Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
AI-Assisted Ultrasound for the Early Diagnosis of Antibody-Negative Autoimmune Thyroiditis
Authors Yao S, Zhang B, Fei X, Xiao M, Lu L, Liu D, Zhang S, Cui J
Received 10 February 2023
Accepted for publication 12 May 2023
Published 28 June 2023 Volume 2023:16 Pages 1801—1810
DOI https://doi.org/10.2147/JMDH.S408117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Shengsheng Yao,1,* Bo Zhang,2,* Xiang Fei,3 Mingming Xiao,4 Li Lu,5 Daming Liu,6 Siyuan Zhang,7 Jianchun Cui3
1China Medical University - Department of Thyroid and Breast Surgery, Liaoning Provincial People’s Hospital, Shenyang, Liaoning Province, 110015, People’s Republic of China; 2Department of Science and Education, The 10th Division of Xinjiang Production and Construction Corps, Beitun General Hospital, Beitun City, Xinjiang Province, 831300, People’s Republic of China; 3Department of Thyroid and Breast Surgery, People’s Hospital of China Medical University (Liaoning Provincial People’s Hospital), Shenyang, Liaoning Province, 110015, People’s Republic of China; 4Department of Pathology, People’s Hospital of China Medical University (Liaoning Provincial People’s Hospital), Shenyang, Liaoning Province, 110015, People’s Republic of China; 5Department of Endocrinology, People’s Hospital of China Medical University (Liaoning Provincial People’s Hospital), Shenyang, Liaoning Province, 110015, People’s Republic of China; 6Department of Ultrasound, People’s Hospital of China Medical University (Liaoning Provincial People’s Hospital), Shenyang, Liaoning Province, 110015, People’s Republic of China; 7Department of Thyroid and Breast Surgery, The 10th Division of Xinjiang Production and Construction Corps, Beitun General Hospital, Beitun City, Xinjiang Province, 831300, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianchun Cui, Department of Thyroid and Breast Surgery, People’s Hospital of China Medical University (Liaoning Provincial People’s Hospital), No. 33 of Wenyi Road, Shenhe District, Shenyang, Liaoning Province, 110015, People’s Republic of China, Tel +86 24 24016014, Fax +86 24 24016014, Email [email protected]
Abstract: The prevalence of antibody-negative chronic autoimmune thyroiditis (SN-CAT) is increasing. The early diagnosis of SN-CAT can effectively prevent its further development. Thyroid ultrasound can diagnose autoimmune thyroiditis and predict hypothyroidism. Primary hypothyroidism with a hypoechoic pattern suggested by thyroid ultrasound and negative thyroid serum antibodies is the main basis for the diagnosis of SN-CAT. However, for early SN-CAT, only hypoechoic thyroid changes and serological antibodies are currently available. This study explored how to achieve an accurate and early diagnosis of SN-CAT and prevent the development of SN-CAT combined with hypothyroidism. The diagnosis of a hypoechoic thyroid by artificial intelligence is expected to be a breakthrough in the accurate diagnosis of SN-CAT.
Keywords: antibody negative, autoimmune thyroiditis, hypothyroidism, hypoechoic thyroid, artificial intelligence, Hashimoto’s disease
Introduction
Clinically, a considerable number of patients diagnosed with primary hypothyroidism have been found to be negative for thyroid serological antibodies, whereas ultrasound suggests a hypoechoic pattern, ie serum antibody-negative chronic autoimmune thyroiditis (SN-CAT).1 Artificial intelligence (AI) has been a research hotspot in recent years, gradually entering the field of imaging and achieving certain development. The thyroid has become a precursor to the development of AI in the field of ultrasound due to its unique characteristics, such as its unique body surface position and relatively easy collection of standard images.2,3 It has also been shown that sonographers combined with AI diagnosis can predict the nature of thyroid nodules in ultrasound images more effectively than sonographers alone.4 At present, it is considered that ultrasonography can diagnose SN-CAT and predict hypothyroidism. However, it is uncertain whether early SN-CAT can be diagnosed by ultrasonography or thyroid ultrasonography assisted by AI before SN-CAT becomes combined with hypothyroidism. Therefore, providing favourable opportunities for effective intervention in SN-CAT and hypothyroidism has become a research focus.
