Back to Journals » Infection and Drug Resistance » Volume 15
Alarming Antibiotic Resistance in Pediatric Oncology Patients: A Three-Year Prospective Cohort Study from Oman
Authors Al Battashi A, Al Harrassi B, Al Maskari N, Al Hashami H, Al Awaidy S
Received 8 April 2022
Accepted for publication 11 July 2022
Published 25 July 2022 Volume 2022:15 Pages 3939—3947
DOI https://doi.org/10.2147/IDR.S369909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Abeer Al Battashi,1 Bishara Al Harrassi,2 Nawal Al Maskari,3 Hilal Al Hashami,3 Salah Al Awaidy4
1Department of Pediatric Hematology and Oncology, The National Oncology Centre, Royal Hospital, Ministry of Health, Muscat, Oman; 2Oman Medical Specialty Board, Muscat, Oman; 3Pediatric Infectious Diseases, Child Health Department, Royal Hospital, Ministry of Health, Muscat, Oman; 4Office of Health Affairs, Ministry of Health, Muscat, Oman
Correspondence: Salah Al Awaidy, Office of Health Affairs, Ministry of Health, Al Khuwair Street, Muscat, Oman, Tel +968 99315063, Fax +968 24946381, Email [email protected]
Background: Bloodstream infections (BSI) are severe and challenging oncological complications, with a consequent high morbidity and mortality in the immunocompromised. We reviewed the profile and susceptibility of bacteria associated with infections in children under 13 years of age receiving chemotherapy.
Methods: Prospective cohort study of pediatric oncology patients was conducted between January 2015 and October 2017 at the Royal Hospital in Oman. Patient demographics, clinical data, laboratory parameters, microbial etiology and susceptibility, and outcomes were retrieved and analyzed.
Results: A total of 74 episodes of positive bacterial blood cultures were detected in 38 oncology patients (positive blood culture rate of 51%). Fifty-seven percent were positive for gram-negative organisms with Klebsiella (21%) being the most common gram-negative organism cultured, and the most common gram-positive organism was Staphylococcus (coagulase negative Staphylococcus (CONs) and S. Aureus) (30%). The majority of patients had gastrointestinal complaints (74%), and almost half (51%) had prolonged periods of neutropenia (> 7 days). One third of gram-negative organisms were resistant to four or more antibiotics with a major resistance of 31% to piperacillin-tazobactam. Of the gram-positive organisms, 38% were resistant to at least four antibiotics and 30% were pan-resistant (except for vancomycin).
Conclusion: The gram-negative organisms were dominant in BSIs with Klebsiella being the most common culprit. Bacteremia was prevalent, however, high resistance to first-line antibiotics was documented amongst gram-negative isolates, demanding strategies to ensure our patients’ safety.
Keywords: neutropenia, pediatric oncology, bloodstream infections, antibiotic susceptibility, Oman
Corrigendum for this paper has been published.
Study Summary
Bloodstream infections (BSIs) are serious and difficult oncologic complications, resulting in high morbidity and mortality. In this study, we found that gram-negative organisms dominated BSIs among pediatric oncology patients, with Klebsiella being the most prevalent culprit. We also observed that resistance to multiple antibiotics exists in gram-negative and gram-positive bacterial groups, which is worrisome because these antibiotics are commonly used as a frontline in managing febrile neutropenic cancer patients. There is a high level of resistance of gram-negative bacteria towards piperacillin-tazobactam. Oncology pediatric cancer patients with gram-negative blood stream infections commonly presented with gastrointestinal symptoms. A first-line combination of anti-gram-negative coverage is justifiable based on our data. Vancomycin needs to be used very cautiously due to the increased resistance of gram-positive bacteria among other alternatives.
Introduction
Bloodstream infections (BSIs) remain an important cause of morbidity and mortality in chemotherapy oncology patients.1,2 Chemotherapy induced by bone marrow suppression is a major risk factor for developing various mild to severe infections including sepsis and septic shock. Other risk factors include the presence of indwelling central venous catheters (CVC), leukemia as a diagnosis, malnutrition and recent chemotherapy administration.3 Moreover, bacterial infections may sometimes coexist with invasive and non-invasive fungal and viral infections, as different immune pathways are compromised during treatment.4 Significant changes in the spectrum of microorganisms isolated from blood cultures have been reported in cancer patients over the last few years.2
A prospective study in southeastern England (2002) found that 70% of positive blood cultures in childhood cancer patients treated with febrile neutropenia grew gram-negative organisms.5 Another study in the Middle East revealed that 55% of pediatric oncology patients had gram-positive bacterial infections compared to 45% gram-negative infections.1 We speculate that the pattern of growth that is reported in the literature depends on the individual institutional antimicrobial practice and the infection control policy implementation.
