interval (CI) for the OR of LowC was between 5.1998 and 5.5355.
A history of smoking demonstrated a coefficient of 0.4566, with an OR of 1.5788. The 95% CI for the OR of smoking was between 1.4888 and 1.6742. This variable presents a level of significance at the limit of what is considered significant. A previous history of smoking was considered smoking. Neither the pack-year value nor the current smoking status was significant in the analysis. The age variable presented a coefficient of 0.0262, with an OR of 1.0266. The 95% CI for the OR of age ranged from 0.2392 to 4.4054. The sex variable was also explored, but it was not significant.
These findings suggest that low serum concentrations, as well as smoking, increase the likelihood of emphysema. The age variable seems to have a modest positive effect, as illustrated by the width of the confidence interval for the OR (Table 6).
 |
Table 6 Logistic Regression for Risk of Pulmonary Emphysema |
Discussion
Our study reveals an association between some variants in the alpha-1 antitrypsin gene that do not cause severe deficiency and the presence of PE, assessed by CT. This correlation is statistically significant with variants that are associated with reductions in serum AAT values. Although these reductions do not reach levels that would be considered serious, they do appear to have a clinically relevant effect on predisposition to emphysema. The results of this study suggest that even moderate levels of AAT deficiency could predispose individuals to develop pulmonary emphysema.
Although more than 150 variants of the SERPINA1 gene have been described, their study has focused on the most frequent.1,7 In a population-based study, Ferkingstad et al observed that the risk of emphysema was especially striking in patients with the ZZ phenotype (OR 28 [18–44]) versus those with MZ variant (1.4 [1.2–1.7]).8 In fact, in a longitudinal study of heterozygous MZ and SZ patients who had not smoked, carriers of these variants did not have an increased risk of lung disease, but the risk was higher in smokers.9,10 Nonetheless, this association has not been confirmed by other studies.11–14 Franciosi et al compared 486 participants from an AAT registry to determine whether SZ was associated with pulmonary results that were more comparable to MZ or ZZ. These authors found no significant differences between MZ and SZ individuals, regardless of whether they had ever smoked or not (P>0.05 in all cases).15
However, the risk attributable to SZ remains a matter of debate. The ZZ variant was associated with lower FEV1 than SZ, regardless of whether the patients had ever smoked. Furthermore, an increase in OR was observed when PE was assessed visually by CT (P≤.002 in all cases). In this registry cohort, SZ was associated with a risk of lung disease comparable to MZ, but not ZZ.15 In short, the results for the PI*SZ genotype have been contradictory, although a meta-analysis based on 6 studies observed increased risk (OR: 3.26; 95% CI: 1.24–8.57) for developing COPD.16 Patients with SS variant may have reduced AAT levels, but there is no evidence to confirm a greater susceptibility to develop emphysema in SS homozygotes. However, the S allele is involved in increased hepatic degradation of AAT.17
Our results show a clear relationship between the different variants that cause reduced AAT values and PE. Results from the literature have been contradictory, possibly due to several reasons. In this study, alpha-1 antitrypsin (AAT) deficiency was defined within a range from normal to severe deficiency, specifically with serum values between 90 and 60 mg/dL. Given that AAT levels are continuous variables, it is likely that varying values correlate with different levels of risk. Moreover, prevalence has frequently been analyzed together with several respiratory diseases. When this problem has been analyzed in patients with COPD, the diagnosis has been either clinical or based on spirometry, but with no detailed assessment of pulmonary emphysema. Veith et al studied the prevalence of variants in a large sample of 29465 testing kits (AlphaKit®). The diagnosis of AAT deficiency was made by measuring serum levels, with phenotyping or genotyping depending on the need for a more detailed analysis. The authors jointly analyzed a COPD/emphysema group and reported similar findings to patients with bronchiectasis, but the emphysema population was not analyzed in detail.18 In fact, although AAT deficiency can also affect the airway, the paradigm of lung injury due to AAT deficiency is PE.19
Our results have unquestionable clinical implications. In severe forms, the clinical manifestations of the pulmonary disorders associated with AAT deficiency are indistinguishable from non-hereditary emphysema. This is possibly one of the main factors responsible for the high rate of underdiagnosis of this disease. This situation may be accentuated in the variants that are present with non-severe deficiency, so their identification as a risk factor must be evaluated proactively. So far, the primary objective has been to detect patients who are candidates for AAT augmentation therapy. However, our study confirms that even variants that cause a moderate decrease in serum AAT levels can also have clinical implications since their early detection allows preventive measures to be established in populations at higher risk. Furthermore, these results support the fact that various factors influence the pathogenesis of PE, which in some cases includes the presence of genetic susceptibility. Thus, a more detailed assessment of the AAT gene could be useful to identify individuals at risk of developing emphysema, even if they do not require augmentation therapy, since moderate reductions in serum AAT levels seem to contribute to a greater risk of the illness.