We searched the PubMed and HowNet databases using the keywords “antibody negative”, “autoimmune thyroiditis” (CAT), “hypothyroidism”, “hypoechoic thyroid”, “AI” and “Hashimoto’s disease”. The related literature was summarised and expanded on the topic of AI-assisted ultrasound early diagnosis of antibody-negative autoimmune thyroiditis.
SN-CAT and Classic Hashimoto’s Thyroiditis
Hashimoto’s thyroiditis (HT) is a chronic inflammation of thyroid follicular cells. It is an autoimmune disease in which the antigen is the normal thyroid tissue of the thyroid gland. The pathology of HT is the lymphocyte infiltration of thyroid tissue.5 Studies have shown that the prevalence of HT in China is 1%~2%, the incidence of HT in men is 0.8/1000, the incidence of HT in women is 3~4 times as high as that in men, and most of them are 30~50 years old.6 Hashimoto’s thyroiditis is the most common cause of hypothyroidism,7 and the clinical manifestations of the disease are complex and varied. With the progress of HT come many clinical manifestations, including fatigue, lethargy, chills, oedema, memory loss, constipation and dry skin, seriously affecting patient’s daily lives.8 Because of the lack of early clinical manifestations and specific diagnostic indicators, it is often overlooked by patients, leading to a high rate of missed diagnosis; often, at the time of diagnosis, the disease has entered the stage of clinical hypothyroidism, with missed opportunities for early intervention.9
Many studies have shown that HT can affect the nervous system of the brain, causing brain metabolic disorders and reducing neuronal activity in the normal brain.10,11 It has also been shown that impaired brain bioelectrical activity occurs in patients with HT without central nervous system deficits, suggesting that impaired brain bioelectrical activity during hormone therapy may be related to ongoing autoimmune processes. Increased visual evoked potential (VEP) amplitude during hormone therapy may indicate increased cerebral cortical activity, and VEP and brain-stem auditory evoked potential analysis may be helpful in evaluating brain bioelectrical activity during hormone therapy.12
The diagnosis of HT is based on clinical manifestations, thyroid hormone levels, the presence of serum anti-thyroid antibodies and thyroid ultrasound assessment13 (see Figures 1–5). Clinically, the sensitivity, specificity and stability of thyroid peroxidase autoantibodies, including serum anti-thyroid peroxidase antibody (TPOAb) and serum anti-thyroglobulin antibody, have been significantly improved. Positive TPOAb and anti-thyroglobulin antibodies (TgAb) can be used to diagnose HT before hypothyroidism.14 Cases of HT that are positive for thyroid autoantibodies are also known as classic HT (CHT).1 In the past few years, it has been shown that other anti-thyroid immunoglobulins, including thyroid-stimulating hormone (TSH) receptor blocking or stimulating antibodies, thyroxine antibodies, triiodothyronine and megalin (a transmembrane protein belonging to the low-density lipoprotein receptor family), are also detectable in patients with autoimmune thyroiditis.13
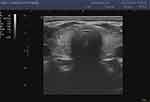 |
Figure 1 Hashimoto’s disease without hypothyroidism. |
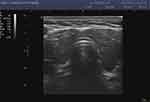 |
Figure 2 Hashimoto’s disease with mild subclinical hypothyroidism. |
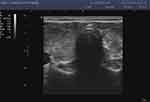 |
Figure 3 Hashimoto’s disease with severe subclinical hypothyroidism. |
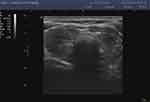 |
Figure 4 Hashimoto’s disease with clinical hypothyroidism. |
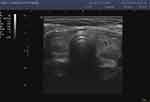 |
Figure 5 Hashimoto’s hyperthyroid stage. |
In 1988, Baker et al15 reported the first case of SN-CAT. The authors found histological findings consistent with Hashimoto’s disease in a specimen from a patient who underwent thyroidectomy because of neck compression caused by a goitre; however, the patient’s preoperative serological test was negative for autoantibodies. Since then, increasing numbers of scholars have started to study SN-CAT, which is defined as a hypoechoic pattern on thyroid ultrasonography in patients with primary hypothyroidism who are negative for TPOAb and TgAb.1 In fact, TPOAb and TgAb negativity can be used to identify SN-CAT and CHT, and hypoechoic patterns on primary hypothyroidism and thyroid ultrasounds can be used to diagnose autoimmune thyroiditis.16 Although the early diagnosis of SN-CAT can effectively block its further development,15 presently, the only effective indicator for the diagnosis of SN-CAT is the hypoechoic mode of thyroid ultrasound. Therefore, relevant studies are not easy to perform, and relevant literature is very limited.
