Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Affective Symptoms and Health-Related Quality of Life Among Women with Stress Urinary Incontinence: Cross-Sectional Study
Authors Steibliene V , Aniuliene R, Aniulis P , Raskauskiene N, Adomaitiene V
Received 27 October 2019
Accepted for publication 15 January 2020
Published 24 February 2020 Volume 2020:16 Pages 535—544
DOI https://doi.org/10.2147/NDT.S236234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Vesta Steibliene,1,2 Rosita Aniuliene,3 Povilas Aniulis,4 Nijole Raskauskiene,2 Virginija Adomaitiene1
1Clinic of Psychiatry, Lithuanian University of Health Sciences, Kaunas, Lithuania; 2Neuroscience Institute, Lithuanian University of Health Sciences, Kaunas, Lithuania; 3Department of Obstetrics and Gynaecology, Lithuanian University of Health Sciences, Kaunas, Lithuania; 4Department of Urology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Correspondence: Vesta Steibliene
Lithuanian University of Health Sciences, Mickeviciaus Str. 9, Kaunas LT432060, Lithuania
Tel +370 687 39116
Fax +370 460 30011
Email [email protected]
Purpose: To evaluate the relationship between affective symptoms, clinical variables of uro-gynaecological history and health-related quality of life (QoL) among women with stress urinary incontinence (SUI) in comparison to healthy controls.
Patients and Methods: In a cross-sectional study, 80 women 30 to 80 years of age diagnosed with SUI and 97 controls without symptoms of SUI provided sociodemographic data and answered the King’s Health Questionnaire (KHQ) for assessing the QoL among individuals with urinary incontinence. Symptoms of anxiety and depression were assessed by Hospital Anxiety and Depression (HAD) scale with a threshold ≥ 7. A multiple regression was performed to reveal the cross-sectional predictors of affective symptoms and QoL among women with SUI.
Results: Women with SUI had a significantly higher prevalence of symptoms of anxiety and depression than the controls (50% vs 11% and 29% vs 3.1%, respectively; both p< 0.001) and worse health-related QoL on all domains of the KHQ. In multiple logistic regression models adjusted for sociodemographic and clinical variables of uro-gynaecological history, perceived symptoms of mild-to-severe depression were associated with a higher amount of leakage (OR=3.59; 1.04– 12.4), older age (≥ 55 years old vs < 55 years old) (OR=5.82; 1.47– 23.1) and higher BMI (OR=1.13; 1.01– 1.27). In addition, when controlled for all domains of the KHQ, perceived depressive symptoms were associated with the “emotions” domain of the KHQ (OR=1.06; 1.02– 1.09). Perceived anxiety symptoms (independent of age) were related to shorter duration of SUI, low parity, absence of comorbidities and to higher scores on the “personal relationships” and “emotions” domains of the KHQ.
Conclusion: Women with SUI have a significantly poorer QoL than their counterparts without SUI. It was determined that one-half of women with SUI had anxiety symptoms, while one-third of women with SUI had depressive symptoms. In addition, this study indicated that QoL was associated with anxiety symptoms in middle-aged women and with depressive symptoms in older women, especially those with a shorter duration of SUI.
Keywords: urinary incontinence, anxiety, depression, King’s Health Questionnaire
Introduction
Stress urinary incontinence (SUI) is urine leakage on effort, or sneezing, coughing, or physical exertion as defined by the International Continence Society (ICS).1 It is known as related to aging, obesity and multiparity. SUI is an important multifactorial health problem that negatively affects women’s personal hygiene, leads to feelings of a loss of body control, promotes a behaviour associated with social interruption, and lowers general functioning and health-related quality of life (QoL).2,3
Many factors other than the severity of incontinence and the burden of symptoms contribute to morbidity. Previous studies mostly used the quantity of leaking urine as an objective clinical outcome for urinary incontinence (UI). In recent years, studies have focused on comorbid psychosocial conditions. The associations between SUI and mental disorders, especially anxiety and depression, have been receiving increasing attention recently. It is known that SUI is associated with anxiety and depressive symptoms, and women with SUI and depression have worse QoL as compared to women without affective symptoms.4–6 In the EPINCONT study, both depression and anxiety were shown to be the risk factors for developing UI, and UI is associated with an increased incidence of depression and anxiety.7 However, there is a lack of information about the relationship between affective symptoms and QoL in different age groups of women with SUI.
