Back to Journals » Clinical Interventions in Aging » Volume 18
Age-Related Study of Anthropometry Indicators, Body Composition, Strength and Vital Capacity at Masters Athletics: How to Postpone Sarcopenia
Authors Safonicheva O , Zaborova V, Lazareva I, Kryuchkova K , Bolotskaya A, Ovchinnikova M, Popova C, Putilo V, Rybakov V , Kotovskiy S , Nikitin M
Received 10 September 2023
Accepted for publication 1 December 2023
Published 19 December 2023 Volume 2023:18 Pages 2155—2164
DOI https://doi.org/10.2147/CIA.S433944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Olga Safonicheva,1 Victoria Zaborova,1 Irina Lazareva,1 Kira Kryuchkova,1 Anastasia Bolotskaya,1 Marina Ovchinnikova,1 Christina Popova,1 Victor Putilo,1 Vitaly Rybakov,2 Sergey Kotovskiy,3 Mikhail Nikitin3
1Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia; 2Moscow Institute of Physics and Technology (National Research University), Dolgoprudny, Moscow Region, Russia; 3National Medical Research Center of Rehabilitation and Balneology, Moscow, Russia
Correspondence: Olga Safonicheva, Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), 119991, Trubetskaya Street, 8/2, Moscow, Russia, Tel +7 926 534-62-65, Fax +7 495 609-14-00, Email [email protected]
Purpose: The purpose of this study was to compare the anthropometric indicators of sports veterans, former athletes who stopped training, and non-sports people aged 40 years and older to assess the impact of regular sports on the stability of the body.
Patients and Methods: 100 athletes and 31 people non-sports were included in the study. Athletes were divided into two groups depending on the mode of motor activity. The first group (n=75) continued their regular sports activities. The second group (n=25) stopped training. Height, weight, chest circumference, mobility, waist, shoulder circumference, forearm, hip, ankle, fat mass, and muscle mass were measured, and dynamometry was performed.
Results: Body weight is statistically significantly (p< 0.05) less in those who continue sports (70.7± 10.2) classes after 60 years compared with the control group (82.4± 9.3). In sports veterans, the chest excursion and the shoulder circumference is statistically significantly (p< 0.05) greater than in the control group. In the subjects of the first group aged from 40 to 49 (4551± 612) and from 50 to 59 (4242± 416), the FVC index was statistically significantly (p< 0.05) higher than in the control group (3890± 344 and 3786± 401, respectively). The body composition of veterans is characterized by a high level of muscle mass and a low level of fat mass. At the age of 40– 49, the percentage of muscle tissue in sports veterans was statistically significantly higher (46.32± 2.74) (p< 0.05) than in the group of athletes who stopped sports activities (44.09± 5.29).
Conclusion: Veterans of sports demonstrate higher indicators of limb girth and muscle strength compared to untrained people of the same age. In addition, sports veterans have a lower content of adipose tissue and a greater expression of muscle mass. Thus, the data obtained by us show that sports prevent the development of sarcopenia and can also affect cardiovascular risk.
Keywords: regular motor activity, muscle mass, resilience, sarcopenia
Introduction
The increase in life expectancy is characterized by an increase in the burden on the health care system and an increase in global demand for its resources, which is associated with the accumulation of many concomitant diseases in old age as well as the appearance of concomitant conditions. Thus, strategies aimed at reducing the gap between health and life expectancy are of paramount importance.1
Sports bring incalculable health benefits to all age groups. It has been proven that regular physical activity can reduce the development of several chronic degenerative diseases associated with old age, such as metabolic and cardiovascular disorders.2 In addition, aging affects the histological composition of muscle fibers. It has been found that regular exercise in old age can contribute to the deposition and preservation of high-performance fibers.3 Physical activity brings undoubted benefits to young people since sports ensure the preservation of metabolic health in adulthood, ie, contribute to a significant delay in the development of osteoporosis.4 In addition, sport has a long-term impact on psychological health, improving social activity and playing a crucial role in the treatment of severe mental illnesses such as depression or anxiety.5
Despite all the benefits of playing sports, only 13% of people aged 65 and older regularly engage in physical activity more than 3 days a week, and the obesity rate among adults over 60 increases by 45%.6 Physical activity helps prevent chronic diseases and improves the quality of life, but few people lead an active lifestyle in old age. The exception is made by athletes and masters of sports who participate in sports competitions in middle and older age. Sports veterans have the same lipid profile as young people, which reduces the risk of heart disease. Master athletes also have better glucose tolerance and a lower waist-to-hip ratio than adults who lead a sedentary lifestyle, which reduces the risk of metabolic syndrome and type 2 diabetes.7 According to several dietary studies conducted, experienced athletes consume more nutritional energy while maintaining a lower body weight than adults who lead a sedentary lifestyle.