Limitations
Although the sample size is sufficient to detect significant associations, larger populations and longitudinal follow-ups are necessary to increase the robustness of the findings. Furthermore, it would be necessary to assess the individualized impact of the variants that we have evaluated jointly (MZ, SS, SZ). However, the most determining factor in our study was the reduction in AAT concentrations, which, although associated with the type of variant, may present variations in each patient group. Secondly, although CT is a reference diagnostic tool for PE, the lack of correlation with pulmonary function tests leaves a gap in our understanding of the clinical impact of these variants on a general COPD population. Nonetheless, COPD is an umbrella that encompasses very diverse disorders; thus, studies are needed that focus on specific conditions, like PE. In fact, it is even likely that different patterns of parenchymal destruction may exist within emphysema, and that this genetic disorder is especially relevant in the more diffuse forms of lung destruction.20,21
To conclude, our study demonstrates that variants associated with non-severe reductions in serum AAT levels act as predisposing factors for pulmonary emphysema. These findings expand our understanding of genetic risk factors for emphysema and offer new insight for early detection and treatment.
Funding
This project has been funded by an unrestricted scholarship from GRIFOLS Lab and the Chair of Inflammatory Diseases of the Airways, University of Alcalá.
Disclosure
José Luis Izquierdo received professional fees from Astra Zeneca, Bayer, Boehringer Ingelheim, Chiesi, GSK, Grifols, Menarini, Novartis, Orion, Pfizer, Sandoz, Teva, and Zambon. He is also the director of the Chair of Inflammatory Diseases of the Airways, University of Alcalá, during the conduct of the study. José Miguel Rodríguez received professional fees from Astra Zeneca, Bayer, Boehringer Ingelheim, Chiesi, GSK, Grifols, Menarini, Novartis, Orion, Pfizer and Teva. The authors report no other conflicts of interest in this work.
References
1. Strnad P, McElvaney NG, Lomas DA, Longo DL. Alpha 1 -antitrypsin deficiency. N Engl J Med. 2020;382(15):1443–1455. PMID: 32268028. doi:10.1056/NEJMra1910234
2. Aiello M, Marchi L, Ferrarotti I, et al. Distribution of the clinical manifestations of alpha 1 antitrypsin deficiency in respiratory outpatients from an Area of Northern Italy. Respiration. 2022;101(9):851–858. PMID: 35793662. doi:10.1159/000525549
3. Duk K, Zdral A, Szumna B, Roży A, Chorostowska-Wynimko J. Frequency of rare alpha-1 antitrypsin variants in polish patients with chronic respiratory disorders. Adv Exp Med Biol. 2016;910:47–53. PMID: 26987331. doi:10.1007/5584_2016_213
4. Duk K, Zdral A, Szumna B, Roz Y. Frequency of rare alpha-1 antitrypsin variants in polish patients with chronic respiratory disorders. Respir Medi Sci. 2016;47–53. doi:10.1007/5584_2016_213
5. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidance for reporting observational research. Available from: http://strobe-statement.org/index.php?id=strobe-home.