The Prevalence and Clinical Characteristics of SN-CAT
At first, scholars thought that cases of SN-CAT were not rare, and based on the positive rate of thyroid autoantibodies in hypothyroidism, the prevalence of SN-CAT was estimated to be about 5%.17 However, no definite clinical epidemiological data is available. In recent years, the prevalence of subclinical hypothyroidism and SN-CAT has increased significantly, and with the use of TSH levels in TSH serology and the widespread use of thyroid ultrasound in physical examinations, clinical epidemiological evidence of SN-CAT has recently emerged. In a cross-sectional study of SN-CAT in 581 patients with CAT associated with hypothyroidism in 2020, L. Croce et al found a prevalence of 20.8% for SN-CAT, a result that far exceeded the previous estimate of 5%. In a subsequent longitudinal follow-up study of 8.9 ± 4.6 years, serum TPOAb and/or TgAb testing was positive in 8 of 49 (16.3%) patients with SN-CAT, perhaps reminding us that “SN-CAT is an early stage of CHT!” More in-depth research is required.
In addition, a study on the clinical manifestations of SN-CAT found that at the time of initial diagnosis, patients in an SN-CAT group had lower serum TSH levels and higher serum FT4 levels; low-dose LT4 corrected hypothyroidism in the SN-CAT group, but the thyroid volume in the CHT group was significantly larger than that in the SN-CAT group. After LT4 treatment, the thyroid volume in the CHT group was significantly decreased, and no change was observed in the SN-CAT group. Therefore, SN-CAT is considered more moderate than CHT.18
Thyroid Ultrasound and Autoimmune Thyroiditis
Theoretical Basis of Autoimmune Thyroiditis Ultrasound in Low-Echo Mode
Thyroid follicles are the structural and functional units of the thyroid gland. Normal thyroid cells condense into a rosette-like ring-shaped follicular structure. The follicles are filled with glia and are 200–400 μm in diameter, forming a site for synthesising and secreting thyroid hormone. The interface between the thyroid cells and the glia has high acoustic impedance, and high-frequency sound waves are reflected to the probe, allowing the normal echo density of the thyroid gland to be distinguished from that of the surrounding neck muscles. In 2003, Italian Paolo Vitti et al19 showed that in patients with HT, the interface between thyroid cells and glia is reduced due to a large lymphocyte infiltration in the thyroid tissue, resulting in hypoechoic thyroid tissue.
Low-Echo Mode Ultrasonography of Autoimmune Thyroiditis Thyroid
In 2001, Rago et al20 of Italy studied thyroid ultrasound as a diagnostic tool for thyroid autoimmune disease and a predictor of thyroid dysfunction to in comparison with the available methods hypoechoic patterns of the thyroid; they specified in detail the methods of thyroid ultrasound examination. First, they adjusted the overall gain of the device at the time of the ultrasound, taking the absence of echo in the internal jugular vein as a criterion. Then, they determined that under these conditions, the structurally normal thyroid had a moderate greyscale uniform echo pattern, with an echo level higher than that of the surrounding muscles; when the echo density was significantly lower than that of the normal subjects, it was considered to be hypoechoic and was classified as mild (I), moderate (II) or severe (III). In evaluating the low-echo mode of HT in 2021,21 only “Diffuse heterogeneous thyroid disease” or “Hypoechoic thyroid” were used as evaluation criteria, and no more in-depth typing of “Heterogeneous” or “Hypoechoic thyroid” was performed.
Therefore, the Rago20 hypoechoic pattern triad classification is the only method in the current literature to further classify the hypoechoic pattern on ultrasonography in thyroid autoimmune diseases.
Limitations of Hypoechoic Thyroiditis Thyroid in the Diagnosis of Autoimmune Disorders
Although Rago T’s hypoechoic three-degree classification gives diagnostic criteria for the diagnosis of thyroid autoimmune diseases by ultrasonography, such criteria are more subjective and difficult to quantify, and different ultrasound doctors will draw different conclusions on the same patient, directly affecting the development of more research in this area.
