Back to Journals » Journal of Inflammation Research » Volume 16
Aerobic Exercise Ameliorates Liver Injury in Db/Db Mice by Attenuating Oxidative Stress, Apoptosis and Inflammation Through the Nrf2 and JAK2/STAT3 Signalling Pathways
Authors Sun M , Zhao X , Li X, Wang C, Lin L, Wang K, Sun Y, Ye W, Li H, Zhang Y, Huang C
Received 6 July 2023
Accepted for publication 12 October 2023
Published 24 October 2023 Volume 2023:16 Pages 4805—4819
DOI https://doi.org/10.2147/JIR.S426581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Meiyan Sun,1,2,* Xiaoyong Zhao,2,* Xingyue Li,2 Chunling Wang,2 Lili Lin,3 Kaifang Wang,2 Yingui Sun,2 Wei Ye,3 Haiyan Li,3 Ye Zhang,1 Chaolu Huang2,4
1Department of Anesthesiology and Perioperative Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, 261053, People’s Republic of China; 3The First Clinical Medical College, Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 4Department of Clinical Medicine, Qiandongnan Ethnic Vocational and Technical College, Kaili, 556000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Zhang, Department of Anesthesiology and Perioperative Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, People’s Republic of China, Email [email protected] Chaolu Huang, Department of Clinical Medicine, Qiandongnan Ethnic Vocational and Technical College, Kaili, 556000, People’s Republic of China, Email [email protected]
Objective: Diabetes mellitus (DM) implicates oxidative stress, apoptosis, and inflammation, all of which may contribute liver injury. Aerobic exercise is assured to positively regulate metabolism in the liver. This project was designed to investigate whether and how aerobic exercise improves DM-induced liver injury.
Methods: Seven-week-old male db/db mice and age-matched m/m mice were randomly divided into a rest control group or a group that received 12 weeks of aerobic exercise by treadmill training (10 m/min). Haematoxylin and eosin (HE) staining, electron microscopy, Oil Red O staining and TUNEL assays were used to evaluate the histopathological changes in mouse liver. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TRIG), cholesterol (CHOL) were analyzed by serum biochemical analysis. Interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), and tissue levels of malondialdehyde (MDA) and superoxide dismutase (SOD) were analyzed via ELISA. Nuclear factor E2-associated factor-2 (Nrf2), nuclear factor κB (NF-κB) and JAK2/STAT3 pathway-related proteins were measured by immunofluorescence, Western blotting and q-PCR. F4/80 expression in liver tissues was assessed by immunohistochemistry.
Results: In diabetic mice, exercise training significantly decreased the levels of serum TRIG, CHOL, IL-6, TNF-α, ALT and AST; prevented weight gain, hyperglycaemia, and impaired glucose and insulin tolerance. Morphologically, exercise mitigated the diabetes-induced increase in liver tissue microvesicles, inflammatory cells, F4/80 (macrophage marker) levels, and TUNEL-positive cells. In addition, exercise reduced the apoptosis index, which is consistent with the results for caspase-3 and Bax. Additionally, exercise significantly increased SOD activity, decreased MDA levels, activated Nrf2 and decreased the expression of NF-kB, phosphorylated JAK2 and STAT3 proteins in the livers of diabetic mice.
Conclusion: This study demonstrated that aerobic exercise reversed liver dysfunction in db/db mice with T2DM by reducing oxidative stress, apoptosis and inflammation, possibly by enhancing Nrf2 expression and inhibiting the JAK2/STAT3 cascade response.
Keywords: db/db mice, aerobic exercise, inflammation, apoptosis, Nrf2, JAK2/STAT3
Introduction
Type 2 diabetes mellitus (T2DM) is a global life-threatening disease characterized pathologically by chronic hyperglycaemia associated with pancreatic β cell collapse and insulin resistance (IR).1 The assault of diabetes is accompanied by the development of glycation end products (AGEs), oxidative stress, lipotoxicity and endoplasmic reticulum stress (ERS), which contribute to hepatocyte inflammation, damage, liver tissue necrosis, and serious liver disease.2–4 Furthermore, it has been reported that hepatomegaly, elevated liver enzyme levels, steatosis and fibrosis are closely related to diabetes.5,6 Consequently, an effective therapeutic strategy is desirable to control liver damage caused by diabetes.
Accumulating evidence suggests that oxidative stress occurs by increasing cellular reactive oxygen species (ROS) and depleting the antioxidant defensive mechanism.7,8 Additionally, lipid accumulation in the liver can also cause oxidative stress damage, which subsequently leads to hepatic inflammation and cell apoptosis.9,10 In addition, it was recently shown that changes in oxidative status in diabetes could induce inflammatory signal transduction.11 Nrf2 is a redox-sensitive transcription factor encoded by the NFE2L2 gene that protects against oxidative damage induced by ROS by regulating oxidative stress and antioxidant mechanisms.12 Recent evidence suggested that Nrf2 was the desired therapeutic target for T2DM13 because Nrf2 activation markedly enhanced glucose homeostasis and decreased the inflammatory response in diabetic mice.14 Moreover, Nrf2 plays a key role in preventing hepatic oxidative injury and inflammation.15,16
Excessive nutrient uptake and physical inactivity affect the hepatic metabolism of glucose and aggravate oxidative stress. Conversely, persistent physical training has many unexpected effects. For example, it ameliorates diabetes-induced liver dysfunction by increasing levels of antioxidant enzymes and hepatic ROS scavenging,17 and preventing metabolic syndrome, obesity18 and nonalcoholic fatty liver disease.19 If aerobic exercise training lasts more than 6 weeks, the inhibitory effect of insulin on endogenous glucose production will be enhanced to ameliorate hepatic glucose metabolism.20 In addition, a recent study showed that aerobic exercise attenuated ethanol-induced hepatotoxicity in Wistar rats by inhibiting inflammation, apoptosis and oxidative stress.21 Nonetheless, the function and regulatory mechanisms of aerobic exercise in diabetic hepatic damage remain to be clarified. The janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling pathway is an evolutionarily conserved regulatory pathway for many cytokines, interferons and growth factors.22 STAT3 has been demonstrated to be closely correlated with hepatic lipometabolism and involved in the regulation of liver injury progression.23 Blockade of the JAK2/STAT3 pathway impedes apoptotic and inflammatory responses, which contributes to hepatic protection.24 Intriguingly, Wang et al found that STAT3 was presumed to be the downstream target of Nrf2.25 Similar studies have found that JAK2/STAT3, as a downstream pathway of the IL-6/Nrf2 crosstalk pathway, participates in the antioxidant effect of vinicidin B.26 Therefore, it was reasonable to postulate that activating Nrf2 and inhibiting the JAK2/STAT3 pathway contribute to liver dysfunction restoration. In this study, we investigated the protective effect of aerobic exercise on inflammatory reactions and apoptosis in diabetic liver injury to assess a new therapeutic choice for T2DM and to expand the effects of aerobic exercise.