A preventative approach to the management of serious bacterial infections in pediatric cancer patients can help to reduce the associated morbidity and even mortality. Some treatment protocols advocate for the prophylactic use of antimicrobials (for example, cefixime alongside irinotecan).6 Moreover, patients who undergo allogeneic bone marrow transplantation need to be on long-term prophylactic antibiotics, antifungals, and antivirals until they are immune reconstituted.7 The cornerstone of BSI management is the early identification of septicemia clinical signs combined with the rational use of broad-spectrum antibiotics and the implementation of uniform catheter care practices and infection control counter-measures.8 This approach improves outcomes and reduces mortality. The standard management of febrile neutropenia prompts administration of empirical antibiotics until the fever subsides with an evident recovery in the neutrophil count.9 The rate of response to empirical antibiotics in febrile neutropenia ranges between 40% and 90%.2 Therefore, the advancement of successful treatment and practical strategies to treat bloodstream infections requires a cautious understanding of the local positive blood cultures with their sensitivity and resistance outline.
The current national practice is the use of a combination of a beta lactam antibiotic in the form of Pipracillin-Tazobactam with an aminoglycoside in the form of amikacin or gentamicin within thirty minutes of presentation to the emergency unit or upon fever onset. The addition of other antibiotics is based on the clinical presentation and complications like central-line infection, previous positive blood culture or bacterial colonization.
The purpose of this study is to describe the nature of the organisms detected in central or peripheral blood cultures and associate them with various clinical and laboratory parameters and treatment phase (low or highly immunosuppressive chemotherapy phases). It will also provide data on the microbial susceptibility and resistance patterns that can guide national clinical practice and management guidelines.
Materials and Methods
Study Design and Setting
A prospective cohort study was conducted from January 2015 to October 2017 at the Royal Hospital.
Study Population
The study included all oncology patients aged 13 or younger who were hospitalized between January 2015 and October 2017.
Data Collection
Data were collected using the electronic Al-Shifa system, an integrated electronic health care management platform managed by the Omani Ministry of Health. Positive blood cultures for all patients receiving chemotherapy with or without neutropenia were retrieved retrospectively. Patients’ demographics (age, sex and nationality), clinical characteristics (constitutional symptoms like fever, shortness of breath or cough, abdominal pain and distension, nausea and vomiting, constipation, or diarrhea), type of cancer, mortality (outcome) related to BSIs neutrophil counts and microbial etiology and susceptibility pattern were extracted.
Fever is defined in this study as a single axillary temperature greater than or equal to 37.8°C, or a sustained temperature of 37.5–37.7°C for at least one hour with no antipyretic coverage. BSI is considered to be the presence of a bacterial microorganism in the blood culture. All blood cultures were processed by the hospital clinical microbiology laboratory and the antimicrobial susceptibility test was determined using the disk diffusion assay.10 Neutropenia is defined as an absolute neutrophil count (ANC) of less than 1500 cells/microliter. In severe neutropenia, the ANC was less than 500 cells/microliter.7,8 Positive blood cultures were categorized into two groups, gram-positive and gram-negative organisms with sensitivity and resistance patterns as reported.
Statistical Analysis
The data were then transferred to the Microsoft Excel program for analysis. The data were analysed using descriptive statistical analysis and presented as continuous and categorical data.
Ethical Approval
Ethical clearance was obtained from the Royal Hospital Ethical Approval Committee (SRC#22/2018). Written informed consent was waived after the researchers analyzed the data anonymously, no potential risk to patients was foreseen and the study complies with the Declaration of Helsinki.