In addition, morbid obesity is the most important disease that needs to be identified in the ultrasound diagnosis of autoimmune thyroiditis and hypothyroidism because up to 10–15% of morbidly obese patients have elevated serum TSH under the condition of a negative thyroid autoimmune antibody test. In addition, because of a large amount of fatty tissue in the neck of morbidly obese patients, up to 65% of such patients exhibited a hypoechoic thyroid appearance on thyroid ultrasound examination; this was unrelated to the change in the thyroid tissue structure, meaning that SN-CAT can be easily misdiagnosed.22,23
Based on the above issues, thyroid ultrasound diagnosis and the early prediction of SN-CAT are very important, but there are many complex problems to be solved.
Artificial Intelligence-Assisted Thyroid Ultrasound Diagnosis System
The clinical manifestation of SN-CAT is similar to that of CHT. Often, SN-CAT is overlooked in the early stage of the disease due to the lack of typical thyroid autoantibodies. Ultrasonography suggests that a hypoechoic appearance is the only valid indicator for the early diagnosis of SN-CAT; however, because of the complexity of this hypoechoic feature, it is not easy to achieve accurate graduation with conventional ultrasound for diagnosis; it is less likely to interrupt the course of autoimmune thyroiditis at an early stage and prevent the development of hypothyroidism. However, it is possible to achieve an early accurate diagnosis of SN-CAT using AI-assisted thyroid ultrasound, which provides a more favourable opportunity for the accurate prevention and control of hypothyroidism.
Introduction to Artificial Intelligence
Artificial intelligence is a general description of human intelligent activities, such as computers imitating human thinking and behaviour, and is a general term that includes methods or technologies, such as machine learning, artificial neural networks and deep learning (DL).24 Computer-aided diagnosis (CAD) can improve the detection rate of focus areas and reduce the false-negative rate by locating abnormal or suspicious areas in images.25 In CAD, machine learning methods are used to analyse imaging and non-imaging data from past case samples of patient populations and to develop a model to correlate extracted information with certain disease outcomes. As data from the new case is entered, the developed model is expected to predict the outcome of the new unknown case. If properly trained and validated, CAD predictions can be used as a second opinion or supporting information in clinical decision-making processes.26 The use of machine learning techniques to analyse patient data for decision support is applicable to any patient care process, with examples including disease or lesion detection, characterisation, cancer staging, treatment planning, treatment response assessment, recurrence monitoring and prognostication.27,28 In general, imaging data plays an important role at each stage, so image analysis is a major component of CAD.29
Deep learning does not rely on manual feature extraction and can generate advanced features directly from an original image.30 Driven by arithmetic, data and algorithms, DL can remember the visible features of tumour focus, such as tumour size, boundary, blood flow, calcification and aspect ratio. At the same time, many non-visual features, such as the greyscale, which cannot be recognised by human eyes, are found, and real-time dynamic analysis is performed to effectively improve the diagnostic efficacy of observers.29 In addition, DL must be combined with big data to continuously improve and eventually achieve or exceed the level of the human brain. Deep learning has become the most advanced machine learning method. Deep learning iteratively adjusts the weight layers in deep neural network architecture to learn multiple levels of representation from training data. It has been successful in many fields, including speech and text recognition, natural language understanding and translation, object detection and classification.