Materials and Methods
Reagents
D-(+)-Glucose was purchased from Sigma-Aldrich (Shanghai, China). p-JAK2 (#3776), p-STAT3(#4093), NF-κB p-P65 (#3033), Caspase-3 (#9662), and Bax (#14796) antibodies were bought from Cell Signaling Technology (Beverly, MA, USA). Nrf2 (ab62352), F4/80(ab300421), NF-κB P65 (ab16502), JAK2 (ab108596), STAT3 (ab68153) antibodies were obtained from Abcam (Cambridge, UK). β-actin (sc-8432), Tubulin (sc-23950), and the horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibodies (sc-2357) were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The fluorescein-conjugated anti-rabbit IgG antibodies (SA00003-1) were purchased from Proteintech Group, Inc. (Rosemont, IL, USA). ApopTag® peroxidase in situ apoptosis detection kit was from Roche (Chemicon, CA, USA). Hematoxylin-eosin (HE) Staining Kit was delivered from Solarbio (Beijing, China). Triglyceride, Total cholesterol, AST, ALT were obtained from Beijing Zhuoyue Biotechnology Co., Ltd. (Beijing, China). MDA and SOD were delivered from Elabscience Biotechnology (Wuhan, China).
Animals and Groups
Seven-week-old male db/db mice (BKS-Leprem2Cd479/Nju, n=20) and age-matched m/m mice (C57BL/KsJNju, n=20) were purchased from GemPharmatech LLC (Jiangsu, China). The mice were housed in specific pathogen-free (SPF) conditions with a 12 h light/dark cycle and were allowed free access to water and conventional feed. All procedures on mice were approved by the Animal Care and Use Committee of Weifang Medical University (Approval no. 2021SDL064), and were in accordance with the USA National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23, revised in 1996). After one week of acclimatization, the m/m mice were randomly divided into two groups (10/group): m/m mice that remained sedentary (MC) and m/m mice that underwent aerobic exercise training (ME). The db/db mice were randomly divided into two groups (10/group): db/db mice that remained sedentary (DC) and db/db mice that underwent aerobic exercise training (DE).
Aerobic Exercise Training
All mice in the exercise groups underwent treadmill pretraining for 5 days. Treadmill exercise intensity was based on previous research but was slightly modified.27 During acclimation, all mice were trained on a motorized rodent treadmill at a speed of 5 m/min (0° slope, 30 min) on day one. The speed was then increased 1 m/min each day, and the treadmill duration was slowly increased from 30 min to 60 min (0° slope). The mice were tested 5 days/week at a speed of 10 m/min for 12 weeks based on the target training protocol. The OGTT and ITT were preformed 24 h after the last exercise training, and then all animals were fasted (10–12 h) and anaesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Body weights and fasting blood glucose (FBG) were measured. Blood samples were obtained from the retroorbital sinus and centrifuged to separate serum. Then, the mice were euthanized by cervical dislocation, and the livers were immediately excised and briefly rinsed in PBS. The right lobe of the livers was collected into 4% paraformaldehyde for histological assessment, and the remaining liver tissues were stored at −80 °C for molecular analyses.
Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
The OGTT and ITT were performed as outlined previously.28 The overnight fasted mice were given glucose via gavage at 2 g/kg body weight. Glucose values were determined by a ROCHE glucometer (Roche, Basel, Switzerland) from tail vein samples at 0, 15, 30, 60 and 120 min post gavage. For the ITT, after the baseline blood glucose level of 6-h-fasted mice was measured, an intraperitoneal injection of insulin (0.75 u/kg body weight) was administered. The blood glucose levels were then measured at 0, 15, 30, 60, 90, and 120 min postinjection.
Serum Biochemical Analysis and ELISA Analysis
The serum levels of ALT, AST, TRIG, and CHOL were tested with an automated chemical analyser (AU5400, Tokyo, Japan). The cytokine (interleukin-6 (IL-6) and tumour necrosis factor-a (TNF-a)) levels in serum were evaluated by ELISA kits strictly according to the manufacturer’s instructions. Absorbance at 450 nm was measured by using a microplate reader (Thermo Fisher, USA).
Detection of Oxidative Stress Markers
Fresh liver samples were homogenized with PBS at 4 °C, and the supernatant was extracted after centrifugation for 10 minutes to determine MDA content and SOD activity as described.29 The optical density was measured at 405 nm (MDA) and 450 nm (SOD).