Results
Patient’s Demographic Characteristics
A total of 74 positive bacterial blood culture episodes were found in 38 oncology patients during the study period, resulting in a positive blood culture rate of 51%. Females represented 53% of the cases (n = 39) and 97% (n = 72) were Oman citizen. The overall mean age of the cohort was 51.76 months with a standard deviation (SD 33.38 months), with age ranging between 2 weeks and 12 years of age (Table 1).
 |
Table 1 Demographic Characteristics of Pediatric Oncology Patients with Bacteremia |
In terms of diagnosis, 20 patients (53%) had the diagnosis of acute leukemia (including B-cell and T-cell lineage, and myeloid leukemia), 6 (16%) patients had neuroblastoma, 3 (7.8%) patients had central nervous system embryonic tumors (medulloblastoma and atypical teratoid rhabdoid tumor), 3 (8%) had Ewing’s sarcoma, and 2 (5%) had lymphomas (Hodgkin’s and non-Hodgkin’s). Other malignancies included kidney tumors, rhabdomyosarcoma, soft tissue sarcoma, and peripheral nerve sheath tumor (3% one patient each).
Out of the 74 episodes of positive blood cultures, the majority (n = 47, 64%) were in patients treated for acute leukemia. Fourteen (19%) episodes were in patients during the intensive phase of therapy (eg, induction), 13 (18%) episodes were in patients in the maintenance phase (milder immunosuppressive phase), and 8 (11%) episodes were for patients treated for acute myeloid leukemia (intensive throughout). Twelve (16%) positive cultures were recorded in patients treated with a relapse regimen for leukemia (containing cytarabine and clofarabine). Six (8%) patients were treated for high-risk neuroblastoma with different agents, including anthracyclines and alkylator agents. Seven (9.5%) episodes were reported in patients treated for different types of sarcomas (Ewing’s sarcoma, rhabdomyosarcoma, and soft tissue sarcoma) with regimens containing high doses of methotrexate and anthracyclines. Also, 7 (9.5%) were detected in patients treated for lymphoma (Hodgkin’s and non-Hodgkin’s lymphoma). Bacteremia episodes were less commonly observed in patients undergoing palliative care (n = 4, 5%) and central nervous system tumors (n = 3, 4%).
Clinical Characteristics
Thirty patients (79%) experienced febrile neutropenia episodes. Furthermore, 54 of the BSIs (80%) were associated with neutropenia, with 46 (62%) of all BSI episodes categorized as severe neutropenia, with an absolute neutrophil count of fewer than 500 cells/μL. Furthermore, 38 (51%) of the patients exhibited neutropenia for at least seven days. At the time of presentation, 68 (92%) of the bacteremia episodes had a body temperature greater than 38.3°C.
Patients presented with different clinical presentations, noted that some presented with a mix of multiple symptoms and signs. Six patients (8%) had no symptoms other than fever at the time of presentation. Patients mainly presented with gastrointestinal complaints (n = 55, 74%), including abdominal pain and distension, nausea and vomiting, constipation, and/or diarrhea. Forty-three (58%) patients had respiratory symptoms, associated with shortness of breath, cough, and upper respiratory tract infections. In 21 (29%) of the episodes, the patients presented with local infections, including ear discharge, cellulitis, and central venous catheter (CVC) site infection. Other manifestations included septic shock (n = 16, 21%), constitutional symptoms (n = 19, 26%) like lethargy and insufficient oral intake, and only one patient had a generalized maculopapular skin rash.
Microbial Etiology and Susceptibility Pattern
Gram-negative organisms were more commonly grown (n = 42, 57%) in children receiving chemotherapeutic agents for cancer (Table 2). Among the gram-negative microorganisms, Klebsiella were the most dominant (21%, n = 9), followed by Pseudomonas (19%, n = 8), followed equally by Escherichia coli(E. coli) and Salmonella (17%, n = 7) (Figure 1). Among the gram-positive organisms, the majority (69%, n = 22) were Staphylococcus (Coagulase negative Staphylococcus (n = 2), Staphylococcus epidermidis (n = 11), Staphylococcus haemolyticus (n = 1), Staphylococcus hominis (n = 3) and Staphylococcus aureus (n = 5)) followed by Streptococcus (28%, n = 9) and Aerococcus (3%, n = 1) (Figure 2).