Deep learning can learn directly from raw data and leverage an output layer with multiple hidden layers.31 Compared with expert systems, the DL method is easier and more accurate. The increase in computing power and the popularity of algorithms, such as convolutional neural networks (CNNs), full convolution networks, recurrent neural networks and generation countermeasure networks, have facilitated multiple studies on the use of DL-based AI in pathology. The application of AI in pathology has helped to overcome the limitations of pathologists’ subjective visual assessments and integrate multiple measurements for precision tumour treatment.32,33 However, most of the current DL applications are far from exhibiting the desired intelligent characteristics of humans, and developers and users are committed to marking computer-aided technologies as AI.33
Application of AI in Clinical Medicine
In 1998, the US FDA approved the clinical use of molybdenum target AI, increasing the detection rate of breast cancer by 8%, while in 2017, it approved the clinical use of breast AI, reducing the missed diagnosis rate of breast cancer by 39%.34 In 2019, Dan and Zhan et al35 indicated that thyroid nodule AI-assisted ultrasound diagnosis had a sensitivity of 86.20% and a specificity of 85.48%. The 2022 China Society of Clinical Oncology guidelines for the diagnosis and treatment of breast cancer state that AI is useful in the diagnosis of benign and malignant breast diseases, second only to the comprehensive interpretation of plain and enhanced images by breast radiologists with 20 years’ experience. Furthermore, AI has been applied to the prediction of triple-negative breast cancer and the automatic Her-2 scoring of pathology. In conclusion, there is a high rate of agreement with the physician’s diagnosis.36
On 1 February 2021, Beijing medical quasi-intelligence obtained a national medical device registration certificate for breast ultrasound image data management software (GUIJI NOTE: 20212210021). On 29 July 2021, Wuxi produced intelligent access ultrasound-assisted processing software (Xiangji note: 20212211483 [national medical device registration certificate]). This indicates that after more than 20 years of research and catch-up, China’s AI-assisted ultrasound diagnosis technology has undoubtedly entered the clinical application stage.
Application of AI in Thyroid Ultrasound Diagnosis
In June 2022, Bing et al37 successfully applied pulsed smart dynamic real-time AI to thyroid nodule diagnostics. They pointed out that dynamic AI can perform real-time and synchronous dynamic analysis of the nodules from multi-layers and multi-angles and assess the nature of nodules, thus further improving diagnostic efficiency and reducing unnecessary punctures. This was the first clinical research report on a dynamic AI-assisted real-time diagnosis system in China to effectively assist surgeons to formulate scientific and reasonable individualised diagnosis and treatment strategies for patients.
In addition, large-sample studies on AI-assisted thyroid inflammation have also been reported. Zhang et al38 established a DL database model (HT-Net) for the largest sample of AI-assisted diagnoses of HT to date (106,513 ultrasound images from 17,934 patients); in all patients, pathological examination was used as the gold standard for the diagnosis of HT. In this study, serological markers were examined to improve their diagnostic performance, and their diagnostic efficacy was comprehensively evaluated by internal and external tests. The three independent test sets were (1) the training set, which included 37,424 ultrasound images from 6143 patients with HT and 69,089 ultrasound images from 11,791 controls (obtained from 1 January 2012 to 30 December 2017 in our unit); (2) internal tests, which included 48,803 images from 4303 patients (from 1 January 2018 to 28 March 2019) and 185 videos from 185 patients (from 1 April 2021 to 10 May 2021); (3) external testing, which included 5304 ultrasound images from 563 patients. The results of our study suggests that HT-Net has surpassed ultrasound physicians in sensitivity and specificity in diagnosing HT and that the presence or absence of thyroid nodules and the different types of ultrasound equipment have no impact on diagnostic performance. However, because the study was retrospective, the pathologic diagnosis on which it was based did not grade HT; additionally, there is no classification of thyroiditis other than HT, such as Graves’ disease and subacute, postpartum, sporadic and suppurative thyroiditis. Therefore, the diagnostic efficacy of HT-Net needs to be verified by follow-up prospective studies.
Similarly, another recent study by Zhao et al39 investigated the efficiency of a DL model in the automatic diagnosis of HT using real ultrasound data from CAD and AI ultrasound. Two CNNs were integrated as the final model for HT classification (CAD-HT), and it was found that compared with advanced radiologists, the CAD-HT model achieved higher performance and improved accuracy by nearly 9%. There was no change in the diagnostic efficacy for different hospitals and thyroid hormone levels (hyperthyroidism, hypothyroidism and normal thyroid function). Peng et al40 developed a DL AI model (ThyNet) to distinguish between malignant and benign thyroid nodules and investigated how it can help radiologists improve diagnostic performance and avoid unnecessary puncture tests. Images and videos were used in the study. In the simulation scenario, the number of punctures using the ThyNet adjuvant strategy decreased from 61.9% to 35.2%, while the number of missed malignancies decreased from 18.9% to 17.0%. The ThyNet adjuvant strategy was shown to significantly improve the diagnostic ability of radiologists.