By the eighth decade of life, the mass of skeletal muscles decreases by about 18% in men and 27% in women, and accompanied by this loss of muscle mass, sarcopenia develops.8,9 Sarcopenia is characterized by a decrease in the cross-sectional area of muscle fibers, the content of satellite cells, remodeling of motor units, infiltration of adipose and connective tissue, changes in microcirculation,9–12 and a decrease in oxidative capacity.13 At the same time, the question of the role of regular physical activity in the development of sarcopenia remains open. There are suggestions that lifelong sports (so-called “chronic” sports training) can reduce or even prevent age-related deterioration of physical functions.1 Understanding whether regular exercise throughout life is a way to maintain weight, the strength and morphology of muscles, and the prevention of the development of sarcopenia is of great importance for taking appropriate measures to counteract the age-related deterioration of health.14
Thus, the purpose of this study was to compare the anthropometric indicators of sports veterans, former athletes who stopped training, and people who do not engage in sports at the age of 40.
Materials and Methods
The study was conducted in accordance with the provision of the Declaration of Helsinki and approved by the local ethical committee of Sechenov Moscow State Medical University (protocol №173 of October 30, 2020). Informed written consent was obtained from all patients before their inclusion in this study.
One hundred athletes, members of national teams, were included in the study. All subjects were under the supervision of a sports medicine physician. Inclusion criteria: male sex, age over 40 years, past participation in high-performance sports, athlete level (highest sport qualification, participation in international competitions – World Championships, European Championships), sport specialization (sports games), total sport experience – 10 to 20 years or more. Only men were included in the study because the mechanisms and risk factors of sarcopenia vary slightly depending on gender.15
100 athletes made up the main group. A control group of 31 persons, consisting of persons of similar age who had not participated in sports before. The selection of athletes was regulated by the current regulatory documents on the basis of Federal Law No. 329-FZ dated 04.12.2007 (ed. dated 02.07.2021) “On Physical Culture and Sports in the Russian Federation”.
100 athletes were divided into 2 groups depending on the mode of motor activity. The first group consisted of 75 veteran athletes who continued regular sports activities. The second group consisted of 25 people who had completely stopped training or had only occasional, haphazard physical activity.
Most of the sports veterans (53.3%) trained daily for 1.5–2.5 hours, 17.6% trained 5 times a week, and 27.1% trained 3–4 times a week.
To measure the height and weight of the subjects, mechanical column-type scales SECA-700 were used, supplemented with a SECA-220 height meter (seca GmbH & co. KG, Germany). The measurement of the chest circumference was carried out by applying a centimeter tape from behind, under the lower corners of the shoulder blades, and in front, at the level of attachment of four ribs to the sternum. The study was conducted using a spirometer from MIR Spirodoc (Medical International Research, Italy).
The muscular strength of the grip of the hand was measured using a manual dynamometer. The measurement of the strength of the muscles of the back was carried out using stand-up dynamometry using the Takei 5002 Back Muscle Analog Dynamometer. The measurement of the circumference of the limbs was carried out according to generally accepted methods of anthropometry. The circumference of the forearm was measured along a line passing 5 cm below the projection of the elbow joint. The shoulder circumference was measured along a line passing through the middle of the distance between the acromial process of the scapula and the ulnar process of the ulna. The circumference of the thigh was measured by the gluteal fold, and the lower leg was measured by the most prominent part of the calf muscle.