From a public health perspective, advancing age is associated with a higher prevalence of SUI, which is linked to worse health outcomes and an increase in hospitalizations and costs of treatment.8 Therefore, healthcare should be concentrated not only on providing appropriate medical care but also mental education, psychosocial support and treatment of affective disorders.9,10 Throughout, the impact of SUI on the QoL of the ageing population as well as interface with anxiety and depressive symptoms in different ages needs more research.
The aim of our study was to evaluate the relationship between affective symptoms, clinical variables of uro-gynaecological history, sociodemographic variables and health-related QoL among women with SUI in comparison to healthy controls.
Materials and Methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki. All study subjects signed an informed consent (permission of the Bioethics Committee of Lithuanian University of Health Sciences Nr-BEC-LSMU(R)-94 (27 October 2014)).
Study Population and Setting
This cross-sectional study enrolled women 30 to 80 years of age diagnosed with SUI who were consecutively admitted for uro-gynaecological consultation in the outpatient department of the Clinic of Obstetrics and Gynaecology at University Hospital between November 2014 and September 2015. Diagnosis of SUI was performed by gynaecologist All patients had typical medical history of stress incontinence and they filled in 3 days diaries. The degree of the incontinence was 2–3 according to the Ingelman-Sundberg score. For confirmation of diagnosis, a gynaecological examination, a cough test and stress provocation test were performed for all patients.
The control group consisted of women who came in for preventive check-ups from a family physician at the Clinic of Family Medicine. At first, all the women were asked if they had been diagnosed with UI or had specific complaints about UI. If they answered no, they were invited to participate as study controls.
The exclusion criteria for this study were: urogenital prolapse greater than stage three (Pelvic Organ Prolapse Quantification system (POP–Q)), urinary retention, overactive bladder, onco-gynaecological diseases, extreme obesity (BMI more than 40 kg/m2), significant cognitive impairment, severe unstable mental disorders or inability to understand the purpose of this study and sign the informed consent form.
Of 115 women with SUI invited to participate in the study, 24 declined to participate and 11 were excluded due to exclusion criteria or incomplete data in the questionnaires. Of 115 controls, 18 women declined to participate. The final study sample consisted of 80 women diagnosed with SUI and 97 women designated as controls.
Patient Characteristics
Sociodemographic characteristics included age, marital status, and education. Clinical health-related factors, known as predictors of SUI, were body mass index (BMI) and comorbidities, such as concomitant chronic illnesses. Gynaecological history included questions about menopause, types of delivery, parity and symptoms of SUI.
Assessments
Hospital Anxiety and Depression Scale (HADS)
The HADS, a 14-item questionnaire, was used to assess mental health, which includes seven items to assess anxiety and seven items to assess depressive symptoms. The HADS has good psychometric properties in various clinical settings. Each question was scored on a Likert-like scale from 0 to 3 points. The total sum of points in assessing anxiety and depression can vary from 0 to 21, respectively. Women were asked to choose one response from the four given for each interview and were expected to give an immediate response that was the most appropriate to describe their feelings in the last week. A score of >7 for both the HADS-Anxiety (HADS-A) and HADS-Depression (HADS-D) was suggested as an optimal threshold, in terms of sensitivity and specificity, to indicate clinically relevant levels of anxiety and depressive symptoms.11 We also used a cut-off of HADS≥11 for moderate/severe anxiety/depression. To quantify the severity of anxiety/depression symptoms, we analysed the HADS-D and HADS-A data as continuous variables. In the current study, Cronbach’s alpha was 0.79 for anxiety and 0.83 for depression.