Another study showed that of 25,445 biopsy samples, approximately 20% were pathologically diagnosed as atypical lesions of unknown significance or undetermined malignancy, with an average malignancy risk of 15.9% and 75.2%, respectively.41 Through the combined application of AI technology and medical image data, a static AI ultrasonic intelligent-assistant diagnosis system was generated for the diagnosis of thyroid nodules; it can improve diagnosis efficiency while ensuring accuracy.42–46
However, static AI can only diagnose a single view of a nodule and cannot determine the nature of the nodule in real time. The development of a dynamic AI ultrasound assisted diagnosis system provides a new method for thyroid nodule ultrasound diagnosis. It can perform real-time and synchronous dynamic analysis of nodules from multiple cross-sectional views and different angles to further improve the diagnostic efficiency of clinical examination. Studies have found that dynamic AI examination has a high diagnostic value for benign and malignant thyroid nodules and can effectively assist surgeons in developing scientific and rational individualised diagnostic and treatment strategies for patients.47,48
Establishment of an AI Database of SN-CAT Cases
How to collect SN-CAT cases is a very important step in the establishment of an SN-CAT database for AI-assisted diagnosis. It is an effective method to collect SN-CAT cases by detecting thyroid antibodies and thyroid ultrasonography in patients with hypothyroidism, although there is no doubt that cases in the middle and late stages are associated with hypothyroidism. Therefore, we need to find new ways and methods of identifying SN-CAT cases without hypothyroidism, ie early SN-CAT cases.
The American Baker designed this method for us as early as 1988. Autoimmune thyroiditis has been found in patients with thyroid surgery by lymphocyte infiltration, a typical pathological feature of thyroid surgery; subsequently, SN-CAT and hypothyroidism can be detected by thyroid serological antibody testing, which may be the only effective way to identify early cases of SN-CAT.
Conclusion and Prospects
Primary hypothyroidism, a hypoechoic pattern of thyroid ultrasound and negative serum antibodies are the three necessary diagnostic criteria for SN-CAT; however, for early SN-CAT, only hypoechoic thyroid changes and serological antibodies are available. Therefore, we must determine how best to combine the two methods based on the three-degree classification of low echo under SN-CAT ultrasound to further realise the accurate diagnosis and the early diagnosis of SN-CAT and prevent the development of SN-CAT with hypothyroidism. The diagnosis of hypoechoic thyroid by AI is expected to be a breakthrough in the accurate diagnosis of SN-CAT. At present, AI is yet to be applied in the diagnosis of SN-CAT, so the AI-assisted ultrasound diagnosis of SN-CAT is significant. In subsequent studies, we will verify the real value of AI-assisted ultrasound diagnosis of SN-CAT to effectively prevent the further development of the disease.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Review
An ethics statement is not applicable because this study is based exclusively on published literature.
Funding
Applied Basic Research Program of Liaoning Science and Technology Department (No. 2022020247-JH2/1013). Project of Beitun Science and Technology Bureau of the 10th Division of Xinjiang Production and Construction Corps (No. 2022-179-03).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately for this work.
References
1. Croce L, De Martinis L, Pinto S, et al. Compared with classic Hashimoto’s thyroiditis, chronic autoimmune serum-negative thyroiditis requires a lower substitution dose of L-thyroxine to correct hypothyroidism. J Endocrinol Invest. 2020;43(11):1631–1636. doi:10.1007/s40618-020-01249-x
2. Adler DD, Carson PL, Rubin JM, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. 1990;16(6):553–559. doi:10.1016/0301-5629(90)90020-D
3. Wen T, Qingqing H, Kewei J, et al. Surgical expert consensus in the clinical practice of hyperparathyroidism secondary to chronic renal failure. Chin J Pract Surg. 2016;36(5):481–486.
4. Zhang R, Zhang Z, Huang P, et al. Diagnostic performance of ultrasonography, dual -phase 99mTc -MIBI scintigraphy, early and delayed 99mTc -MIBI SPECT/CT in preoperative parathyroid gland localization in secondary hyperparathyroidism. BMC Med Imaging. 2020;20(1):91. doi:10.1186/s12880-020-00490-3
5. Jian L. The value of color Doppler ultrasound in the diagnosis of Hashimoto's thyroiditis with normal thyroid function. Chin J IntegrTradit West Med. 2019;17(05):492–496.
