Back to Journals » Journal of Inflammation Research » Volume 16
Alopecia Universalis in an Elderly Chinese Man Induced by Sacubitril/Alisartan, a Novel Angiotensin Receptor-Neprilysin Inhibitor
Authors Teng Y , Fan Y, Shang D, Tao X, Sun D
Received 28 June 2023
Accepted for publication 16 August 2023
Published 19 August 2023 Volume 2023:16 Pages 3519—3522
DOI https://doi.org/10.2147/JIR.S427937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yan Teng,1 Yibin Fan,1 Danying Shang,1 Xiaohua Tao,1,* Dongsheng Sun2,*
1Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China; 2Geriatric Medicine Center, Department of Geriatric Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongsheng Sun, Geriatric Medicine Center, Department of Geriatric Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected] Xiaohua Tao, Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China, Email [email protected]
Abstract: Drug-induced alopecia areata is a rare adverse event wherein medications such as antimicrobials, anticonvulsants, and biologics, trigger the premature transition of actively growing hairs into the telogen phase. Herein, a unique case of alopecia universalis observed during a clinical trial involving sacubitril/alisartan, a novel angiotensin receptor-neprilysin inhibitor (ARNI) has been reported. This case contributes to the range of cutaneous reactions that might be observed in association with ARNI therapy.
Keywords: alopecia universalis, sacubitril/alisartan, angiotensin receptor-neprilysin inhibitor
Introduction
Alopecia areata (AA) is a chronic, autoimmune condition characterized by non-scarring hair loss, which can manifest in any hair-bearing area. It is considered to be a complex genetic, immune-mediated disease that targets anagen hair follicles.1 Negative prognostic indicators include severe nail abnormalities, atopic tendency, the onset of extensive disease in children below 5 years of age, as well as the persistence of alopecia totalis or universalis for over 2 years. AA reported affects 1% to 2% of the population, carrying a lifelong risk of 2.1%.2 However,drug-induced AA (DIAA) have rarely been reported. Herein, our case reported an elderly Chinese man who experienced alopecia universalis induced by sacubitril/alisartan, a novel angiotensin receptor-neprilysin inhibitor (ARNI).
Case Presentation
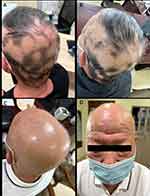
A 68-year-old elderly male patient, with a 5-year history of resistant hypertension, participated in a Phase III clinical trial involving the oral administration of sacubitril/alisartan tablets, a novel ARNI. Approximately 1 month after treatment initiation with a daily dosage of 240 mg of sacubitril/alisartan, the patient experienced patches of hair loss on the scalp. No prior occurrence of AA was evident in the patient’s medical or family history. Dermoscopy revealed black dots, exclamation mark hairs, and tapered hairs, while microscopic examination ruled out fungal involvement. Discontinuation of sacubitril/alisartan administration and topical application of corticosteroids and minoxidil tincture over a month yielded no improvement. Instead, hair loss intensified, resulting in complete scalp hair loss and nearly complete eyebrow loss within 6 months (Figure 1). Based on the clinical assessment, alopecia universalis was diagnosed. The patient commenced baricitinib therapy; however, no observable improvement was noted even after 4 weeks of treatment.
Discussion
DIAA exhibits similar demographic characteristics and disease outcomes as idiopathic AA. The clinical manifestations of DIAA range from well-defined focal patches to alopecia totalis or alopecia universalis, affecting all hair-bearing areas. Reported instances indicate a mean age of 42.5 years and a mean onset time of 3.8 months.3 Primarily, most documented cases are associated with biologics such as chemotherapy, antimicrobials, and anticonvulsants.4–7 However, reports of antihypertensive-induced alopecia universalis are scarce.