A 120-caliper Lange (Beta Technology Inc., USA) was used to measure the thickness of fat folds. The total fat mass (FM) was calculated by the formula 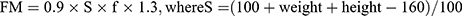 is the body surface, f is the average thickness of subcutaneous fat, and
is the body surface, f is the average thickness of subcutaneous fat, and 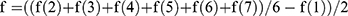 . In calculating the fat mass, 8 skin-fat folds were taken into account, which is recommended to be used in anthropology in man.
. In calculating the fat mass, 8 skin-fat folds were taken into account, which is recommended to be used in anthropology in man.
Muscle mass was determined by the formula 6.5×height×R×R, where R is the average radius of the muscles and is calculated in turn by the formula  . C(1): forearm girth; C(2): shoulder girth; C(3): hip girth; and C(4): shin girth. The average thickness of the fat and skin layers on the body segments is f1. f1 was calculated by the formula
. C(1): forearm girth; C(2): shoulder girth; C(3): hip girth; and C(4): shin girth. The average thickness of the fat and skin layers on the body segments is f1. f1 was calculated by the formula 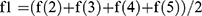 , where f(2) is the forearm, f(3) is the shoulder behind, f(4) is the hip in front, and f(5) is the lower leg in back.
, where f(2) is the forearm, f(3) is the shoulder behind, f(4) is the hip in front, and f(5) is the lower leg in back.
We assessed normality by Kolmogorov–Smirnov test using SPSS program. The data were processed using Microsoft Excel and Statistica 8.0 for Windows. The results of the study were processed by the method of variation statistics with estimation of reliability according to the Student’s criterion (t). The relationship of the signs was evaluated by Pearson correlation coefficient. The probability of error not exceeding 5% (p<0.05) was considered significant.
Results
Analyzing the anthropometry data, it should be noted that there are statistically significant (p < 0.05) differences between the height rates of the subjects in the first two age groups compared with the control group (Figure 1). No statistically significant differences (p > 0.05) were found when comparing the height of subjects over 60 years of age with a similar indicator of non-sports people of the same age.
 |
Figure 1 Growth indicators of subjects of different ages, depending on the motor mode. |
Body weight is statistically significantly (p < 0.05) less in those who continue sports (70.7±10.2) classes after 60 years compared with the control group (82.4±9.3) (Figure 2). When comparing the body weight of subjects over 60 who stopped sports training, as well as other age groups with a similar indicator of non-sports people of the same age, no statistically significant differences (p > 0.05) were found.
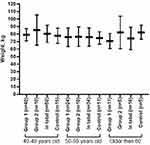 |
Figure 2 The body weight of the subjects of different ages depends on the motor mode. |
The anthropometric examination showed that the chest excursion was statistically significantly (p <0.05) greater in sports veterans than in the control group (Figure 3). At the same time, in former athletes who stopped training, the index of chest excursion does not statistically significantly differ from the similar indicator in subjects who do not engage in sports (p > 0.05).
 |
Figure 3 Indicators of the chest circumference of subjects of different ages, depending on the motor mode. |
It should be noted that there are statistically significant (p < 0.05) differences between the indicators of chest circumference in the age groups of 50–59 years and over 60 years in the subjects of the first and control groups. In active athletes, the chest circumference on exhalation is statistically significantly larger in comparison with the same indicator in subjects who stopped regular training. In addition, in the first group of patients over 60 years of age, chest mobility is higher (8.3±1.9) than in the second group (6.8±1.3), which is reflected in the statistically significant difference in chest excursion in these groups.
In the subjects of the first group aged from 40 to 49 (4551±612) and from 50 to 59 (4242±416), the FVC index was statistically and significantly (p < 0.05) higher than in the control group (3890±344 and 3786±401, respectively) (Figure 4). However, there were no statistically significant differences in this indicator from the control group in subjects older than 60 years (p > 0.05). Athletes who have stopped sports activities have a decrease in body fat with age. At the same time, at the age of 40–49, former athletes had a statistically significant (p < 0.05) higher FVC compared to people who do not engage in sports, and in the age groups of 50–59 years and older than 60 years, there were no statistically significant differences from the control group by a similar indicator (p > 0.05).