 |
Table 2 Types of Gram-Positive Bacteria Which is Isolated in Blood Cultures for Patients Aged 13 Years or Less Receiving Chemotherapy and Its Antimicrobial Resistance Pattern |
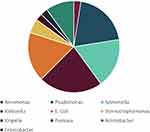 |
Figure 1 Distribution of gram-negative bacterial growth within blood cultures of children aged less than 13 years receiving chemotherapy. |
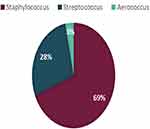 |
Figure 2 Distribution of gram-positive bacterial growth within blood cultures of children aged less than 13 years receiving chemotherapy. |
CVCs were present in 60% (n = 44) of the patients at the time of obtaining the blood cultures. About 42% (31 cultures) of the total bacterial specimens came from cultures obtained from CVC. Of these, gram-positive organisms were most common, with Staphylococcus representing 35%, followed by Streptococcus in 16%. For gram-negative organisms, both Pseudomonas and Klebsiella were found equally in 13% of the cultures each, followed by Salmonella (10%), E. coli(6%), Acinetobacter (3%), and Aeromonas (3%). Patients were given one or two antibiotics at first, depending on the clinical scenario and judgment. The majority were given antibiotics with two agents, the most prevalent of which were piperacillin-tazobactam and amikacin (n = 22, 30%).
Cefepime and amikacin were another commonly used combination (n = 10, 14%). Other less common combinations were meropenem and vancomycin, piperacillin-tazobactam and vancomycin, cefepime and vancomycin, and ceftazidime and amikacin. Monotherapy with piperacillin-tazobactam was administered imperially in 11% (n = 8) of the cases. Other antibiotics given as monotherapy are cefepime, meropenem, ceftriaxone and cloxacillin. Metronidazole was used in 18% (n = 13) of patients combined with other agents.
Thirty-three percentage (n = 14%) of the gram-negative organisms were resistant to at least four antimicrobials (Table 3). They were highly resistant to beta-lactam antibiotics even in combination with beta-lactamase. Around 31% (n = 13) were resistant to piperacillin-tazobactam, and 21% (n = 9) were resistant to Amoxicillin/clavulanic acid. Only 17% (n = 7) were resistant to amikacin, while 27% (n = 11) were resistant to gentamicin. Meropenem had the least resistance, with only 7% (n = 3) of the bacteria being affected. For gram-positive bacteria (Table 3), the resistance rate was high for all antimicrobial groups tested, including beta-lactams, aminoglycosides, sulphonamides, and clindamycin. That is except for vancomycin, which exhibited no resistance at all.
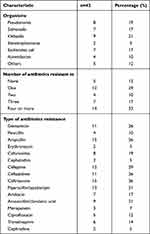 |
Table 3 Types of Gram-Negative Bacteria Isolated in Blood Cultures for Patients and Its Antimicrobial Resistance Pattern |
In terms of antimicrobial sensitivity, around 66.6% (n = 6) of Klebsiella was sensitive to gentamicin, and 33% (n = 3) was sensitive to amikacin. Moreover, there was an equal sensitivity of around 22% (n = 2) to imipenem, tazocin, meropenem, colistin and ciprofloxacin. Pseudomonas showed a higher sensitivity to antimicrobials with 62.5% (n = 5) out of the total being sensitive to ceftazidime, ciprofloxacin and gentamicin. 50% (n = 4) were sensitive to meropenem, tazocin, and cefepime. Only 37.5% were sensitive to amikacin. Gram-positive bacteria showed a high sensitivity to vancomycin (100% of Staphylococcus and 88% of Streptococcus).
Mortality Related to BSIs
Over the study period, six patients died of BSIs (case fatality rate, 16%). Four cases died due to gram-negative infections: two due to Pseudomonas aeruginosa, and the others due to Stenotrophomonas and Enterobacter. One patient died of Staphylococcus (methicillin-resistant) bacteremia who had Down’s syndrome with acute myeloid leukemia. Another patient with acute lymphoblastic leukemia was in maintenance and died as a result of Streptococcus septicemia. Out of the six who died, three cases had CVC in situ. Four of the deaths had leukemia as a primary diagnosis, and the other two were treated for rhabdomyosarcoma and neuroblastoma.