Liver Histology and TUNEL Staining
The liver tissues were fixed with 4% paraformaldehyde for 24 hours and then embedded in paraffin. For morphological evaluation, liver slices 4 μm in thickness were obtained and stained with haematoxylin-eosin. The stained sections were examined with a Nikon microscope and photographed with a digital camera (Nikon, Japan). An ApopTag® peroxidase in situ apoptosis detection kit was utilized to identify apoptotic cells on paraffin sections (4 µm). Photographs of the sections were captured by fluorescence microscopy (Nikon, Japan), and TUNEL-positive cells were counted.
Immunohistochemistry (IHC)
Immunohistochemistry was performed as outlined previously.30 F4/80 antibody was used at a 1:5000 dilution. Three representative photos were randomly captured.
Oil Red O Staining and Immunofluorescence
Fresh liver tissues fixed in 4% paraformaldehyde for 24 hours were transferred to 15% and 30% sucrose solutions for 24 hours at 4 °C. Then, the tissues were embedded in OCT medium and sliced at a thickness of 7 µm. These sections were subjected to oil red O staining and immunofluorescence. Before the liver sections were stained with Oil red O, Oil red O stains A and B were mixed at a 3:2 ratio and filtered to form a working solution. The sections were washed with PBS, prewetted with 60% isopropanol, stained with Oil Red O working solution for 15 minutes and counterstained with haematoxylin for 3 minutes. The liver sections were observed and photographed under a light microscope. Immunofluorescence staining was utilized to assess Nrf2 protein distribution in the liver. Briefly, frozen liver sections were fixed with 4% paraformaldehyde for 10 minutes before permeabilization with 0.3% Triton X-100. The sections were blocked with 5% normal goat serum for one hour and incubated overnight at 4 °C with Nrf2 (1:200) primary antibody. The sections were washed and treated with secondary antibodies in the dark for 2 hours at room temperature. Then, the sections were mounted with an anti-fluorescence quencher containing 6-diamidino-2-phenylindole (DAPI) and imaged with a fluorescence microscope.
Electron Microscopic Analysis
Fresh liver tissues (1 mm ×1 mm ×1 mm) were immersed in 3% buffered glutaraldehyde overnight at 4 °C, and the residual fixative was washed with 0.1 mol/L PBS. The specimens were dehydrated with ethanol and acetone and then embedded in resin. Ultrathin sections were obtained via an ultramicrotome (RMC-PXL, New York, USA), stained with uranium acetate for 15 minutes and lead citrate for 5 minutes, and observed with a transmission electron microscope (Hitachi, H-7500, Japan).
Western Blot Analysis
Western blotting was conducted as described previously.30 The bands were developed with an enhanced chemiluminescence (ECL) substrate (Bio-Rad, USA), and the protein levels were quantified by Image J.
Quantitative Real-Time PCR (qPCR)
Total RNA was extracted from the liver tissues from each group utilizing a TRIzol Kit (Takara, Japan) per the manufacturer’s protocol. Purified RNA (1 μg) was reverse-transcribed into cDNA by a PrimeScriptTM RT Master Mix Kit (Takara, Japan) in a 20 µL reaction volume, and quantitative PCR (qPCR) was directly monitored by a 7500 Fast Real-Time PCR System (ThermoFisher, USA) with a TB GreenTM Premix EX TaqTMIIkit (TaKaRa, Japan). The relative mRNA expression levels were calculated using the 2−ΔΔCT method and quantified relative to β-actin (Table 1).
 |
Table 1 Forward and Reverse Primer Sequences Used for qPCR |
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 8.0 (GraphPad, San Diego, CA, United States), and the results are reported as the means ± standard deviations. The Shapiro‒Wilks test was used to evaluate the normality of the data distribution. Two-way ANOVA was used to make multiple comparisons followed by the post hoc Tukey’s test, and Student’s t-test was used to analyse two groups. Values of P < 0.05 and P < 0.01 were considered statistically significant.
Results
Aerobic Exercise Reduces Body Weight and Fasting Blood Glucose and Improves Glucose and Insulin Tolerance
Body weight, fasting blood glucose, OGTT and ITT results were recorded after 12 weeks of aerobic exercise. The body weights of mice from the MC, ME, DC, and DE groups were 25.34 g ± 1.40, 25.05 g ± 2.06, 62.14 g ± 2.92, and 57.32 g ± 4.43, respectively, which showed that the DC group weighed more than the MC and ME groups (p < 0.01). However, the DE group mice weighed less than the DC group mice (p < 0.05) (Figure 1A). As expected, the FBG in the DC and DE groups was much higher than that in the MC and ME groups (p < 0.01) (Figure 1B). Compared with the DC group, the FBG in the DE group decreased significantly because of exercise training (p < 0.05). Next, we examined the effects of exercise training on metabolic disorders with the OGTT and ITT after 12 weeks of exercise. Both the DC and DE groups exhibited metabolic syndrome (p < 0.01). Strikingly, exercise training obviously ameliorated this situation compared to the DC group (p < 0.05) (Figure 1C and D).