6. Haibo X. The value of two-dimensional ultrasound and color Doppler ultrasound in the differential diagnosis of Hashimoto's’s thyroiditis and hyperthyroidism. Electron J Mod Med Health Res. 2021;5(04):124–126.
7. Ragusa F, Fallahi P, Elia G, et al. Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101367. doi:10.1016/j.beem.2019.101367
8. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646–2655. doi:10.1056/NEJMra021194
9. Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19(10):102649. doi:10.1016/j.autrev.2020.102649
10. Bladowska J, Waliszewska-Prosół M, Ejma M, Sąsiadek M. The metabolic alterations within the normal appearing brain in patients with Hashimoto's’s thyroiditis are correlated with hormonal changes. Metab Brain Dis. 2019;34(1):53–60. PMID: 30242734; PMCID: PMC6351519. doi:10.1007/s11011-018-0318-z
11. Waliszewska-Prosół M, Bladowska J, Budrewicz S, Sąsiadek M, Dziadkowiak E, Ejma M. The evaluation of Hashimoto's’s thyroiditis with event-related potentials and magnetic resonance spectroscopy and its relation to cognitive function. Sci Rep. 2021;11(1):2480. PMID: 33510336; PMCID: PMC7843607. doi:10.1038/s41598-021-82281-6
12. Waliszewska-Prosół M, Ejma M, Cavaliere C. Assessment of visual and brainstem auditory evoked potentials in patients with Hashimoto's’s thyroiditis. J Immunol Res. 2021;2021:3258942. PMID: 33763490; PMCID: PMC7946475. doi:10.1155/2021/3258942
13. Waliszewska-Prosół M, Ejma M. Hashimoto encephalopathy-still more questions than answers. Cells. 2022;11(18):2873. PMID: 36139446; PMCID: PMC9496753. doi:10.3390/cells11182873
14. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. 2022;132(3). doi:10.20452/pamw.16222
15. Baker JR
16. Dingchang W, Chaolin H, Ting X. Clinical significance of serum TGAb and TPOAb tests in the diagnosis of autoimmune thyroid diseases. Lab Med Clin Med. 2013;10(19):2530–2531.
17. Takamatsu J, Yoshida S, Yokozawa T, et al. Correlation of antithyroglobulin and antithyroid-peroxidase antibody profiles with clinical and ultrasound characteristics of chronic thyroiditis. Thyroid. 1998;8(12):1101–1106. doi:10.1089/thy.1998.8.1101
18. Rotondi M, de Martinis L, Coperchini F, et al. Serum negative autoimmune thyroiditis displays a milder clinical picture compared with classic Hashimoto's’s thyroiditis. Eur J Endocrinol. 2014;171(1):31–36. doi:10.1530/EJE-14-0147
19. Vitti P, Rago T. Thyroid ultrasound as a predicator of thyroid disease. J Endocrinol Invest. 2003;26(7):686–689. doi:10.1007/BF03347031
20. Rago T, Chiovato L, Grasso L, et al. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24(10):763–769. doi:10.1007/BF03343925
21. Bearing support, Chiang Kai-shek’s troops, Shgg Yang, et al. Consistency analysis of Hashimoto's’s thyroiditis serology, ultrasonography and histopathology. J Nanjing Med Univ Sci. 2021;41(12):1806–1810.
