Back to Journals » OncoTargets and Therapy » Volume 16
Alectinib-Induced Severe Hemolytic Anemia in a Patient with ALK-Positive Non-Small Cell Lung Cancer: A Case Report
Authors Misawa K , Nakamichi S, Iida H, Nagano A, Mikami E, Tozuka T , Matsumoto M, Miyanaga A , Noro R, Kubota K, Yamaguchi H, Seike M
Received 24 November 2022
Accepted for publication 12 January 2023
Published 24 January 2023 Volume 2023:16 Pages 65—69
DOI https://doi.org/10.2147/OTT.S398375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Kazuhito Misawa,1 Shinji Nakamichi,1 Hiroki Iida,1 Atsuhiro Nagano,1 Erika Mikami,1 Takehiro Tozuka,1 Masaru Matsumoto,1 Akihiko Miyanaga,1 Rintaro Noro,1 Kaoru Kubota,1 Hiroki Yamaguchi,2 Masahiro Seike1
1Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, 113-8603, Japan; 2Department of Hematology, Nippon Medical School, Tokyo, 113-8603, Japan
Correspondence: Shinji Nakamichi, Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, 1-1-5, Sendagi, Bunkyo-ku, Tokyo, 113-8603, Japan, Tel +81-3-3822-2131, Email [email protected]
Abstract: Alectinib is a selective anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor as standard therapy for ALK-rearranged non-small cell lung cancer (NSCLC). Hemolytic anemia is considered as a rare but significant adverse event with alectinib. Here, we report a case of a 73-year-old female with lung adenocarcinoma, harbouring an ALK fusion gene, who received alectinib as second-line therapy and developed gradually progressive grade 4 (6.4 g/dL) drug-induced hemolytic anemia (DIHA) after complete response. We discontinued alectinib and performed a blood transfusion for the severe anemia. The anemia improved with no recurrence of lung adenocarcinoma over 10 months. Regular hematologic monitoring and the possibility of DIHA should be considered in case of progressive hemolytic anemia during alectinib treatment.
Keywords: non-small cell lung cancer, ALK, alectinib, drug-induced hemolytic anemia
Introduction
Drug-induced hemolytic anemia (DIHA) is a rare cause of anemia with an estimated incidence of approximately one case per million inhabitants per year.1 DIHA has been reported with several drugs, especially with antimicrobials. DIHA is classified according to two major mechanisms of action: immune destruction of erythrocytes and destruction due to oxidant injury.2 DIHA is usually mild, but occasionally associated with acute severe hemolytic anemia and death.3
Anaplastic lymphoma kinase (ALK) rearrangement is present in 2–7% of patients with non-small-cell lung cancer (NSCLC).4 Alectinib is a selective ALK tyrosine kinase inhibitor used as standard therapy for NSCLC harbouring an ALK fusion gene with remarkable progression-free survival (PFS) and overall survival (OS), along with manageable safety profile.5,6 Anemia due to alectinib has been reported to account for 6–26.3% of adverse events (AEs).6,7 To our knowledge, only 7 references of DIHA caused by alectinib have been described.8–14 In addition, there are potentially many other similar cases that have not been reported. However, no patient with grade 4 severe hemolytic anemia during treatment with alectinib has been reported previously. Here, we report a case of a 73-year-old female with lung adenocarcinoma harbouring an ALK fusion gene who developed severe grade 4 anemia due to DIHA with alectinib treatment.
Case Report
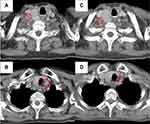
A 73-year-old woman complained of epicardial pain with a nodular shadow in the left lower lobe of the lung by chest computed tomography (CT). We diagnosed the patient with lung adenocarcinoma of the left lower lobe and clinical T1aN3M0 stage IIIB. She received concurrent chemoradiation (CCRT) (60 Gy in 2 Gy daily fraction with 2 cycles of cisplatin plus pemetrexed) without maintenance therapy by pemetrexed. About 2 years later, positron emission tomography-computed tomography (PET-CT) revealed bilateral supraclavicular lymph node recurrence. Alectinib (600 mg) was administered orally twice daily as second-line treatment, because an EML4-ALK fusion gene was identified using reverse transcription polymerase chain reaction (RT-PCR) analysis of the free test by Pfizer. About 2 months after alectinib initiation, grade 2 anemia was observed with an unknown cause (Figure 1). The anemia continued for about 6 months, with the hemoglobin level then spontaneously improving to a normal level; indeed, the patient showed a complete response to treatment. About 3 years later, she was admitted to our hospital due to gradually progressive anemia with edema. Laboratory testing of blood showed the hemoglobin level decreased from 12.3 g/dL to 6.4 g/dL, along with elevated levels of T-Bil (1.87 mg/dL) and LDH (386 U/L) and low levels of reticulocytes (3.8%) and haptoglobin (2 mg/dL) (Table 1). Folic acid and vitamin B12 levels were normal. Both the direct antiglobulin test (DAT) and indirect antiglobulin test were negative. There was no evidence of active bleeding as determined by upper and lower gastrointestinal endoscopy, and no irregular bleeding. We also confirmed no recurrence of lung cancer and no splenomegaly by whole-body contrast-enhanced CT (Figure 2).