 |
Figure 4 Vital lung capacity subjects of different ages depending on the motor mode. |
Statistically significant differences (p < 0.05) in the dynamometry of veterans and subjects from the control group, but only in the first age group (Figure 5). In the remaining age groups of 50–59 years and older than 60 years, dynamometry indicators have no statistically significant differences (p > 0.05).
 |
Figure 5 Dynamometry indicators of various muscle groups in subjects of different ages, depending on the motor mode. |
In sports veterans of all age groups, the shoulder circumference is statistically significantly (p < 0.05) higher than in the control group (Figure 6). Athletes who have stopped playing sports also have statistically significant (p < 0.05) differences from the control group in shoulder circumference, but these differences are not observed in the age group over 60 years. In addition, sports veterans have shoulder and forearm circumferences that are statistically significantly lower (p < 0.05) compared to former athletes who stopped training. Statistically significant differences (p < 0.05) in hip circumference with persons who have never engaged in sports are observed in sports veterans and former athletes who have stopped sports activities at the age of 40–49 years. The circumference of the lower leg in both sports veterans and those who stopped classes of all age groups are statistically significantly greater (p < 0.05) than in the control group. The results obtained may be associated with low-fat content and hypertrophy of limb muscles in sports veterans.
 |
Figure 6 The circumference of the limbs of subjects of different ages, depending on the motor mode. |
According to the study, the body area of all subjects has no statistically significant (p > 0.05) differences from the control group.
The body composition of veterans is characterized by a high level of muscle mass and a low level of fat mass (Figure 7). The percentage of adipose tissue was statistically significantly (p < 0.05) lower in the age groups over 50 years in veterans (13.94±2.38) compared with former athletes who stopped training (15.92±2.22). There is also a statistically significant (p < 0.05) increase in both absolute and relative indicators of muscle mass in all age groups of sports veterans compared with the control group. At the same time, at the age of 40–49 years, the percentage of muscle tissue in sports veterans is statistically significantly higher (46.32±2.74) (p < 0.05) than in the group of athletes who stopped sports activities (44.09±5.29).
 |
Figure 7 Body composition of subjects of different ages, depending on the motor mode. |
Discussion
A study conducted by Eime et al showed that older people make up about 10% of people involved in sports.6 At the same time, it was demonstrated that regular physical activity in old age is an effective means of improving the quality of life, psychophysical well-being, development and maintenance of participation in society, and social ties.16,17
Studies show that playing sports even at an older age is a preventive measure for many pathological conditions.5 So, in our previous study, the data obtained showed that sports veterans are less likely and to a lesser extent than people of the same age who have not previously engaged in sports; age-related changes and diseases of the cardiovascular system are detected with higher functional capabilities of the circulatory system and preservation of ways of adaptation to physical exertion characteristic of a younger age.
Aging has a detrimental effect on physiological functions, muscle mass, and muscle strength, which can be aggravated by low physical activity or adverse changes in body composition. As is known, in elderly patients, a decrease in muscle mass and low physical activity lead to lower metabolism, which, in turn, leads to weight gain and an increase in abdominal fat. A study by Perna et al,18 found that the phenotype of sarcopenic obesity is associated with inflammation, an increased risk of fractures, and a worse metabolic profile in general. At the same time, it is known that the accumulation of adipose tissue and/or the presence of an excessive number of macrophages infiltrating adipocytes is accompanied by an increase in the secretion of proinflammatory cytokines, such as interleukin-1, IL-6, and tumor necrosis factor-alpha (TNF-α). In addition, adipose tissue produces pro-inflammatory adipokines, due to which the effect of lipotoxicity against skeletal muscle cells is mediated, which makes a significant contribution to the pathophysiology of sarcopenia.19 Schrager et al20 showed in a large sample of elderly patients that the presence of central obesity positively correlates with the severity of systemic inflammation and negatively with muscle strength. In addition, there is a hypothesis according to which the infiltration of skeletal muscles by adipose tissue is associated with more pronounced inflammation compared to muscle tissue without such infiltration, thus establishing an important pathogenetic relationship between an increase in fat mass, the accumulation of triglycerides in muscles, and inflammation. The increase in leptin levels observed with age may be accompanied by the development of leptin resistance and a decrease in fatty acid oxidation, which contribute to the ectopic deposition of fat along with other tissues and into skeletal muscles, leading to their atrophy.21 According to the dynamometry results obtained by us, the muscle strength of sports veterans was statistically significantly different from similar indicators in the control group, which suggests a decrease in the development of sarcopenia and the process of inflammation in muscle tissue.