Discussion
The study found that gram-negative organisms dominated the BSIs among pediatric oncology patients, with Klebsiella being the most prevalent culprit. Multiple antibiotics resistance do exist in both gram-negative and gram-positive bacterial groups, which is a concern because these antibiotics are commonly used as front-line life-saving therapies in the management of febrile neutropenic cancer patients. Children treated for malignancy have a high risk of serious BSIs as one of the most serious complications leading to a high mortality during treatment. Risks included the type and duration of chemotherapy, type of malignancy (mainly leukemia),11 neutropenia, and the presence of CVC.12,13
The severity and duration of neutropenia remained the most important risk factors, as shown by previous studies.10,12,14 Our study had equivocal result, with 51.3% (n = 38) of BSIs episodes were associated with neutropenia of 7 days or more (and the rest were less than 7 days duration). Kuo et al, identified neutropenia among patients aged <18 years diagnosed with acute myeloid leukemia or acute lymphocytic leukemia as a risk factor in 83.5% of BSIs episodes, with 73% being categorized as severe neutropenia.15 Additionally, in our study, most positive bacterial cultures exhibited severe neutropenia (62%) of less than 500 cells/μL.
In fact, dual antibiotic therapy (mainly piperacillin-tazobactam and amikacin) was a very common practice at our center. However, there were few occasions when monotherapy was deemed appropriate. The most common choice was either single-agent piperacillin-tazobactam or single-agent cefepime. This was mainly driven by clinical judgment and experience of the treating physicians. That variation has been noticed worldwide, with some centers advocating for mono-antibiotics and others for dual antibiotics therapy.16
The use of double anti-gram-negative antibiotics is justified due to the increased piperacillin-tazobactam resistance among gram-negative bacteria and the fact that Pseudomonas is a common pathogen among gram-negative bacteria. The addition of amikacin instead of gentamicin is a good option due to amikacin’s better bacterial susceptibility. Meropenem has a low comparative resistance in our cohort of patients; however, this needs to be taken into consideration as an important step for septic unstable patients except for those colonized with carbapenem-resistant Enterobacteriaceae (CRE).
The high incidence of gram-negative BSI in this cohort of patients indicates the need to implement strict infection prevention and control practices in these high-risk patients. It also emphasizes the importance of having a local antimicrobial stewardship program to guide the rational use of antibiotics and to implement a central-line care bundle to prevent hospital-acquired central-line infection.
In our study, we showed that gram-positive organisms exhibit increased resistance to antimicrobials in general. However, none were resistant to vancomycin. Additionally, the use was primarily for patients with a local CVC site infection, patients with gram-positive growth, or stubbornly unexplained prolonged fever in a patient with stay CVC. The fact that vancomycin is the only antibiotic that remains effective against gram-positive advocates for restricting its use based on true clinical need.
In terms of clinical presentation, gastrointestinal manifestations were the most common association with BSIs. Around 73.6% of the children had various symptoms, including abdominal distension, diarrhea, vomiting, and pain. Abdominal manifestations are known to be associated with gram-negative and anaerobic bacterial infections due to disturbances in the gastrointestinal mucosal barrier as a side effect of chemotherapy, possibly due to mucositis. Those patients received anti-gram-negative antibiotics as a first line, followed by adding metronidazole only in 17.5% of the patients in conjunction with other antimicrobials.
Limitations of the Study
Febrile neutropenia patients are managed at our center and sometimes at other local secondary care centers. Thus, we might not have captured all BSI episodes, making it a significant limitation as not all blood cultures from a single patient have been reported in this study. This study is also limited due to unexclusively including all patients in Oman as many of them get treatments in other non-governmental institutions. An additional limitation of the study is that the cohort is seven years of age. However, trends and practices have remained the same over time.
Conclusion and the Way Forward
This is the first study from Oman that reports crucial data concerning bacterial BSIs etiology and susceptibility to pediatric febrile neutropenia due to chemotherapy and cancer. A major finding is the dominance of gram-negative organisms among BSIs, with Klebsiella being the most common culprit. In addition, there is a high level of resistance among gram-negative bacteria towards piperacillin-tazobactam, calling for strategies to ensure the safety of our patients.
Undertaking antibiotic stewardship rounds to provide a clear path for limiting the use of broad-spectrum agents, especially in low-risk cases and over-seeing the handling of antibiotics would be invaluable. Moreover, strict infection-control and prevention practice is another cornerstone for the improvement of the outcome of patients. It is highly recommended that pediatric oncology and infectious disease practitioners in Oman work together to create national guidelines and policies for addressing febrile neutropenia in children with cancer at all centers in Oman.