Based on the clinical presentation and hair loss onset observed in our case, a temporal association between oral sacubitril/alisartan tablets and alopecia universalis development is apparent. Exclusion of other potential causes such as nonscarring alopecia, scarring alopecia, genetic conditions, tinea capitis, and trichotillomania was based on typical clinical manifestations, medical history, and disease progression. Sacubitril/alisartan, a novel single-molecule ARNI, combines the molecular moieties of EXP3174 (the active metabolite of the angiotensin receptor blocker losartan) and sacubitril (a prodrug neprilysin inhibitor) in a 1:1 M ratio. It was developed in China to treat chronic heart failure (HF) and hypertension. A preclinical animal model study conducted by Sun et al8 indicated the efficacy of sacubitril/alisartan in treating myocardial ischaemia-induced chronic HF. Additionally, a Phase I clinical trial conducted by Hu et al,9 which involved randomised, double-blind, placebo-controlled, and single and multiple dose-escalation, demonstrated the overall tolerability of sacubitril/alisartan in healthy Chinese volunteers across various doses. Although antihypertensive agents typically cause pruritus and rashes, rare instances link them to other forms of alopecia. Mangkorntongsakul et al10 reported a case of prazosin-induced AA caused by a selective alpha-1-adrenoceptor blocker commonly used for managing hypertension. This study presents the first case of ARNI-induced alopecia universalis, notably absent in other ARNIs such as sacubitril/valsartan. The underlying mechanisms remain under investigation. It is hypothesised that the components of ARNI might induce vasodilation by blocking angiotensin receptors on smooth muscle cells in blood vessels, which could result in increased vascular permeability and potential antigen presentation, leading to this idiopathic reaction. Recent years have seen the development of biologics and Janus kinase (JAK) inhibitors as therapies for AA.11,12 In this case, baricitinib, a Food and Drug Administration-approved JAK1/2 inhibitor for severe AA, was administered but showed limited efficacy, possibly due to the short treatment duration. Long-term follow-up is necessary to evaluate the long-term efficacy and safety file of baricitinib.
Conclusion
This case report presents an elderly Chinese male who developed sacubitril/alisartan-induced alopecia universalis, broadening the spectrum of potential cutaneous reactions associated with ARNI therapy.
Ethics Statement
Patient consent for case image publication was obtained, and the Hospital Ethics Committees of the Affiliated People’s Hospital of Hangzhou Medical College approved the publication of case details.
Consent Statement
Informed consent was obtained from the patient for the publication of this case.
Funding
There is no funding to report.
Disclosure
Dongsheng Sun and Xiaohua Tao are co-corresponding authors for this study. The authors have no conflicts of interest to declare for this work.
References
1. Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–1525. doi:10.1056/NEJMra1103442
2. Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134(4):1141–1142. doi:10.1038/jid.2013.464
3. Murad A, Maguire J, Bergfeld W. Drug-induced alopecia areata? Clin Exp Dermatol. 2021;46(2):363–366. doi:10.1111/ced.14381
4. Martora F, Vastarella M, Fattore D, et al. Oral minoxidil for chemotherapy-induced alopecia. Skin Appendage Disord. 2022;8(6):508–510. doi:10.1159/000525463
5. Zimmermann J, Buhl T, Müller M. Alopecia universalis following alemtuzumab treatment in multiple sclerosis: a barely recognized manifestation of secondary autoimmunity-report of a case and review of the literature. Front Neurol. 2017;8:569. doi:10.3389/fneur.2017.00569
6. Chung J, Slaught CL, Simpson EL. Alopecia areata in 2 patients treated with dupilumab: new onset and worsening. JAAD Case Rep. 2019;5(8):643–645. doi:10.1016/j.jdcr.2019.03.019
7. Yalici Armagan B, Atakan N. New onset alopecia areata during secukinumab therapy. Dermatol Ther. 2019;32(5):e13071. doi:10.1111/dth.13071
8. Sun J, Xu W, Hua H, et al. Pharmacodynamic and pharmacokinetic effects of S086, a novel angiotensin receptor neprilysin inhibitor. Biomed Pharmacother. 2020;129:110410. doi:10.1016/j.biopha.2020.110410
9. Hu Y, Zhang H, Li X, et al. A randomized, double-blind, placebo-controlled, single, and multiple dose-escalation Phase I clinical trial to investigate the safety, pharmacokinetic, and pharmacodynamic profiles of oral S086, a novel angiotensin receptor-neprilysin inhibitor, in healthy Chinese volunteers. Expert Opin Investig Drugs. 2022;31(9):977–985. doi:10.1080/13543784.2021.1985464
10. Mangkorntongsakul V, Thilagarajan C, Arianejad F, et al. Alopecia areata: a rare side effect of prazosin. Australas J Dermatol. 2021;62(3):443–445. doi:10.1111/ajd.13648
11. Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35(4):e15346. doi:10.1111/dth.15346
12. Martora F, Villani A, Ocampo‐Garza SS, et al. Alopecia universalis improvement following risankizumab in a psoriasis patient. J Eur Acad Dermatol Venereol. 2022;36(7):e543–e545. doi:10.1111/jdv.18017
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.