 |
Table 1 Laboratory data |
We noted that T-Bil and LDH were gradually elevated after the start of alectinib despite the absence of anemia progression. Considering the possibility of other drugs such as antibiotics, we finally diagnosed her as having DIHA caused by alectinib. Blood transfusion for the severe anemia was performed along with discontinuation of alectinib. She was discharged on day 9 without further blood transfusion. Hemoglobin was gradually increased, and T-Bil and LDH were decreased after hospital discharge (Figure 1). There was no relapse of anemia and no recurrence of lung adenocarcinoma for approximately 10 months after discontinuation of alectinib.
Discussion
To our knowledge, this is the first reported case of grade 4 anemia caused by treatment of alectinib in a patient with ALK-positive NSCLC.
Alectinib is considered to invariably induce subclinical hemolysis in all patients with morphological changes such as acanthocytes or spherocytes of erythrocytes.9,10 The US package insert of alectinib mentions potential for hemolytic anemia. Nevertheless, hemolytic anemia is underreported because many physicians are unaware of its existence. Most of the cases reported developed mild and acute (within 90 days) hemolysis and anemia is generally reversible with discontinuation of alectinib.11 In our case report, although grade 4 anemia first appeared after about 3 years, T-Bil and LDH were already elevated above baseline one month after the start of alectinib, followed by a decrease in hemoglobin level. Therefore, the anemia that appeared about 2 months after alectinib administration was considered the first hemolytic event in this patient.
Only one case of DIHA by alectinib from Japan has been reported.8 Previous reports have not mentioned the dose of alectinib. The dosage of alectinib overseas is double the dosage used in Japan. Anemia has been considered a rare adverse event in clinical trials from Japan.15 A small number of reports of DIHA by alectinib in Japan suggests that hemolysis could be dose-dependent.
In a similar vein to previous reports of alectinib-induced hemolytic anemia, the results of DAT were consistently negative.8,9,12,13 The DAT demonstrates the presence of antibodies or complement on the surface of red blood cells.16 The negative result for DAT suggested that the hemolysis observed was occurring via a nonimmune mechanism. Autoimmune hemolytic anemia (AIHA) was ruled out because of a negative DAT in this case. Increases in T-Bil and LDH are the main indicators of hemolytic anemia. However, we did not initially recognize hemolytic anemia because these factors were elevated before the hemoglobin decreased in this patient. We finally concluded this patient as having hemolytic anemia due to the absence of hemorrhagic disease and a decrease in haptoglobin. Regular hematologic monitoring (eg, bilirubin, LDH, reticulocyte, etc) should be examined during alectinib treatment for early detection of hemolytic anemia.
There have been no reports to date examining the mechanism of hemolytic anemia due to alectinib, although differences in EML4-ALK variants status do not appear to have any effect on the hemolytic response.9 Most DIHA events are due to drug-induced immune hemolytic anemia (DIIHA), caused by an immune mechanism against red blood cells with positivity for DAT seen.1 Hemolysis due to alectinib differs from DIIHA in that there are morphological changes in the erythrocytes and the DAT is negative. Eosin-5-maleimide staining (EMA) is decreased in patients who receive alectinib.10 EMA is a fluorescent dye which intercalates with a number of erythrocyte cytoskeletal proteins.10 EMA is generally decreased in hereditary spherocytosis (HS) and found at normal levels in acquired hemolytic anemias such as AIHA and DIIHA. The morphology of erythrocytes in peripheral blood smears suggests that the erythrocyte cytoskeleton is affected upon alectinib treatment. Hemolysis was not found to occur in patients who received alectinib for only a few days.10 It is assumed that alectinib-induced alterations in the hematopoietic process causes erythrocyte deformation, although the detailed mechanism is unknown. Based on the above, the mechanism of hemolytic anemia caused by alectinib may be different from the characteristics of DIHA. There have been no prior reports of DIHA caused by other selective ALK-TKIs such as ceritinib, lorlatinib and brigatinib, although there has been one report of crizotinib-induced hemolytic anemia.17 If alectinib must be discontinued due to severe anemia, a switch to an alternative ALK-TKI could be an option.