The King’s Health Questionnaire (KHQ)
The instrument is known as the disease-specific questionnaire and evaluates the impact of lower urinary tract symptoms on women’s health-related QoL.12,13
The KHQ is a patient self-administered self-report questionnaire and has two parts consisting of 21 items. Part 1 contains general health perception and overall health related to urinary symptoms (one item each). Part 2 includes 19 questions divided into seven domains of quality of life: role limitations, physical limitations, social limitations (two items each), personal relationships (three items), emotions (three items each), sleep/energy (two items) and severity measures (five items). The responses to the KHQ have a 4-point rating system. The section on personal relationships included a fifth possible answer: “The question is not suitable for me,” which is for women who are divorced, have no current sexual partner or are not sexually active. The nine subscales (“domains”) were scored between 0 (best) and 100 (worst). Lower scores indicate patient wellbeing and higher scores indicate that the woman is severely affected by the disease condition. The minimally important difference, or the smallest change in score that subjects perceived as beneficial, was 5 points for all the KHQ domains.13
Statistical Analysis
The statistical software package SPSS, Version 17.0 was used for statistical analysis. Descriptive statistics were expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)) for continuous variables, according to normality, and categorical variables were represented as percentages. For comparisons regarding the sociodemographic and clinical variables, Pearson-χ2-tests, Mann–Whitney-U-tests and T tests were conducted. Spearman correlation was used for correlation analyses.
A reliability analysis for the nine KHQ items using Cronbach’s alpha was performed. The analysis revealed a Cronbach’s alpha of 0.89 (more than acceptable). Internal consistency of the KHQ was strong. The subscale alphas were ranging between 0.86 and 0.92.
To find the best cross-sectional predictors of perceived mild-to-severe symptoms of anxiety and depression, a separate multiple logistic regression was performed in two steps, with the primary predictor of interest (symptoms of SUI) and covariates entered in the first step and all domains of the KHQ entered in the second step.
Cross-sectional predictors of disease-specific quality of life in both middle-aged (<55 years old) and older (≥55 years old) women with SUI were assessed using multiple linear regressions. A multiple linear regression model was conducted for the two summary scores (Part 1 and Part 2) of the KHQ: Part 1 – sum of domains 1 and 2 of the KHQ, and Part 2 – sum of domains 3 to 9 of the KHQ. The following covariates were assessed and used as independent variables: age, spouse status, education, BMI, parity, comorbidities, SUI symptoms, HADS-A and HADS-D (continuous). The level of significance was set at 5% (p≤0.05).
Results
The comparison of the two study groups (ie, demographics, urinary symptoms, presence of medical comorbidities and filled questionnaires), women with SUI and the controls, is presented in Table 1. There was no significant mean age (±SD) difference between women with SUI and the controls (51.5±11 years old vs 49±7 years old, respectively; p=0.104). In the univariate analysis, differences according to participants’ characteristics were statistically significant in the spouse status, education, type of delivery and parity. As expected, women with SUI had significantly worse symptoms of SUI as compared with the controls. In the SUI group, the mean duration of SUI was 8.9±5.6 years.
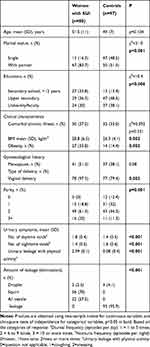 |
Table 1 Sociodemographic and Clinical Characteristics (Uro-Gynaecological History) Among Women with SUI and the Controls |
The comparison of self-reported anxiety and depressive symptom severity (HADS) and health-related QoL (KHQ) among women with SUI and controls is shown in Table 2. Women with SUI had a significantly higher prevalence of self-reported symptoms of anxiety or depression (HADS>7) than the controls (50% vs 11% and 29% vs 3.1%, respectively; both p<0.001). By the way, women with SUI reported higher mean scores in severity of depression (5.4±3.3 vs 2.4±2.1) and severity of anxiety (7.2±3.6 vs 3.8±3.0) on the HADS as compared to the controls without SUI (both p<0.001). As expected, the women with SUI had worse scores than the control group for all the KHQ domains. In women with SUI, the KHQ domains such as role limitation, physical/social limitations and measures for severity had the highest averages (70 or more) (Table 2).
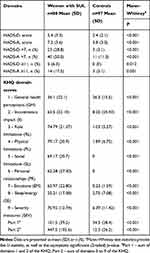 |
Table 2 The Comparison of Self-Reported Anxiety and Depression Symptoms on HADS Scores and Health- Related QoL in the KHQ Scores Among Women with SUI and the Controls |
With the objective to evaluate the relation of age and affective symptoms among women with SUI and their impact on QoL, women with SUI were divided into those below or above the cut-off age of 55, 60 or 65 years old. The difference in impairment of quality of life was greatest when the cut-off age was 55 years old. The age of the examined women with SUI ranged between 30 and 80 years old, with the majority aged between 41 and 55 years old (n=35 (43.8%)), followed by the group of women aged ≥56 years old (n=30 (37.5%)) and finally, women aged between 30 and 40 years old (n=15 (18.8%)). Among women with SUI, 47 of 80 (58.7%) women were aged 30 to 55 years old, and 33 of 80 (41.3%) women were aged ≥55 years old. Table 3 demonstrates the results of the comparison between the HADS and KHQ scores of patients with SUI, according to age groups <55 and ≥55 years old.