22. Radetti G, Kleon W, Buzi F, et al. Thyroid function and structure are affected in childhood obesity. J Clin Endocrinol Metab. 2008;93(12):4749–4754. doi:10.1210/jc.2008-0823
23. Rotondi M, Magri F, Chiovato L. Thyroid and obesity: not a one-way interaction. J Clin Endocrinol Metab. 2011;96(2):344–346. doi:10.1210/jc.2010-2515
24. Gore JC. Artificial intelligence in medical imaging. Magn Reson Imaging. 2020;68:A1–A4. doi:10.1016/j.mri.2019.12.006
25. Czajkowska J, Borak M. Computer-aided diagnosis methods for high-frequency ultrasound data analysis: a review. Sensors. 2022;22(21):21. doi:10.3390/s22218326
26. Wani IM, Arora S. Computer-aided diagnosis systems for osteoporosis detection: a comprehensive survey. Med Biol Eng Comput. 2020;58(9):1873–1917. doi:10.1007/s11517-020-02171-3
27. Ahmad OF, Soares AS, Mazomenos E, et al. Artificial intelligence and computer-aided diagnosis in colonoscopy: current evidence and future directions. Lancet Gastroenterol Hepatol. 2019;4(1):71–80. doi:10.1016/S2468-1253(18)30282-6
28. Jahmunah V, Oh SL, Wei JKE, et al. Computer-aided diagnosis of congestive heart failure using ECG signals - a review. Phys Med. 2019;62:95–104. doi:10.1016/j.ejmp.2019.05.004
29. Chan HP, Hadjiiski LM, Samala RK. Computer-aided diagnosis in the era of deep learning. Med Phys. 2020;47(5):e218–e227. doi:10.1002/mp.13764
30. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–157. doi:10.3322/caac.21552
31. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. doi:10.1038/nature14539
32. Jain RK, Mehta R, Dimitrov R, et al. Atypical ductal hyperplasia: interobserver and intraobserver variability. Mod Pathol. 2011;24(7):917–923. doi:10.1038/modpathol.2011.66
33. Jiang Y, Yang M, Wang S, et al. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun. 2020;40(4):154–166. doi:10.1002/cac2.12012
34. Choi JS, Han BK, Ko ES, et al. Effect of a deep learning framework-based computer-aided diagnosis system on the diagnostic performance of radiologists in differentiating between malignant and benign masses on breast ultrasonography. Korean J Radiol. 2019;20(5):749–758. doi:10.3348/kjr.2018.0530
35. Dan W, Zhan H, Jingping W. Ultrasonic ai diagnosis of thyroid nodule. Chin J Ultrasound Med. 2019;3(12):1070–1072.
36. Guidelines for the diagnosis and treatment of breast cancer (2022 edition). Ration Drug China. 2022;19(10):1–26.
37. Bing W, Mingbo Z, Zheng W, et al. Study on the value of dynamic artificial intelligence ultrasonic assistant diagnosis system in thyroid nodule diagnosis. Chin J Pract Surg. 2022;42(06):680–684.
38. Zhang Q, Zhang S, Pan Y, et al. Deep learning to diagnose Hashimoto's’s thyroiditis from sonographic images. Nat Commun. 2022;13(1):3759. doi:10.1038/s41467-022-31449-3
39. Zhao W, Kang Q, Qian F, et al. Convolutional neural network-based computer-assisted diagnosis of Hashimoto's’s thyroiditis on ultrasound. J Clin Endocrinol Metab. 2022;107(4):953–963. doi:10.1210/clinem/dgab870
40. Peng S, Liu Y, Lv W, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study. Lancet Digit Health. 2021;3(4):e250–e259. doi:10.1016/S2589-7500(21)00041-8
41. Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120(23):3627–3634. doi:10.1002/cncr.29038
42. Nguyen DT, Pham TD, Batchuluun G, et al. Artificial intelligence-based thyroid nodule classification using information from spatial and frequency domains. J Clin Med. 2019;8(11):11. doi:10.3390/jcm8111976
43. Han M, Ha EJ, Park JH. Computer-aided diagnostic system for thyroid nodules on ultrasonography: diagnostic performance based on the thyroid imaging reporting and data system classification and dichotomous outcomes. AJNR Am J Neuroradiol. 2021;42(3):559–565. doi:10.3174/ajnr.A6922
44. Thomas J, Haertling T. AIBx, artificial intelligence model to risk stratify thyroid nodules. Thyroid. 2020;30(6):878–884. doi:10.1089/thy.2019.0752
45. Sorrenti S, Dolcetti V, Radzina M, et al. Artificial intelligence for thyroid nodule characterization: where are we standing? Cancers. 2022;14(14):3357. doi:10.3390/cancers14143357
46. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14(5):587–595. doi:10.1016/j.jacr.2017.01.046
47. Wang B, Wan Z, Li C, et al. Identification of benign and malignant thyroid nodules based on dynamic AI ultrasound intelligent auxiliary diagnosis system. Front Endocrinol. 2022;13:1018321. doi:10.3389/fendo.2022.1018321
48. Wildman-Tobriner B, Taghi-Zadeh E, Mazurowski MA. Artificial Intelligence (AI) tools for thyroid nodules on ultrasound, from the AJR special series on AI applications. AJR Am J Roentgenol. 2022;219(4):1–8. doi:10.2214/AJR.22.27430
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