Other disorders have also been described, such as mitochondrial dysfunction, oxidative stress, and impaired function of stem cells in muscles.22 In addition, the contribution of inflammatory markers, including those secreted by adipocytes, to the development and progression of such classical CHD risk factors as obesity and hypertension is now reliably shown. Convincing evidence of the significant role of IL-6, CRP, homocysteine, and several other cytokines has been published.23 At the same time, regular physical activity, especially endurance exercises, contributes to a decrease in heart rate and blood pressure at rest, which acts protectively on the vessel wall and reduces cardiovascular risk.24 According to our previous study, the main hemodynamic indicators identified in the majority of sports veterans are characteristic not only of the lower limits of age variations but also of hemodynamic indicators in younger people. At the same time, the results of this study demonstrate that athletes who continue to train even in old age have a body composition with a lower proportion of adipose tissue and greater severity of the muscle frame. In general, conclusions can be drawn about the favorable cardiovascular profile of sports veterans compared to untrained individuals.
Thus, the lack of sufficient physical activity against the background of excessive energy consumption in old age increases the likelihood of adverse changes in body composition (ie, a decrease in muscle mass and an increase in body fat). In addition, periods of low physical activity disrupt the anabolism of muscle proteins and accelerate the progression of sarcopenia.25 Our data show that sports veterans have a higher skeletal muscle mass index and less body fat than untrained individuals of the same age. In addition, the data obtained by measuring the circumference of the limbs also indirectly reflect the development of skeletal muscles. According to Figure 6, it is noticeable that the circumference of the limbs of untrained people is lower than that of sports veterans. This can be explained by the greater severity of muscle tissue atrophy in the control group of subjects. Muscle atrophy in the absence of regular training is related to the results of the study by McKengry et al. Colleagues have shown that sports veterans have a larger area of MHC I muscle fibers than untrained people of the same age. In addition, individuals who continue sports activities in old age demonstrate greater capillarization of MHC I and II fibers compared to the control group.1 Therefore, the continuation of structured endurance training promotes a favorable body composition and can bring important metabolic health benefits.
Some limitations of the study should be taken into account. Using the total muscle mass equation offers an approximate calculation. Despite this, it is an easy-to-use method that does not require expensive equipment and that has been tested and is widely used.
Conclusion
Our results show that veterans of gaming sports with an average training experience of 20 years or more demonstrate higher indicators of limb girth and muscle strength compared to untrained people of the same age. In addition, veterans of sports have a lower content of adipose tissue and greater severity of muscle. So, in the age group over 60, athletes who continue regular training have 1.5 times less total adipose tissue mass than in the control group. Also, the indicators of muscle mass in sports veterans are 1.2 times higher than similar indicators in the control group. The data presented by us shows the possibility of maintaining the physical function of skeletal muscles in elderly people. Sports activity throughout life allows you to maintain physical tone and prevent the development of sarcopenia. Thus, the data obtained by us show that sports activities contribute to an increase in the specific and nonspecific stability of the body, the maximum deployment of the capabilities of functional systems, including the circulatory system, and their long-term preservation, postponing the age-related decline in vital activity, and thus are essential for strengthening and preserving human health.
Abbreviations
CHD, coronary heart disease; FM, fat mass; FVC, vital capacity.
Acknowledgments
We would like to thank all participants included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McKendry J, Joanisse S, Baig S, et al. Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults. J Gerontol Ser A. 2020;75:1079–1088. doi:10.1093/gerona/glz142
2. Khan KM, Thompson AM, Blair SN, et al. Sport and exercise as contributors to the health of nations. Lancet. 2012;380:59–64.