 |
Table 3 Comparison Between the HADS and KHQ Scores Among Women with SUI According to Age Groups |
The majority of women with SUI consider their general health perception (GH) to be good. In middle-aged women (<55 years old), the KHQ GH domain scores were similar in those with SUI and controls (30.8±20.9 vs 24.3±14.9, p=0.081; control data not shown). The middle-aged women were less likely to suffer impairment of their GH due to SUI as compared with the older women with SUI (≥55 years old) (30.8 ±20.9 and 50.7 ±18.2, respectively, p<0.001) (Table 3).
In the disease-specific QoL assessment, middle-aged women with SUI differed in seven out of the nine KHQ domains (GH, II, RL, PL, PR, EM and SE) as compared with older women with SUI and showed a better quality of life. Middle-aged women with SUI were in lower-quality “personal relationships” than older women with SUI (68.8±22.7 vs 53±30.7, p=0.028). Both age groups were not different from each other only in relation to the “social limitations” and “severity measures” domains (Table 3).
As presented in Table 3, older women with SUI (≥55 years old) were more likely to experience mild-to-severe anxiety and depressive symptoms as compared with middle-aged women with SUI (<55 years old): 45% vs 17%, p=0.006; 63.6% vs 40.4%, p=0.041, respectively.
Cross-sectional predictors of anxiety and depressive symptoms in women with SUI in multiple logistic regression models (Table 4), when controlled for potentially influential characteristics, included the association between depressive symptoms (HADS-D>7) and shorter duration of SUI (OR=0.81; 0.69–0.94), higher amount of urine (OR=3.59; 1.04–12.4), older age (≥55 vs <55 years old) (OR=5.82; 1.47–23.1) and higher BMI (OR=1.13; 1.01–1.27)—all of which were statistically significant. In addition, model 2 controlled for all domains of the KHQ perceived depression symptoms associated with the “emotions” domain of the KHQ (OR=1.06; 1.02–1.09) (Table 4).
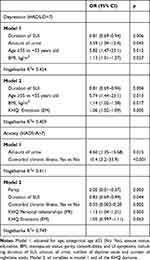 |
Table 4 Cross-Sectional Predictors of Anxiety and Depression in Women with SUI (n=80) |
The association of anxiety symptoms (HADS-A>7) was found only with a higher amount of urine and comorbid chronic illness. In addition, when controlled for all domains of the disease-specific KHQ, perceived anxiety symptoms were related to low parity, shorter duration of SUI, absence of comorbidities, and to higher scores on the “personal relationships” and “emotions” domains of the KHQ (Table 4). Age was not associated with perceived anxiety.
Cross-sectional predictors of SUI-specific quality of life are presented in Table 5. Linear multiple regression analysis with the stepwise method was conducted to determine the predominant cross-sectional predictors of QoL in both middle-aged (<55 years old) and older (≥55 years old) women with SUI. The predictive fit of all models was quite good.
 |
Table 5 Predictors of Disease-Specific Quality of Life in Both Middle-Aged (<55 Years) and Older (≥55 Years) Women with SUI (n=80) |
In middle-aged women with SUI, the frequency of daytime voids (β=0.244; p=0.025), comorbid chronic illness (β=0.314; p=0.009) and symptoms of anxiety (β=0.430; p=0.001) were associated with the score on Part 1 of the KHQ. These variables explained 54% (adjusted R2= 0.544; p<0.001) of the variance for QoL in the middle-aged women in SUI. Anxiety (β=0.723; p<0.001) and frequency of daytime voids (β=0.243; p=0.008) were associated with the score on Part 2 of the KHQ, and the model explained 67% of the variance (p<0.001) (Table 5).
Regression analysis showed that for older women with SUI (≥55 years old), depression (β=0.411; p=0.003) and BMI (β=0.359; p=0.023) were associated with the score on Part 1 of the KHQ, and the model explained 34% of the variance (p<0.001) (Table 5). Depression (β=0.460; p=0.010), lower education (β=–0.408; p=0.007) and spouse status (β=0.321; p=0.030) were associated with the score on Part 2 of the KHQ, and the model explained 39.7% of the variance (p<0.001) (Table 5).