6. Lynch DA, Austin JH, Hogg JC, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277(1):192–205. PMID: 25961632; PMCID: PMC4613878. doi:10.1148/radiol.2015141579
7. Ortega VE, Li X, O’Neal WK, et al. NHLBI subpopulations and intermediate outcomes measures in COPD Study (SPIROMICS). The effects of rare SERPINA1 variants on lung function and emphysema in SPIROMICS. Am J Respir Crit Care Med. 2020;201(5):540–554. PMID: 31661293; PMCID: PMC7047460. doi:10.1164/rccm.201904-0769OC
8. Ferkingstad E, Oddsson A, Gretarsdottir S, et al. Genome-wide association meta-analysis yields 20 loci associated with gallstone disease. Nat Commun. 2018;9(1):5101. doi:10.1038/s41467-018-07460-y
9. Molloy K, Hersh CP, Morris VB, et al. Clarification of the risk of chronic obstructive pulmonary disease in α 1 -antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189(4):419–427. doi:10.1164/rccm.201311-1984OC
10. Foreman MG, Wilson C, DeMeo DL, et al. Alpha-1 antitrypsin PiMZ genotype is associated with chronic obstructive pulmonary disease in two racial groups. Ann Am Thorac Soc. 2017;14(8):1280–1287. doi:10.1513/AnnalsATS.201611-838OC
11. Lieberman J, Winter B, Sastre A. Alpha 1‐antitrypsin Pi‐types in 965 COPD patients. Chest. 1986;89:370–373. doi:10.1378/chest.89.3.370
12. Shigeoka JW, Hall WJ, Hyde RW, et al. The prevalence of alpha antitrypsin heterozygotes (Pi MZ) in patients with obstructive pulmonary disease. Am Rev Respir Dis. 1976;114(6):1077–1084. doi:10.1164/arrd.1976.114.6.1077
13. Cox DW, Hoeppner VH, Levison H. Protease inhibitors in patients with chronic obstructive pulmonary disease: the alpha‐antitrypsin heterozygote controversy. Am Rev Respir Dis. 1976;113(5):601–606. doi:10.1164/arrd.1976.113.5.601
14. Morse JO, Lebowitz MD, Knudson RJ, Burrows B. Relation of protease inhibitor phenotypes to obstructive lung diseases in a community. N Engl J Med. 1977;296(21):1190–1194. doi:10.1056/NEJM197705262962102
15. Franciosi AN, Carroll TP, McElvaney NG. SZ alpha-1 antitrypsin deficiency and pulmonary disease: more like MZ, not like ZZ. Thorax. 2021;76(3):298–301. PMID: 32917839. doi:10.1136/thoraxjnl-2020-215250
16. Dahl M, Hersh CP, Ly NP, Berkey CS, Silverman EK, Nordestgaard BG. The protease inhibitor PI*S allele and COPD: a meta‐analysis. Eur Respir J. 2005;26(1):
17. Stoller JK, Aboussouan LS. A review of α 1 -antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):
18. Veith M, Tüffers J, Peychev E, et al. The distribution of alpha-1 antitrypsin genotypes between patients with COPD/emphysema, asthma and bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2020;15:2827–2836. PMID: 33192056; PMCID: PMC7654539. doi:10.2147/COPD.S271810
19. Toumpanakis D, Usmani OS. Small airways disease in patients with alpha-1 antitrypsin deficiency. Respir Med. 2023;211:107222. PMID: 36965591. doi:10.1016/j.rmed.2023.107222
20. Kim WD, Eidelman D, Izquierdo JL, Ghezzo H, Saetta MP, Cosio MG. ”Centrilobular and Panlobular emphysema in smokers. Two distinct morphological and functional entities”. Amer Rev Respir Dis. 1991;144(6):1385–1390. doi:10.1164/ajrccm/144.6.1385
21. Saetta M, Kim WD, Izquierdo JL, Ghezzo H, Cosio MG. Extent of centrilobular and panacinar emphysema in smokers’ lungs: pathological and mechanical implications. Eur Respir J. 1994;7:664–671. doi:10.1183/09031936.94.07040664
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
