Back to Journals » OncoTargets and Therapy » Volume 9
ALK-positive anaplastic large cell lymphoma with soft tissue involvement in a young woman
Authors Gao K, Li H, Huang C, Li H, Fang J, Tian C
Received 2 April 2016
Accepted for publication 16 May 2016
Published 1 July 2016 Volume 2016:9 Pages 3993—3996
DOI https://doi.org/10.2147/OTT.S109746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
This paper has been retracted
Kehai Gao, Hongtao Li, Caihong Huang, Huazhuang Li, Jun Fang, Chen Tian
Department of Orthopaedics, Yidu Central Hospital, Shandong, People’s Republic of China
Introduction: Anaplastic large cell lymphoma (ALCL) is a type of non-Hodgkin lymphoma that has strong expression of CD30. ALCL can sometimes involve the bone marrow, and in advanced stages, it can produce destructive extranodal lesions. But anaplastic large cell lymphoma kinase (ALK)+ ALCL with soft tissue involvement is very rare.
Case report: A 35-year-old woman presented with waist pain for over 1 month. The biopsy of soft tissue lesions showed that these cells were positive for ALK-1, CD30, TIA-1, GranzymeB, CD4, CD8, and Ki67 (90%+) and negative for CD3, CD5, CD20, CD10, cytokeratin (CK), TdT, HMB-45, epithelial membrane antigen (EMA), and pan-CK, which identified ALCL. After six cycles of Hyper-CVAD/MA regimen, she achieved partial remission. Three months later, she died due to disease progression.
Conclusion: This case illustrates the unusual presentation of ALCL in soft tissue with a bad response to chemotherapy. Because of the tendency for rapid progression, ALCL in young adults with extranodal lesions are often treated with high-grade chemotherapy, such as Hyper-CVAD/MA.
Keywords: anaplastic large cell lymphoma, ALK+, soft tissue involvement, Hyper-CVAD/MA
Introduction
In 1988, anaplastic large cell lymphoma (ALCL) was included in the revised Kiel classification, and is nowadays classified as a non-Hodgkin lymphoma of T-cell origin by the World Health Organization with strong expression of CD30.1,2 ALCL is common in Asian countries, which can be divided into three separate groups with different prognosis: anaplastic large cell lymphoma kinase (ALK)-positive ALCL, ALK-negative ALCL, and primary cutaneous ALCL. Systemic ALCL has an aggressive clinical course, and patients frequently present with systemic symptoms, advanced-stage disease, and extranodal localizations.3,4 Response to treatment and overall survival of systemic ALCL in children are good. In adults, however, it is not clear. ALCL sometimes can involve the bone marrow, and in advanced stages, it can produce destructive extranodal lesions. But ALK+ ALCL with soft tissue involvement is very rare. Here, we report a case of ALK-positive ALCL with soft tissue involvement in a young woman.
Case
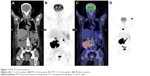
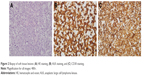
A 35-year-old woman who presented with waist pain for over 1 month was examined in our hospital. The patient had no fever or weight loss. Physical examination revealed hepatosplenomegaly and a lump in the waist but no lymphadenopathy. Serum lactate dehydrogenase was elevated to 1,500 IU/L (normal 200–460 IU/L), and other laboratory data showed anemia. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning showed that accumulation of FDG was observed in the soft tissue lump near the lumbar vertebra 1–5, considering malignant tumor (Figure 1). PET–computed tomography (CT) also showed many FDG-avid mass in mediastinum and mesenterium, suggesting lymphatic metastasis. A CT-guided biopsy of soft tissue lesions revealed ALCL. These cells were positive for ALK-1, CD30, TIA-1, GranzymeB, CD4, CD8, and Ki67 (90%+) and negative for CD3, CD5, CD20, CD10, cytokeratin (CK), TdT, HMB-45, epithelial membrane antigen (EMA), and pan-CK (Figure 2). Bone marrow aspiration and trephine biopsy showed no infiltration. She was diagnosed as ALK-positive ALCL with soft tissue involvement. After two cycles of Hyper-CVAD/MA chemotherapy, her condition showed no significant improvement. After four cycles of Hyper-CVAD/MA chemotherapy, she achieved partially remission (PR). After six cycles of Hyper-CVAD/MA regimen, she still remained in PR. Three months later, she died of disease progression.
Discussion
Malignant lymphoma with prominent soft tissue involvement is an infrequent, and often diagnostically challenging neoplasm, which represents ~3% of all primary malignant bone tumors and 1% of all malignant lymphomas. Among those studies that reported the T- or B-cell phenotype of primary soft tissue lymphoma, B-cell accounted for over 90%. B symptoms such as fever, night sweats, and weight loss, were frequent in ALCL patients. A majority of patients with ALCL had a disseminated disease (Ann Arbor Stage III or IV) and a limited number of extranodal sites.5 Bone marrow involvement has been initially considered a rare event in ALCL.6
As ALCL is a highly curable disease, it is important for it to be differentiated from other causes of lytic bone lesions, such as carcinomas and other primary bone tumors. Although this may include cortical or soft tissue invasion, the diagnosis generally excludes lymph node or distant visceral involvement to be considered a primary lymphoma of bone non-Hodgkin’s lymphoma (NHL) of bone is a rare entity that is limited to the long bones and axial skeleton, with the femur being the most common site of involvement. Besides, ALCL has to be distinguished from classical Hodgkin lymphoma, CD30+ non-Hodgkin B-cell lymphomas, and very rare ALK-1-positive (and eventually CD30-negative) large B-cell lymphomas.