Discussion
UI could affect all areas of women’s health, both somatic and mental health. In our study, women living with SUI showed a significantly lower QoL compared with the controls. In women with SUI, KHQ domains such as role limitation, physical/social limitations and measures for severity had higher averages. Psychiatric symptoms were common in women with SUI and likely had a direct effect on QoL. These women reported having more anxiety and depressive symptoms as compared with the controls.
Incontinence causes a major reduction in the QoL for women of all age groups.14 The instruments used to measure QoL usually include two areas: general aspects of self-reported health and a specific aspect of the effects that a certain pathology or dysfunction causes on an individual’s lifestyle.15 Our study indicated that the negative impact of SUI on general health and lifestyle was associated with anxiety symptoms in middle-aged women (<55 years old) and with depressive symptoms in older women (>55 years old). The majority of women with SUI considered their general health perception to be good. In middle-aged women (<55 years old) with SUI, their KHQ general health perception domain scores were similar to the controls, and they were less likely to suffer impairment of their general health, but not lifestyle perception, due to SUI as compared with older women (>55 years old) with SUI.
According to the World Health Organization Quality of Life (WHOQOL) assessment,16 QoL depends on the subjective perception of UI, and its treatment at social, physical and mental levels. Therefore, UI is not often considered without recognition of the comorbid conditions, such as psychological distress, anxiety or depression.17 Leaving either of these mental disorders (ie, anxiety and depression) undiagnosed/untreated, will clearly have significant impacts on the physical health and QoL of individual patients and the population as a whole.18 Naturally, any type of UI has psychological effects – feelings of insecurity and shame are the common consequences of uncontrolled loss of urine. In length of time, those negative feelings lead to the avoidance of social contacts, to possible isolation and development of depression.14,19
The impact of UI on everyday life can vary, depending on origin and severity of this disorder, as well as personality traits and individual coping strategies. Those with SUI can adapt their lifestyle by avoiding heavy lifting and/or exercising and thus prevent situations that can lead to involuntary loss of urine. The advancement of the clinical symptoms of SUI significantly limited interpersonal contacts and affected the emotional state of the surveyed women due to the unpredictable hyperactivity of detrusor and large volumes of urine leakage.20
Depression
When affective disorders occur together with physical illness, there appears to be an additive effect that affects both physical and mental health, by increasing patient’s negative perceptions of their illness.21 This cross-sectional relationship between depression and UI could be explained by common neurobiological pathways underlying both disorders. The increased activation of the hypothalamic-pituitary-adrenal (HPA) axis seen in depression could also contribute to the physiological changes that result in involuntary urine loss. Melville et al contemplate that prolonged hyper-activation of these systems, resulting in excess catecholamines and cortisol level, might have a physiological effect on bladder function and potentially play a role in the occurrence of UI. On the other hand, the constant embarrassment, social isolation, and symptom burden associated with UI could lead to the development of depressive disorder. For example, when women in their 20s or 30s seek consultation or treatment for their UI symptoms, health professionals should consider the possibility that affective symptoms and a prior history of affective disorder may play a role in the development of the UI.22,23 Also, a psychosomatic background for UI has been presented.24
Melville et al concluded that the presence of depressive symptoms influences women’s health perception and rating of the severity of UI. Women with depressive symptoms had a significantly greater appreciation of the severity of their UI and more emphasis on its impact on QoL and daily functioning than women with similar severity of UI who did not have depressive symptoms.25 Despite the close links between these two disorders, the combined effects of UI and depression outweigh the impact of either condition alone.18,20
UI is a relatively common, but not a life-threatening condition in middle-aged and older women.8 Some studies also specify that the social problems associated with UI are growing with time. However, there is no clear answer, if it is a function of gradually increasing severity of the incontinence or the particular adaptations required for dealing with this condition.20,26,27