Aerobic Exercise Reduced Liver Function Biochemical Markers and Ameliorated Hepatic Steatosis
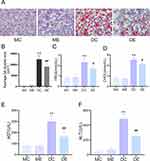
Compared with the m/m mice, hyperglycaemia-induced fat accumulation and liver damage occurred in db/db mice. Histological analyses showed significant hepatocyte ballooning and lipid accumulation in liver slices from the DC group, as revealed by Oil Red O staining (p < 0.01). Conversely, the Oil red O staining area resulting from exercise therapy was significantly reduced to approximately 19.4% of that in the DC group (p < 0.01) (Figure 2A and B). Pathological hepatic lipid accumulation is an obvious characteristic of diabetic liver damage, as shown by an increase in TG and CHOL serum levels (p < 0.01). Exercise training markedly decreased the serum TG and CHOL levels in the DE group compared with the DC group (p < 0.05) (Figure 2C and D). In addition, the AST and ALT levels, as biomarkers for liver function, were distinctly elevated in the DC and DE groups compared with the MC group (p < 0.01). However, the hepatic aminotransferase levels were distinctly lower in the DE group than in the DC group after exercise training (p < 0.01) (Figure 2E and F).
Aerobic Exercise Reverses Oxidative Stress in the Livers of Db/Db Mice
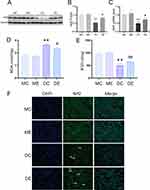
Nrf2 has a wide range of significant hepatoprotective effects. The protein and gene expression of Nrf2 were assessed to explore the possible hepatoprotective mechanisms of aerobic exercise against diabetes. Compared with the MC group, the DC group showed diminished Nrf2 protein and mRNA levels in the liver (p < 0.01). Conversely, aerobic exercise significantly replenished Nrf2 protein and mRNA levels in the DE group compared with the DC group (p < 0.05) (Figure 3A–C). In addition, Nrf2 expression in the liver of the DC group was lower than that in the MC group; this was also proven by weaker green immunofluorescence staining. The DE mouse tissue had significantly stronger positive immunofluorescence compared to the DC mouse tissue (Figure 3F). Next, we measured the levels of MDA and SOD activity. Compared with the MC and ME groups, the MDA levels (p < 0.01) were significantly increased and SOD activity (p < 0.01) was significantly reduced in the livers of the DC group mice. However, after exercise training, the MDA level in the DE group distinctly decreased (p <0.05) and the SOD activity significantly increased (p < 0.01) (Figure 3D and E). Therefore, these results indicated that aerobic exercise protected against diabetes-induced oxidative stress by activating Nrf2.
Aerobic Exercise Improved Diabetes-Induced Liver Apoptosis via the JAK2/STAT3 Pathway
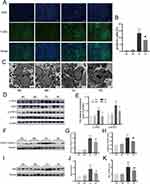
Apoptosis is an essential contributor to liver disease pathogenesis and is related to the JAK2/STAT3 pathway.31 To determine the effect of exercise on hepatic apoptosis, apoptosis staining was performed. Tissue from mice in the MC and ME groups showed very few TUNEL-positive cells. The numbers of TUNEL-positive cells in the liver were substantially higher in the DC group than in the MC group (p < 0.01). At the same time, exercise training diminished the increased number of positive cells in tissue from mice in the DE group (p < 0.01) (Figure 4A and B). The ultrastructure changes of hepatocyte apoptosis were subsequently assessed by transmission electron microscopy. The MC and ME groups clearly had normal hepatocyte structures, including round nuclei, clear karyolemma and round or oval mitochondria. Conversely, the DC group showed marked tissue alterations with hepatocyte nuclear pyknosis, nuclear chromatin condensation and marginalization, and obvious mitochondrial swelling. Aerobic exercise treatment ameliorated hepatic damage, as evidenced by the distinct hepatocyte nucleus and mitochondrial morphology changes (Figure 4C). In addition, Western blotting results indicated that diabetic disease caused hepatocyte apoptosis via activation of p-JAK2, p-STAT, cleaved caspase-3 and Bax (p < 0.01). However, aerobic exercise reversed the protein expression in the DE group (p < 0.05) (Figure 4D–G, I and J). In parallel, q-PCR analysis suggested that the levels Bax and cleaved caspase-3 that were increased by diabetic disease were reduced after exercise treatment (p < 0.01), consistent with the Western blotting results (Figure 4H and K). Therefore, diabetic hepatocyte apoptosis is attenuated by exercise training.
Aerobic Exercise Decreased Diabetes-Induced Liver Inflammation
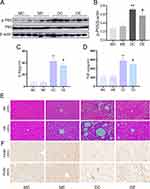
The NF-κB pathway has been reported to regulate the production of various proinflammatory indicators. NF-κB expression was investigated in the liver via Western blot analysis. A substantial increase in NF-κB p-P65 expression was observed in the DC group compared with the MC group (p < 0.01). Aerobic exercise suppressed NF-κB p-P65 expression in the DE group (p < 0.05) (Figure 5A and B). Furthermore, serum inflammatory cytokines, including IL-6 and TNF-α, appeared at significantly higher levels in the DC group (p < 0.01), and aerobic exercise also suppressed the overexpression of these cytokines (p < 0.05) (Figure 5C and D), which support the anti-inflammatory effects of aerobic exercise. Moreover, HE staining was performed to investigate pathological changes in the liver tissue. The DC group exhibited significant lipid accumulation, hepatocyte swelling and inflammatory cell infiltration compared with the normal liver histological structure of the MC and ME groups, and these changes were significantly attenuated by exercise training (Figure 5E). Compared to the MC group, the expression of F4/80 in the liver tissue of the DC group was significantly increased, but in the DE group, aerobic exercise effectively reduced the expression of F4/80 (Figure 5F). Therefore, aerobic exercise alleviated the diabetes-induced liver inflammation response in db/db mice.
Discussion
Published evidence has shown that exercise has a protective effect on the liver,17–21 but the concrete molecular mechanisms have not been fully studied. In this study, we analysed the protective potential of aerobic exercise on the progression of liver injury in T2DM associated with the reduction in serum AST, ALT, TG, and CHOL levels and the mitigation of liver oxidative stress, apoptosis and inflammation in db/db mice. In addition, exercise also corrected low Nrf2 expression in the liver.