3. Trappe S. Master athletes. Int J Sport Nutr Exerc Metab. 2001;11(s1):S196–S207. doi:10.1123/ijsnem.11.s1.s196
4. Faienza MF, Lassandro G, Chiarito M, et al. How physical activity across the lifespan can reduce the impact of bone ageing: a literature review. Int J Environ Res Public Health. 2020;17(6):1862. doi:10.3390/IJERPH17061862
5. Lassandro G, Trisciuzzi R, Palladino V, et al. Psychophysical health and perception of well-being between master badminton athletes and the adult Italian population. Acta Bio Medica Atenei Parm. 2021;92:2021196.
6. Eime RM, Harvey JT, Charity MJ, et al. The contribution of sport participation to overall health enhancing physical activity levels in Australia: a population-based study. BMC Public Health. 2015;15(1):1–12. doi:10.1186/s12889-015-2156-9
7. Soto-Quijano DA. The competitive senior athlete. Phys Med Rehabil Clin N Am. 2017;28:767–776. doi:10.1016/j.pmr.2017.06.009
8. Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–498. doi:10.1016/j.exger.2013.02.012
9. Nederveen JP, Joanisse S, Snijders T, et al. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia, Sarcopenia Muscle. 2016;7(5):547–554. doi:10.1002/jcsm.12105
10. Pollock RD, O’Brien KA, Daniels LJ, et al. Properties of the vastus lateralis muscle in relation to age and physiological function in master cyclists aged 55–79 years. Aging Cell. 2018;17. doi:10.1111/ACEL.12735
11. Piasecki M, Ireland A, Piasecki J, et al. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol. 2018;596:1627–1637. doi:10.1113/JP275520
12. Smeuninx B, McKendry J, Wilson D, et al. Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol Metab. 2017;102(9):3535–3545. doi:10.1210/jc.2017-00869
13. Pollock RD, Carter S, Velloso CP, et al. An investigation into the relationship between age and physiological function in highly active older adults. J Physiol. 2015;593(3):657–680. doi:10.1113/jphysiol.2014.282863
14. Mckendry J, Breen L, Shad BJ, et al. Muscle morphology and performance in master athletes: a systematic review and meta-analyses. Ageing Res Rev. 2018;45:62–82. doi:10.1016/j.arr.2018.04.007
15. Hwang J, Park S. Gender-specific risk factors and prevalence for sarcopenia among community-dwelling young-old adults. Int J Environ Res Public Health. 2022;19(12):7232. doi:10.3390/IJERPH19127232
16. Porto DB, Guedes DP, Fernandes RA, et al. Perceived quality of life and physical activity in Brazilian older adults. Motricidade. 2014;8:33–41.
17. Jenkin CR, Eime RM, Westerbeek H, et al. Sport and ageing: a systematic review of the determinants and trends of participation in sport for older adults. BMC Public Health. 2017;17(1):1–20. doi:10.1186/s12889-017-4970-8
18. Perna S, Spadaccini D, Rondanelli M. Sarcopenic obesity: time to target the phenotypes. J Cachexia Sarcopenia Muscle. 2019;10:710. doi:10.1002/jcsm.12425
19. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi:10.1038/s41574-018-0062-9
20. Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi:10.1152/japplphysiol.00627.2006
21. Shimabukuro M. Leptin resistance and lipolysis of white adipose tissue: an implication to ectopic fat disposition and its consequences. J Atheroscler Thromb. 2017;24(11):1088. doi:10.5551/jat.ED083
22. Barazzoni R, Bischoff S, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11(4):294–305. doi:10.1159/000490361
23. Brinkley TE, Hsu FC, Beavers KM, et al. Total and abdominal adiposity are associated with inflammation in older adults using a factor analysis approach. J Gerontol Ser A. 2012;67:1099–1106. doi:10.1093/gerona/gls077
24. Tso JV, Turner CG, Liu C, et al. Exercise blood pressure changes and aortic dilatation in male Masters endurance athletes. Eur J Prev Cardiol. 2023;30(5):e18–e20. doi:10.1093/EURJPC/ZWAC250
25. Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98(6):2604–2612. doi:10.1210/jc.2013-1502
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