We demonstrated that the main difference between SUI in women with and without depressive symptoms was due to their shorter duration of UI and amount of leakage urine. Higher BMI and older age were also independently associated with depressive symptoms. A higher BMI is still ruled out, as overweight is related to higher abdominal pressures and can cause mild severity UI. A few studies have compared UI patients with depressive symptoms versus UI patients without them.28 Study of Melville et al found no difference in the daily UI episodes or the frequency of moderate/severe UI between UI patients with depressive symptoms and UI patients without them.25 Sung et al studied obese female UI patients and also reported no difference in the amount of UI episodes between obese UI patients with depressive symptoms and obese UI patients without them.29 Seeking help often depends on individual’s beliefs and an understanding of how the disorder can be treated. Older women seem to be able to manage their UI more effectively than working aged women by using hygiene protection. In contrast, due to symptoms of UI, the younger women are more likely to avoid social situations and some of their normal life activities, such as playing sports, dancing or sexual activity.13,30
Anxiety
We found that age was not associated with perceived anxiety symptoms in women with SUI. The association of anxiety symptoms was found with higher amounts of urine leakage and the presence of comorbid chronic illness. About 50% of the women with SUI had anxiety symptoms, and 17.5% of women with SUI had moderate-to-severe anxiety. Similar results were reported in a study on overactive bladder (OAB) patients who presented to clinic.28,31 However, we found that symptoms of anxiety were associated with QoL in middle-aged women with SUI (<55 years old). According to a population-based longitudinal study,32 symptoms of UI may help to recognize persons with at increased risk for a newly-incident anxiety disorder. Symptoms of UI and the associated deterioration of a person’s functioning have been found to be significant stressors that increase a person’s vulnerability and propensity to develop anxiety disorder. Consequently, UI can cause anxiety disorder, and anxiety disorder can be a crucial factor in the deterioration of the QoL and functional disability of a person with UI. Also, anxiety disorders may be a sign of a person’s vulnerability, indicating an increased likelihood of UI. The comorbidity of anxiety disorders and UI is associated with greater UI-related functional disability.32
The relationship between mental disorders and UI is thought to be multidirectional.33 Anxiety, stress-related and other neurotic disorders were considered as the possible causes of unstable bladder contractions and urge UI.34 It is known that alterations in neurotransmitters’ systems could lead to uninhibited contractions of the detrusor muscle and urge UI.33 On the other hand – anxiety and depressive symptoms are associated with decreased serotonergic neurotransmission, leading to a general inhibition in the suppression of bladder contraction and causing an OAB.35 Other researchers have reported that affective symptoms are present in both conditions – SUI and OAB and that mental symptoms are the results of UI.20 Sakakibara et al revised conceptions of bladder dysfunction due to anxiety/depressive symptoms, taking into account frequency of UI, other lower urinary tract symptoms, urodynamic parameters, presumptive leading cause of the disorder, and treatment. Bladder dysfunction resulting from anxiety/depressive symptoms clearly demonstrates that bladder function is closely related to emotions and their control. So, the improvement of bladder dysfunction should be one of the important targets in treatment of patients with anxiety/depressive disorder.36
Since SUI is a condition that impacts QoL (rather than quantity of life), the treatment decisions should be closely linked to the ability to improve the worry caused by the SUI. If the worry about SUI is minimal, non-surgical management of SUI is strongly recommended.37 Anxiety and depressive symptoms are common among patients with SUI, require timely diagnosis and treatment. Stress management therapies and psychopharmacotherapy with antidepressants are appropriate solutions for the treatment of affective disorders and could be beneficial in relieving symptoms of SUI.
Limitations of Our Study
Additionally, our findings are limited by a cross-sectional study design. As a cross-sectional study, our research cannot answer the question about the causes and consequences: whether SUI causes women to be anxious or depressed or whether anxiety or depressive symptoms causes UI. Further longitudinal cohort studies regarding causality between anxiety or depressive symptoms and SUI are therefore needed to answer this question. For clinical practitioners, it is important to keep in mind the wide range of the comorbidity of SUI and anxiety/depressive symptoms. Although the HADS is commonly used instrument to assess the severity of anxiety and depressive symptoms in the outpatient settings, it is not a diagnostic tool for the diagnosis of mental disorders. Diagnosis of anxiety disorders or depression cannot be made without psychiatric evaluation. This evaluation was not performed in our study.