Physical activity, considered a strategy to ameliorate glycaemia and reduce weight, hepatic lipids, and insulin resistance, should be encouraged in human T2DM treatment.32 Chronic hyperglycaemia is the central characteristic of diabetes, leading to oxidative stress and low-grade chronic inflammation, which are involved in diabetic liver damage. Therefore, controlling the blood glucose level is essential. This study showed that maintaining exercise training in db/db mice contributed to reducing body weight and serum glucose levels, indicating the favourable effects of exercise on metabolism in T2DM. Glucose tolerance represents the body’s ability to consume blood sugar. Owing to the devastation of beta cells, insulin deficiency prevents the cells from obtaining glucose for energy, resulting in hyperglycaemia. Previous evidence showed that swim exercise training improved hepatic insulin sensitivity.33 Herein, it is indicated that long-term exercise ameliorated glucose tolerance, consistent with Botezelli et al.34 Diabetes and chronic liver disease are universal metabolic diseases and may coexist for a long time. Hepatic aminotransferases such as AST and ALT in the bloodstream provide direct evidence of liver dysfunction.35 In this study, db/db mice showed a notable increase in serum ALT and AST levels, suggesting liver dysfunction. In addition, we also observed a notable elevation in CHOL and TG levels. However, exercise treatment significantly alleviated liver steatosis and histopathological lesions and decreased serum ALT, AST, CHOL, and TG levels, consistent with previous results.36 Previous studies have suggested that reasonable exercise could also perform its action by decreasing key enzymes of carbohydrate response element-binding protein (ChREBP), such as acetyl-CoA carboxylase and fatty acid synthesis, to recover lipid metabolism in the liver.37,38 In addition, aerobic exercise could prevent diabetes by reducing haemoglobin A1C (A1C) levels, increasing peak oxygen consumption (VO2peak)39 and significantly improving endothelial function,27 cognitive impairment40 and endotoxaemia caused by diabetes.41
Mounting evidence indicates that oxidative stress and subsequent inflammation are strictly related to liver diseases in diabetes,42,43 and the initial cellular reaction to high glucose generates mitochondrial reactive oxygen species (ROS), which rapidly induce apoptotic cell death.44 Nrf2 is a key conditioner for protection against oxidative stress-related damage. Under physiological conditions, Nrf2 is sequestered in the cytosol by elch-like ECH-associated protein 1 (Keap1) until activated by oxidative stimuli and electrophiles and binds to antioxidant response elements (AREs) to initiate the protective mechanism of downstream signalling molecules.45 Damaged Nrf2 signalling in diabetic liver conditions has been reported.46 Due to its anti-inflammatory and antioxidant features, Nrf2 activation offers protection against liver injury produced by various chemicals.47 In vivo and vitro evidence has shown that sulforaphane, as an Nrf2 activator, reduces glucose production in hepatic cells, which results from the nuclear translocation of NRF2 and decreased levels of key enzymes in gluconeogenesis.48 Conversely, Nrf2 knockout increased basal apoptosis in mice, resulting in mitochondrial dysfunction.49 Additionally, decreased levels of Nrf2 were found in HFD-fed tilapia,50 as well as in diabetic mice and human liver tissues.51 In agreement with these results, Nrf2 expression was inhibited in db/db mouse livers, which was reversed after exercise training, implying that exercise exerted antioxidant effects in the diabetic liver by activating Nrf2. In addition, we measured the relative levels of oxidative stress markers (MDA, SOD) in liver tissue. We found that diabetic db/db mice showed an increase in MDA levels and a decrease in SOD activity compared to m/m mice, which was consistent with a previous study.52 However, aerobic exercise effectively protected against these effects of oxidative stress in diabetes. Taken together, these results suggest that aerobic exercise significantly improved diabetic liver injury due to its antioxidant stress effects.
In addition, less TUNEL positivity was observed after exercise training in HFD-fed rats,53 signifying that exercise could defend hepatocytes from hyperglycaemia-induced apoptosis. Excessive ROS levels induce mitochondrial DNA damage and cell death by activating several apoptosis proteins, such as Bax, caspase-3, and cytochrome c. Thus, inhibiting Bax and caspase-3 activation could delay apoptosis.15,53 This study showed that exercise downregulated Bax and cleaved caspase 3 expression, and the results of TUNEL staining were consistent. Therefore, regular aerobic exercise inhibited apoptosis and effectively decreased liver damage.
Unusual activation of the JAK2/STAT3 pathway may be correlated with metabolic diseases such as T2DM.54 Oxidative stress and proinflammatory cytokines stimulate STAT3 activation through a JAK2-dependent mechanism, leading to apoptosis and chronic inflammation.55 Notably, JAK2/STAT3 is the downstream pathway of Nrf2 signalling, and JAK2/STAT3 pathway blockade could enhance insulin sensitivity and protect the liver from fibrosis and inflammation, as extensive studies have shown.56,57 In this study, hyperglycaemia activated the JAK2/STAT3 signalling pathway in db/db mouse livers. Reportedly, downregulation of the JAK2/STAT3 pathway inhibited apoptosis by inhibiting the expression of caspase-3 and correcting the Bcl2/Bax ratio.58 Similarly, it was observed that exercise reduced the phosphorylation of JAK2 and STAT3, which occurred in parallel with caspase 3 and Bax changes, suggesting that the JAK2/STAT3 pathway may participate in liver apoptosis. Therefore, exercise partly inhibits liver apoptosis by downregulating the JAK2/STAT3 signalling pathway.