Abbreviations
BSI, bloodstream infections; CVC, central venous catheters; ANC, absolute neutrophil count; E. coli, Escherichia coli.
Data Sharing Statement
The findings of this study are based on the data that was collected during the study and evaluated using the methods and materials that were specified. The manuscript contains all important information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Al-Mulla NA, Taj-Aldeen SJ, El Shafie S, et al. Bacterial bloodstream infections and antimicrobial susceptibility pattern in pediatric hematology/oncology patients after anticancer chemotherapy. Infect Drug Resist. 2014;7:289–299. PMID: 25395866; PMCID: PMC4226521. doi:10.2147/IDR.S70486
2. Montassier E, Batard E, Gastinne T, et al. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis. 2013;32:841–850. doi:10.1007/s10096-013-1819-7
3. Asturias EJ, Corral JE, Quezada J. Evaluation of six risk factors for the development of bacteremia in children with cancer and febrile neutropenia. Curr Oncol. 2010;17(2):59–63. doi:10.3747/co.v17i2.453
4. Vento D. Infections in patients with cancer undergoing chemotherapy: aetiology, prevention and treatment. Lancet Oncol. 2003;4(10):595–604. doi:10.1016/S1470-2045(03)01218-X
5. Duncan C, Chisholm JC, Freeman S, et al. A prospective study of admissions for febrile neutropenia in secondary paediatric units in South East England. Pediatr Blood Cancer. 2007;49(5):678–681. doi:10.1002/pbc.21041
6. Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: a Phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24(4):563–570. PMID: 16446328. doi:10.1200/JCO.2005.03.2847
7. Lehrnbecher T, Fisher BT, Phillips B, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis. 2020;71(1):226–236. PMID: 31676904; PMCID: PMC7312235. doi:10.1093/cid/ciz1082
8. Choi SW, Chang L, Hanauer DA, et al. Rapid reduction of central line infections in hospitalized pediatric oncology patients through simple quality improvement methods. Pediatr Blood Cancer. 2013;60(2):262–269; PMID: 22522576; PMCID: PMC3720122. doi: 10.1002/pbc.24187
9. Villafuerte-Gutierrez P, Villalon L, Losa JE, et al. Treatment of febrile neutropenia and prophylaxis in hematologic malignancies: a critical review and update. Adv Hematol. 2014;2014:986938. doi:10.1155/2014/986938
10. Humphries RM, Kircher S, Ferrell A, et al. The continued value of disk diffusion for assessing antimicrobial susceptibility in clinical laboratories: report from the clinical and laboratory standards institute methods development and standardization working group. J Clin Microbiol. 2018;56(8):e00437–18. PMID: 29743302; PMCID: PMC6062797. doi:10.1128/JCM.00437-18
11. Ammann RA, Laws HJ, Schrey D, et al. Bloodstream infection in paediatric cancer centres–leukaemia and relapsed malignancies are independent risk factors. Eur J Pediatr. 2015;174(5):675–686. PMID: 25804192. doi:10.1007/s00431-015-2525-5
12. Tural Kara T, Erat T, Yahşi A, et al. Bloodstream infections in pediatric hematology/oncology patients: six years’ experience of a single center in Turkey. Turk J Med Sci. 2019;49(4):1157–1164. PMID: 31342734; PMCID: PMC7018311. doi:10.3906/sag-1812-101
13. Hassan B, Yusoff Z, Othman S. Association of neutropenia onset and severity with chemotherapy regimens and schedules. Asian Pac J Cancer Prev. 2011;12(6):1425.
14. Hua LV, Hing BH, Ning B. Pathogenesis of bloodstream infection in children with blood cancer. Exp Ther Med. 2013;5(1):201–204. doi:10.3892/etm.2012.738
15. Kuo FC, Wang SM, Shen CF, et al. Bloodstream infections in pediatric patients with acute leukemia: emphasis on gram-negative bacteria infections. J Microbiol Immunol Infect. 2017;50(4):507–513. doi:10.1016/j.jmii.2015.08.013
16. Zengin E, Sarper N, Kılıç SC. Piperacillin/tazobactam monotherapy versus piperacillin/tazobactam plus amikacin as initial empirical therapy for febrile neutropenia in children with acute leukemia. Pediatr Hematol Oncol. 2011;28(4):311–320. PMID: 21524156. doi:10.3109/08880018.2011.557144
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