ALCL is characterized by the expression of CD30 on malignant cells, and its prognosis is related with the expression of the ALK protein.7 ALCL is divided into three separate entities based on ALK expression: ALK-positive ALCL, ALK-negative ALCL, and primary cutaneous ALCL. ALCL commonly involves in children and young adults that presents progressive disease with a high incidence of extra-nodal involvement.8 The case we report is ALCL with soft tissue involvement diagnosed with PET–CT, and pathology which is rare. It is known that ALK is an indicator of better responses to treatment in ALCLs. However, this case did not show complete remission (CR) after six cycles of treatment partially because of the soft tissue involvement that affected its response to the treatment. Besides, CD8 expression was reported to link with bad outcomes that may affect the response of this patient.
Although magnetic resonance imaging and CT are the standard imaging modalities for the detection of ALCL with prominent soft tissue involvement, the imaging features are usually nonspecific and the lesion cannot be fully detected because magnetic resonance imaging and CT often were performed in a part of the body. PET-CT plays an important role in the diagnosis, staging, and surveillance of lymphoma.
Although no large comparative studies have been published, most investigators reported that the response of ALCL to chemotherapy was good, ranging from 60% to 90%. The overall survival of localized disease is known to be good, especially in children. More advanced stages have a high relapse rate, and their prognosis in comparison to that of other large cell lymphomas is controversial. Because ALCL belongs to NHL, so it is believed that CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen is generally applicable to ALCL. Besides, modified B-NHL-BFM-90 protocol is shown to be efficacious for Chinese children with ALCL.9 It is reported that both autologous and allogeneic hematopoietic stem cell transplantation (HSCT) can offer the prospect of durable disease-free survival for ALCL in childhood and adolescence. Patients with CR at the time of autologous HSCT had significantly greater event-free survival than patients with non-CR at the time of autologous HSCT.10 Recently, brentuximab vedotin, which is a CD30-targeted antibody, have emerged.9 Novel therapies may soon radically change the treatment paradigm for this disease and hopefully lead to less toxicity and improved outcomes.11 In this case, the patient was treated with high-grade chemotherapy, such as Hyper-CVAD/MA. But there was still no significant improvement and after six cycles of chemotherapy, she only achieved PR. Then she gave up the treatment and died 3 months later.
Conclusion
ALCL is a rare but biologically well-characterized disorder with a wide spectrum of presentation. It may present with soft tissue involvement. Recognition of a combination of symptoms including anemia, renal failure, and bone pain in the presence of normal bone marrow biopsy and serum electrophoresis should trigger aggressive clinical workup to rule out the possibility of lymphoma. A meticulous examination of early biopsies based on PET–CT of deeply situated soft tissue or lymph nodes are recommended to yield an early diagnosis of ALCL.
Acknowledgment
The subject and her parents/guardians gave their informed written consent, and the study protocol was approved by the Ethics Committee of Yidu Central Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
Stansfeld AG, Diebold J, Noel H, et al. Updated Kiel classification for lymphomas. Lancet. 1988;1:292–293. | ||
Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. | ||
Kadin ME. Primary Ki-1-positive anaplastic large-cell lymphoma: a distinct clinicopathologic entity. Ann Oncol. 1994;5(Suppl 1):S25–S30. | ||
Filippa DA, Ladanyi M, Wollner N, et al. CD30 (Ki-1)-positive malignant lymphomas: clinical, immunophenotypic, histologic, and genetic characteristics and differences with Hodgkin’s disease. Blood. 1996;87(7):2905–2917. | ||
Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed–Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–858. | ||
Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013;30(6):509–519. | ||
Lovisa F, Cozza G, Cristiani A, et al. ALK Kinase Domain mutations in primary anaplastic large cell lymphoma: consequences on NPM-ALK activity and sensitivity to tyrosine kinase inhibitors. PLoS One. 2015;10(4):e0121378. | ||
Mitou G, Frentzel J, Desquesnes A, et al. Targeting autophagy enhances the anti-tumoral action of crizotinib in ALK-positive anaplastic large cell lymphoma. Oncotarget. 2015;6(30):30149–30164. | ||
Amin AD, Rajan SS, Liang WS, et al. Evidence suggesting that discontinuous dosing of ALK kinase inhibitors may prolong control of ALK+ tumors. Cancer Res. 2015;75(14):2916–2927. | ||
Fukano R, Mori T, Kobayashi R, et al. Haematopoietic stem cell transplantation for relapsed or refractory anaplastic large cell lymphoma: a study of children and adolescents in Japan. Br J Haematol. 2015;168(4):557–563. | ||
Pillon M, Gregucci F, Lombardi A, et al; NHL committee of the Italian association of pediatric hematology and oncology (AIEOP). Results of AIEOP LNH-97 protocol for the treatment of anaplastic large cell lymphoma of childhood. Pediatr Blood Cancer. 2012;59(5):828–833. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.