Another key factor in liver cell damage is the excessive release of proinflammatory cytokines, and the JAK2/STAT3 pathway plays an important role in this process.30 Shan et al59 have shown that exercise preconditioning could reduce neuronal apoptosis and inflammation by inhibiting the JAK2/STAT3 signalling pathway, thus attenuating cerebral ischaemia‒reperfusion injury. Consistent with their findings, our experimental results show that aerobic exercise reduced the phosphorylation of JAK2 and STAT3. We also found that aerobic exercise repressed the release of the proinflammatory cytokines IL-6 and TNF-α in serum and the expression of F4/80 in the livers of diabetic mice. Previous reports have confirmed that high-intensity intermittent exercise could improve inflammation by decreasing the total macrophage markers F4/80 and TNF-α and IL-6 levels in the liver in T2DM mice.60 Liu’s team found a similar phenomenon in the muscle tissue of diabetic db/db mice after eight weeks of exercise.61 In a previous study, we confirmed that aerobic exercise improves cognitive impairment in T2DM mice by inhibiting the JAK2/STAT3 signalling pathway.27 Therefore, the protective effect of aerobic exercise on the liver may depend on the inhibition of the JAK2/STAT3 signalling pathway. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is activated by oxidative stress in diabetes, which promotes the transcription of proinflammatory cytokines. Furthermore, recent evidence revealed that Nrf2 reduction and deficiency enhanced NF-κB-mediated inflammation and exacerbated histological changes in the liver.58 In the present study, diabetes caused profound enhancement of NF-κB p-P65 in the liver, but this effect was reversed by exercise training. Consistent with other results,32 obesity-related liver lipogenesis and inflammation were reduced by short-term strength training, thereby improving hepatic gluconeogenesis and insulin sensitivity. Similarly, the current histopathological evidence suggests that diabetes intensifies structural and functional damage to the liver and inflammatory cell infiltration. However, aerobic exercise protected the liver from inflammation-related injury. Therefore, JAK2/STAT3 signalling pathway and NF-κB modulation participated in the anti-inflammatory effect of aerobic exercise.
Conclusions
Taken together, this study demonstrates that exercise intervention could protect against diabetic liver injury by activating Nrf2 and inhibiting oxidant responses, inflammation, and apoptosis. Additionally, JAK2/STAT3 signals are an indispensable molecular basis in the protective effect of aerobic exercise. Therefore, aerobic exercise has therapeutic potential in the management of diabetic liver damage (Figure 6).
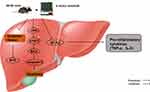 |
Figure 6 Protective mechanisms of aerobic exercise improving liver inflammation, oxidative stress, and apoptosis. |
Institutional Review Board Statement
This study was approved by the Ethics Committee on the use of animal of Weifang Medical University (2021SDL064).
Data Sharing Statement
All data generated and analysed during this study are included in this published article.
Acknowledgments
We are grateful to the Laboratory of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital of Wenzhou Medical University and Medicine and Health Key Laboratory of Clinical Anesthesia, School of Anesthesiology, Weifang Medical University, for technical support.
Funding
This research was funded by the Postgraduate Scientific Research Project of Higher Education Institutions of Anhui Province (YJS20210276); the Shandong Province Medical and Health Science and Technology Development Plan Project Fund (202104110334, 202104010549).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–S113. doi:10.2337/diabetes.54.suppl_2.S108
2. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi:10.1002/hep.20920
3. Manna P, Das J, Ghosh J, Sil PC. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radic Biol Med. 2010;48(11):1465–1484. doi:10.1016/j.freeradbiomed.2010.02.025
4. Bae CS, Lee Y, Ahn T. Therapeutic treatments for diabetes mellitus-induced liver injury by regulating oxidative stress and inflammation. Appl Microsc. 2023;53(1):4. doi:10.1186/s42649-023-00089-2
5. Tiercelin C, Lemoine AY, Ratheau L, Larger E. High frequency of transaminase elevation following diabetic ketoacidosis. Diabetes Metab. 2021;47(1):101123. doi:10.1016/j.diabet.2019.09.001
6. Torbenson M, Chen YY, Brunt E, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30(4):508–513. doi:10.1097/00000478-200604000-00012
7. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. doi:10.1038/cdd.2014.150
8. Peng ML, Fu Y, Wu CW, Zhang Y, Ren H, Zhou SS. Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front Endocrinol. 2022;13:907757. doi:10.3389/fendo.2022.907757
9. Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42(4):585–591. doi:10.1016/j.jhep.2004.11.040
10. Zhang L, Li HX, Pan WS, et al. Administration of methyl palmitate prevents non-alcoholic steatohepatitis (NASH) by induction of PPAR-α. Biomed Pharmacother. 2019;111:99–108. doi:10.1016/j.biopha.2018.12.059
11. Stancic A, Velickovic K, Markelic M, et al. Involvement of ferroptosis in diabetes-induced liver pathology. Int J Mol Sci. 2022;23(16):2–3. doi:10.3390/ijms23169309
12. Zhang Q, Zhao Y, Talukder M, et al. Di(2-ethylhexyl) phthalate induced hepatotoxicity in quail (Coturnix japonica) via modulating the mitochondrial unfolded protein response and NRF2 mediated antioxidant defense. Sci Total Environ. 2019;651:885–894. doi:10.1016/j.scitotenv.2018.09.211
13. Tian S, Zhao H, Song H. Shared signaling pathways and targeted therapy by natural bioactive compounds for obesity and type 2 diabetes. Crit Rev Food Sci Nutr. 2022;3:1–18.