However, anxiety is multi-faceted and includes affective (eg, fear and irritability), cognitive (eg, worry) and physical (eg, palpitations, shortness of breath, and muscle tension) symptoms. Failure to take into account these different groups of anxiety symptoms may limit our understanding of the relationship of SUI to anxiety disorder.38
Conclusion
Women with SUI have a significantly lower quality of life than women without SUI, especially in role limitation and physical/social limitations. It was determined that one-half of women with SUI had anxiety symptoms, while one-third of women with SUI had depressive symptoms. In addition, this study indicated that quality of life was associated with anxiety symptoms in middle-aged women and with depressive symptoms in older women, especially those with a shorter duration of SUI.
Acknowledgments
The authors wish to thank all patients for participation in the study.
Author Contributions
VS and RA designed the study and wrote the study protocol. VS, VA, NR wrote the first draft of the manuscript and undertook the statistical analyses. PA, RA and VA collected and analyzed the data. All authors contributed toward data analysis, critically revised the final manuscript and gave the final approval of the manuscript version to be submitted and published. All authors agreed to be accountable for all aspects of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi:10.1002/nau.20798
2. Padmanabhan P, Dmochowski R. Urinary incontinence in women: a comprehensive review of the pathophysiology, diagnosis and treatment. Minerva Ginecol. 2014;66(5):469–478.
3. Kwon BE, Kim GY, Son YJ, Roh YS, You MA. Quality of life of women with urinary incontinence: a systematic literature review. Int Neurourol J. 2010;14(3):133–138. doi:10.5213/inj.2010.14.3.133
4. Siff LN, Jelovsek JE, Barber MD. The effect of major depression on quality of life after surgery for stress urinary incontinence: a secondary analysis of the Trial of Midurethral Slings. Am J Obstet Gynecol. 2016;215(4):455e451–459. doi:10.1016/j.ajog.2016.04.039
5. Innerkofler PC, Guenther V, Rehder P, et al. Improvement of quality of life, anxiety and depression after surgery in patients with stress urinary incontinence: results of a longitudinal short-term follow-up. Health Qual Life Outcomes. 2008;6:72. doi:10.1186/1477-7525-6-72
6. Yazdany T, Bhatia N, Reina A. Association of depression and anxiety in underserved women with and without urinary incontinence. Female Pelvic Med Reconstr Surg. 2014;20(6):349–353. doi:10.1097/SPV.0000000000000071
7. Felde G, Ebbesen MH, Hunskaar S. Anxiety and depression associated with urinary incontinence. A 10-year follow-up study from the Norwegian HUNT study (EPINCONT). Neurourol Urodyn. 2017;36(2):322–328. doi:10.1002/nau.v36.2
8. Aniuliene R, Aniulis P, Steibliene V. Risk factors and types of urinary incontinence among middle-aged and older male and female primary care patients in Kaunas region of Lithuania: cross sectional study. Urol J. 2016;13(1):2552–2561.
9. Perera J, Kirthinanda DS, Wijeratne S, Wickramarachchi TK. Descriptive cross sectional study on prevalence, perceptions, predisposing factors and health seeking behaviour of women with stress urinary incontinence. BMC Women’s Health. 2014;14:78. doi:10.1186/1472-6874-14-78
10. Bogner HR, Gallo JJ, Swartz KL, Ford DE. Anxiety disorders and disability secondary to urinary incontinence among adults over age 50. Int J Psychiatry Med. 2002;32(2):141–154. doi:10.2190/Y0L8-K2UV-BG4N-VW2J
11. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi:10.1016/S0022-3999(01)00296-3
12. Kelleher CJ, Pleil AM, Reese PR, Burgess SM, Brodish PH. How much is enough and who says so? BJOG. 2004;111(6):605–612. doi:10.1111/j.1471-0528.2004.00129.x
13. Hebbar S, Pandey H, Chawla A. Understanding King‟s Health Questionnaire (KHQ) in assessment of female urinary incontinence. Int J Res Med Sci. 2015;(3):531–538. doi:10.5455/2320-6012.ijrms20150301
14. Debus G, Kastner R. Psychosomatic aspects of urinary incontinence in women. Geburtshilfe Frauenheilkd. 2015;75(2):165–169. doi:10.1055/s-00000020