Our report has several limitations. First, morphological changes in erythrocytes were unknown. In this context, analysis of peripheral blood smears should be performed as an additional test. Second, we cannot directly compare this case with that of overseas patients as the dose of alectinib used in Japan is half that used externally. Third, discontinuation of alectinib may facilitate recurrence; as such, we must follow the course of the patient carefully.
In conclusion, we report the first case with gradually developed progressive grade 4 anemia due to DIHA by alectinib in an ALK-positive NSCLC patient. Most DIHA events are mild and acute, and reversible by discontinuation of alectinib. Regular hematologic monitoring should be performed during alectinib treatment. DIHA should be taken into consideration in the case of the progressive anemia, given the severe anemia that occurred this patient.
Summary
Drug-induced hemolytic anemia is a significant adverse event caused by alectinib treatment in a patient with ALK-positive non-small cell lung cancer.
Ethical Approval
Institutional approval was not required to publish the case details.
Consent for Publication
Informed consents were obtained from the patients for publication.
Disclosure
Dr. Kubota received research fund from Nihon Kayaku, AstraZeneca, Pfizer, and payment or honoraria for lectures and presentations from Chugai Pharmaceutical. Dr. Seike received payment or honoraria for lectures and presentations from AstraZeneca, Chugai Pharmaceutical, Taiho Pharmaceutical, MSD, Ono Pharmaceutical, Bristol–Myers Squibb, Eli Lilly Japan, Takeda Pharmaceutical, Nihon Kayaku, Nippon Boehringer Ingelheim, Pfizer, Kyowa–Hakko Kirin, and Novartis. The other authors report no conflicts of interest in this work.
References
1. Garbe E, Andersohn F, Bronder E, et al. Drug induced immune haemolytic anaemia in the berlin case-control surveillance study. Br J Haematol. 2011;154:644–653.
2. Petz LD, Garratty G. Drug-induced haemolytic anaemia. Clin Haematol. 1975;4:181–197. doi:10.1016/S0308-2261(21)00631-7
3. Garratty G. Drug-induced immune hemolytic anemia. Hematology. 2009;1:73–79. doi:10.1182/asheducation-2009.1.73
4. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi:10.1056/NEJMoa1006448
5. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi:10.1056/NEJMoa1704795
6. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised Phase 3 trial. Lancet. 2017;390:29–39. doi:10.1016/S0140-6736(17)30565-2
7. Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi:10.1016/j.annonc.2020.04.478
8. Okumoto J, Sakamoto S, Masuda T, et al. Alectinib-induced immune hemolytic anemia in a patient with lung adenocarcinoma. Intern Med. 2021;60(4):611–615. doi:10.2169/internalmedicine.4241-19
9. Kunz J, Wiedemann C, Grosch H, et al. Early development of ubiquitous acanthocytosis and extravascular hemolysis in lung cancer patients receiving alectinib. Cancers. 2022;14(11):2720. doi:10.3390/cancers14112720
10. Kuzich JA, Heynemann S, Geoghegan N, et al. Alectinib induces marked red cell spheroacanthocytosis in a near-ubiquitous fashion and is associated with reduced eosin-5-maleimide binding. Pathology. 2021;53(5):608–612. doi:10.1016/j.pathol.2020.10.023
11. Dores GM, Nayernama A, Cheng C, et al. Hemolytic anemia following alectinib reported to the U.S. food and drug administration adverse event reporting system. Am J Hematol. 2022;97(4):E129–E132. doi:10.1002/ajh.26454
12. Yuan Y, Mapp S, Xu W. Two cases of marked red cell anisopoikilocytosis and haemolysis with alectinib, an anaplastic lymphoma kinase inhibitor. Br J Haematol. 2020;190(5):642. doi:10.1111/bjh.16813
13. Gullapalli V, Xu W, Lewis CR, et al. A multi-centre case series of alectinib-related erythrocyte membrane changes and associated haemolysis. J Hematopathol. 2021;14(2):131–136. doi:10.1007/s12308-020-00427-3
14. Legland Ép Dejean AM, Plantamura J, Arnoux I, et al. Acanthocytosis in an alectinib-treated patient. Br J Haematol. 2022;197(2):131. doi:10.1111/bjh.18020
15. Nakagawa K, Hida T, Nokihara H, et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2020;139:195–199. doi:10.1016/j.lungcan.2019.11.025
16. Dhaliwal G, Cornett PA, Tierney LM
17. Crizotinib 2016; Available from: https://link.springer.com/article/10.1007/s40278-016-23836-6.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.