14. Sireesh D, Ganesh M-R, Dhamodharan U, et al. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J Nutr Biochem. 2017;44:11–21. doi:10.1016/j.jnutbio.2017.02.015
15. Feng Y, Cui R, Li Z, et al. Methane alleviates acetaminophen-induced liver injury by inhibiting inflammation, oxidative stress, endoplasmic reticulum stress, and apoptosis through the Nrf2/HO-1/NQO1 signaling pathway. Oxid Med Cell Longev. 2019;2019:7067619. doi:10.1155/2019/7067619
16. Shaw P, Mondal P, Bandyopadhyay A, Chattopadhyay A. Environmentally relevant concentration of chromium activates Nrf2 and alters transcription of related XME genes in liver of zebrafish. Chemosphere. 2019;214:35–46. doi:10.1016/j.chemosphere.2018.09.104
17. Lima TI, Monteiro IC, Valença S, et al. Effect of exercise training on liver antioxidant enzymes in STZ-diabetic rats. Life Sci. 2015;128:64–71. doi:10.1016/j.lfs.2015.01.031
18. Liu SX, Zheng F, Xie KL, Xie MR, Jiang LJ, Cai Y. Exercise reduces insulin resistance in type 2 diabetes mellitus via mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol Ther Nucleic Acids. 2019;18:34–44. doi:10.1016/j.omtn.2019.08.002
19. Fealy CE, Haus JM, Solomon TP, et al. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol. 2012;113(1):1–6. doi:10.1152/japplphysiol.00127.2012
20. Gregory JM, Muldowney JA, Engelhardt BG, et al. Aerobic exercise training improves hepatic and muscle insulin sensitivity, but reduces splanchnic glucose uptake in obese humans with type 2 diabetes. Nutr Diabetes. 2019;9(1):25. doi:10.1038/s41387-019-0090-0
21. Fathi R, Nasiri K, Akbari A, Ahmadi-KaniGolzar F, Farajtabar Z. Exercise protects against ethanol-induced damage in rat heart and liver through the inhibition of apoptosis and activation of Nrf2/Keap-1/HO-1 pathway. Life Sci. 2020;256:117958. doi:10.1016/j.lfs.2020.117958
22. O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66(1):311–328. doi:10.1146/annurev-med-051113-024537
23. Zhao J, Liu X, Chen Y, et al. STAT3 promotes schistosome-induced liver injury by inflammation, oxidative stress, proliferation, and apoptosis signal pathway. Infect Immun. 2021;2021:89.
24. Zhang H, Li H, Ge A, Guo E, Liu S, Zhang L. RETRACTED: long non-coding RNA TUG1 inhibits apoptosis and inflammatory response in LPS-treated H9c2 cells by down-regulation of miR-29b. Biomed Pharmacother. 2018;101:663–669. doi:10.1016/j.biopha.2018.02.129
25. Wang Y, Li H, Huang H, et al. Cardioprotection from emulsified isoflurane postconditioning is lost in rats with streptozotocin-induced diabetes due to the impairment of Brg1/Nrf2/STAT3 signalling. Clin Sci. 2016;130(10):801–812. doi:10.1042/CS20150617
26. Zhong T, Li M, Wu H, et al. Novel Flavan-3,4-diol vernicidin B from Toxicodendron Vernicifluum (Anacardiaceae) as potent antioxidant via IL-6/Nrf2 cross-talks pathways. Phytomedicine. 2022;100:154041. doi:10.1016/j.phymed.2022.154041
27. Wang J, Polaki V, Chen S, Bihl JC, Pagliaro P. Exercise improves endothelial function associated with alleviated inflammation and oxidative stress of perivascular adipose tissue in type 2 diabetic mice. Oxid Med Cell Longev. 2020;2020:8830537. doi:10.1155/2020/8830537
28. Ma Y, Wang L, Yang S, et al. The tissue origin of human mesenchymal stem cells dictates their therapeutic efficacy on glucose and lipid metabolic disorders in type II diabetic mice. Stem Cell Res Ther. 2021;12(1):385. doi:10.1186/s13287-021-02463-x
29. Xu L, Yu Y, Sang R, Li J, Ge B, Zhang X. Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-kappaB signaling pathways in mice. Oxid Med Cell Longev. 2018;2018:8284107. doi:10.1155/2018/8284107
30. Xiong Y, Wang Y, Xiong Y, Teng L. Protective effect of Salidroside on hypoxia-related liver oxidative stress and inflammation via Nrf2 and JAK2/STAT3 signaling pathways. Food Sci Nutr. 2021;9(9):5060–5069. doi:10.1002/fsn3.2459
31. Qaed E, Wang J, Almoiliqy M, et al. Phosphocreatine improves cardiac dysfunction by normalizing mitochondrial respiratory function through JAK2/STAT3 signaling pathway in vivo and in vitro. Oxid Med Cell Longev. 2019;2019:6521218. doi:10.1155/2019/6521218
32. Pereira RM, Rodrigues K, Anaruma CP, et al. Short-term strength training reduces gluconeogenesis and NAFLD in obese mice. J Endocrinol. 2019;241(1):59–70. doi:10.1530/JOE-18-0567
33. Yang Z, Huang G, Zhou P, Zhang Y, Ding J, Sun Q. Hua T: exercise ameliorates high-fat diet-induced insulin resistance accompanied by changes in protein levels of hepatic ATF3-related signaling in rats. Physiol Behav. 2022;249:113766. doi:10.1016/j.physbeh.2022.113766
34. Botezelli JD, Coope A, Ghezzi AC, et al. Strength training prevents hyperinsulinemia, insulin resistance, and inflammation independent of weight loss in fructose-fed animals. Sci Rep. 2016;6(1):31106. doi:10.1038/srep31106
35. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Cmaj. 2005;172(3):367–379. doi:10.1503/cmaj.1040752
36. Sabag A, Way KL, Sultana RN, et al. The effect of a novel low-volume aerobic exercise intervention on liver fat in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(10):2371–2378. doi:10.2337/dc19-2523
37. Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in otsuka long-Evans Tokushima fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–G626. doi:10.1152/ajpgi.00428.2007
38. Zhang Y, Gu M, Wang R, Li M, Li D, Xie Z. Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6 J mice. Nutr Metab. 2020;17(1):14. doi:10.1186/s12986-020-0433-9
39. Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M. Exercise and type 2 diabetes. Adv Exp Med Biol. 2020;1228:91–105.