15. Bushnell DM, Martin ML, Summers KH, Svihra J, Lionis C, Patrick DL. Quality of life of women with urinary incontinence: cross-cultural performance of 15 language versions of the I-QOL. Qual Life Res. 2005;14(8):1901–1913. doi:10.1007/s11136-005-5266-5
16. Whoqol Group. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–1409. doi:10.1016/0277-9536(95)00112-K
17. Heymen S. Psychological and cognitive variables affecting treatment outcomes for urinary and fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S146–S151. doi:10.1053/j.gastro.2003.10.040
18. Vigod SN, Stewart DE. Major depression in female urinary incontinence. Psychosomatics. 2006;47(2):147–151. doi:10.1176/appi.psy.47.2.147
19. Oh SJ, Ku JH. Is a generic quality of life instrument helpful for evaluating women with urinary incontinence? Qual Life Res. 2006;15(3):493–501. doi:10.1007/s11136-005-2487-6
20. Sinclair AJ, Ramsay IN. The psychosocial impact of urinary incontinence in women. Obstet Gynaecol. 2011;13:143–148. doi:10.1576/toag.13.3.143.27665
21. Avery JC, Stocks NP, Duggan P, et al. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol. 2013;13:11. doi:10.1186/1471-2490-13-11
22. Melville JL, Fan MY, Rau H, Nygaard IE, Katon WJ. Major depression and urinary incontinence in women: temporal associations in an epidemiologic sample. Am J Obstet Gynecol. 2009;201(5):490e491–497. doi:10.1016/j.ajog.2009.05.047
23. Mishra GD, Barker MS, Herber-Gast GC, Hillard T. Depression and the incidence of urinary incontinence symptoms among young women: results from a prospective cohort study. Maturitas. 2015;81(4):456–461. doi:10.1016/j.maturitas.2015.05.006
24. Stach-Lempinen B, Hakala AL, Laippala P, Lehtinen K, Metsanoja R, Kujansuu E. Severe depression determines quality of life in urinary incontinent women. Neurourol Urodyn. 2003;22(6):563–568. doi:10.1002/nau.10137
25. Melville JL, Walker E, Katon W, Lentz G, Miller J, Fenner D. Prevalence of comorbid psychiatric illness and its impact on symptom perception, quality of life, and functional status in women with urinary incontinence. Am J Obstet Gynecol. 2002;187(1):80–87. doi:10.1067/mob.2002.124839
26. Coyne KS, Kvasz M, Ireland AM, Milsom I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol. 2012;61(1):88–95. doi:10.1016/j.eururo.2011.07.049
27. Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Prim. 2017;3:17042. doi:10.1038/nrdp.2017.42
28. Lai HH, Shen B, Rawal A, Vetter J. The relationship between depression and overactive bladder/urinary incontinence symptoms in the clinical OAB population. BMC Urol. 2016;16(1):60. doi:10.1186/s12894-016-0179-x
29. Sung VW, West DS, Hernandez AL, Wheeler TL
30. St John W, Griffiths S, Wallis M, McKenzie S. Women’s management of urinary incontinence in daily living. J Wound Ostomy Continence Nurs. 2013;40(5):524–532. doi:10.1097/WON.0b013e3182a2198a
31. Lai HH, Rawal A, Shen B, Vetter J. The relationship between anxiety and overactive bladder or urinary incontinence symptoms in the clinical population. Urology. 2016;98:50–57. doi:10.1016/j.urology.2016.07.013
32. Bogner HR, O’Donnell AJ, de Vries HF, Northington GM, Joo JH. The temporal relationship between anxiety disorders and urinary incontinence among community-dwelling adults. J Anxiety Disord. 2011;25(2):203–208. doi:10.1016/j.janxdis.2010.09.003
33. Morrison LM, Morrison M, Small DR, Glen ES. Psychiatric aspects of female incontinence. Int Urogynecol J. 1991;2(69):69–72. doi:10.1007/BF00376561
34. Frewen WK. An objective assessment of the unstable bladder of psychosomatic origin. Br J Urol. 1978;50(4):246–249. doi:10.1111/bju.1978.50.issue-4
35. Yip SK, Cardozo L. Psychological morbidity and female urinary incontinence. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):321–329. doi:10.1016/j.bpobgyn.2006.12.002
36. Sakakibara R, Ito T, Yamamoto T, et al. Depression, anxiety and the bladder. Low Urin Tract Symptoms. 2013;5(3):109–120. doi:10.1111/luts.12018
37. Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J Urol. 2017;198(4):875–883. doi:10.1016/j.juro.2017.06.061
38. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413–420. doi:10.1093/geronb/gbx020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