40. Lin L, Wang Y, Xu W, et al. Aerobic exercise improves type 2 diabetes mellitus-related cognitive impairment by inhibiting JAK2/STAT3 and enhancing AMPK/SIRT1 pathways in mice. Dis Markers. 2022;2022:6010504. doi:10.1155/2022/6010504
41. Motiani KK, Collado MC, Eskelinen JJ, et al. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc. 2020;52(1):94–104. doi:10.1249/MSS.0000000000002112
42. Chandirasegaran G, Elanchezhiyan C, Ghosh K. Effects of Berberine chloride on the liver of streptozotocin-induced diabetes in albino Wistar rats. Biomed Pharmacother. 2018;99:227–236. doi:10.1016/j.biopha.2018.01.007
43. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–743. doi:10.2337/dc06-1539
44. Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653–2658. doi:10.1073/pnas.0511154103
45. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi:10.1016/j.freeradbiomed.2009.07.035
46. Qi X, Lu XT, Sun XH, Lin CQ, Cui CB. The regulatory effect of total flavonoids of Sedum aizoon L. on oxidative stress in type 1 diabetic mice. Curr Res Food Sci. 2022;5:1140–1147. doi:10.1016/j.crfs.2022.06.010
47. Liu J, Wu KC, Lu YF, Ekuase E, Klaassen CD. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev. 2013;2013:305861. doi:10.1155/2013/305861
48. Axelsson AS, Tubbs E, Mecham B, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9(394):eaah4477.
49. Ariza J, González-Reyes JA, Jódar L, Díaz-Ruiz A, de Cabo R, Villalba JM. Mitochondrial permeabilization without caspase activation mediates the increase of basal apoptosis in cells lacking Nrf2. Free Radic Biol Med. 2016;95:82–95. doi:10.1016/j.freeradbiomed.2016.03.015
50. Jia R, Cao LP, Du JL, et al. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish Immunol. 2020;104:391–401. doi:10.1016/j.fsi.2020.06.025
51. Gokila Vani M, Kumar KJ, Liao JW, et al. Antcin C from antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid Based Complement Alternat Med. 2013;2013:296082.
52. Song JX, An JR, Chen Q, et al. Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered. 2022;13(4):8334–8348. doi:10.1080/21655979.2022.2051858
53. Chen X, Li H, Wang K, et al. Aerobic exercise ameliorates myocardial inflammation, fibrosis and apoptosis in high-fat-diet rats by inhibiting P2X7 purinergic receptors. Front Physiol. 2019;10:1286. doi:10.3389/fphys.2019.01286
54. Yang Y, Zhao J, Song X, et al. Amygdalin reduces lipopolysaccharide-induced chronic liver injury in rats by down-regulating PI3K/AKT, JAK2/STAT3 and NF-κB signalling pathways. Artif Cells Nanomed Biotechnol. 2019;47(1):2688–2697. doi:10.1080/21691401.2019.1634084
55. Zhou GY, Yi YX, Jin LX, et al. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother. 2016;81:318–328. doi:10.1016/j.biopha.2016.04.013
56. El-Nasr NME A, Saleh DO, Mahmoud SS, et al. Olmesartan attenuates type 2 diabetes-associated liver injury: cross-talk of AGE/RAGE/JNK, STAT3/SCOS3 and RAS signaling pathways. Eur J Pharmacol. 2020;874:173010. doi:10.1016/j.ejphar.2020.173010
57. Lu C, Xu W, Shao J, Zhang F, Chen A, Zheng S. Nrf2 induces lipocyte phenotype via a SOCS3-dependent negative feedback loop on JAK2/STAT3 signaling in hepatic stellate cells. Int Immunopharmacol. 2017;49:203–211. doi:10.1016/j.intimp.2017.06.001
58. Li J, Sapper TN, Mah E, et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol Nutr Food Res. 2016;60(4):858–870. doi:10.1002/mnfr.201500814
59. Shan Y, Wang L, Sun J, Chang S, Di W, Lv H. Exercise preconditioning attenuates cerebral ischemia-induced neuronal apoptosis, Th17/Treg imbalance, and inflammation in rats by inhibiting the JAK2/STAT3 pathway. Brain Behav. 2023;13(6):e3030. doi:10.1002/brb3.3030
60. Wang Y, Guo Y, Xu Y, et al. HIIT ameliorates inflammation and lipid metabolism by regulating macrophage polarization and mitochondrial dynamics in the liver of type 2 diabetes mellitus mice. Metabolites. 2022;13(1):13. doi:10.3390/metabo13010013
61. Liu HW, Chang SJ. Moderate exercise suppresses NF-kappaB signaling and activates the SIRT1-AMPK-PGC1alpha axis to attenuate muscle loss in diabetic db/db mice. Front Physiol. 2018;9:636. doi:10.3389/fphys.2018.00636
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.