Back to Journals » Infection and Drug Resistance » Volume 16
Advancing Microbe Detection for Lower Respiratory Tract Infection Diagnosis and Management with Metagenomic Next-Generation Sequencing
Authors Dong Y, Chen Q, Tian B, Li J, Li J, Hu Z
Received 5 September 2022
Accepted for publication 2 January 2023
Published 30 January 2023 Volume 2023:16 Pages 677—694
DOI https://doi.org/10.2147/IDR.S387134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yulan Dong, Qianqian Chen, Bin Tian, Jing Li, Jin Li, Zhidong Hu
Department of Clinical Laboratory, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China
Correspondence: Zhidong Hu, Department of Clinical Laboratory, Tianjin Medical University General Hospital, No. 154 Anshan Road, Heping District, Tianjin, People’s Republic of China, Tel +86 022-60814202, Email [email protected]
Background: Due to limitations of traditional microbiological methods and the presence of the oropharyngeal normal flora, there are still many pathogens that cause lower respiratory tract infections (LRTIs) cannot be detected. Metagenomic next-generation sequencing (mNGS) has the potential capacity to solve this problem.
Methods: This retrospective study successively reviewed 77 patients with LRTI and 29 patients without LRTI admitted to Tianjin Medical University General Hospital, China from August 2020 to June 2021. Pathogens in bronchoalveolar lavage fluid (BALF) specimens were detected adopting mNGS and traditional microbiological assays. The diagnostic performance of pathogens was compared between mNGS and BALF culture. The value of mNGS for aetiological and clinical impact investigation in LRTI was also evaluated.
Results: Among 77 patients with LRTI, 22.1%, 40.3%, and 65.0% of cases were detected as definite or probable pathogens by culture, all conventional microbiological tests, and mNGS, respectively. Using the final diagnosis as a gold standard, mNGS exhibited a sensitivity of 76.6% (95% confidence interval [CI], 65.6– 85.5%), which was considerably superior to that of BALF culture (76.6% vs 18.2%; P < 0.01); specificity of 79.3% (95% CI, 60.3– 92.0%), which was similar (79.3% vs 89.7%; P = 0.38); positive-predictive value of 90.8% (95% CI, 81.0– 96.5%), and negative-predictive value of 56.1% (95% CI, 39.7– 71.5%). According to our data, mNGS identified potential microorganisms in 66.7% (42/63) of culture-negative samples. Among 59 patients with pathogens identified by mNGS, conventional microbiological methods confirmed pathogenic infections in less than half (28/59) cases. Within the 77 patients, 34 (44.2%) patients received pathogen-directed therapy, 7 (9.1%) patients underwent antibiotic adjustment, and 3 (3.9%) patients stopped using antibiotics due to mNGS results.
Conclusion: mNGS exhibits high accuracy in diagnosing LRTI, and combine with traditional microbiological tests, causative pathogens can be detected in approximately 70.0% of cases, thus yields a positive effect on antibiotic application.
Keywords: LRTIs, mNGS, pathogens, culture, diagnosis, infection
Background
Lower respiratory tract infections (LRTIs) have high morbidity and mortality rates around the world. Approximately 2.38 million deaths resulted from LRTIs in 2016.1 A wide variety of pathogens, comprising bacteria, fungi, viruses, and parasites, alone or concurrently can cause LRTIs. Thus, rapid and accurate pathogen identification has become a crucial component of the aetiological diagnosis and appropriate treatment of LRTIs.
The clinical features between LRTIs and noninfectious inflammatory conditions are commonly overlapped, in lack of a definitive microbiological diagnosis, clinicians may assume that symptoms from a noninfectious inflammatory disease and launch empiric corticosteroids as necessary, which may aggravate the possibility of an opportunistic infection.2 Furthermore, even with no positive-supported microbiological testing results, physicians often keep on previous empiric antibiotics due to concerns of falsely negative results, a practice that speeds up the appearance of antibiotic resistance and contributes to susceptibility to Clostridium difficile infection.3
For decades, we have relied on the conventional microbiology methods such as microbial cultures, histopathology, microscopic smears, polymerase chain reactions (PCR), and serological antibody testing to determine the relationship between the potential infectious microorganisms and infectious diseases. Amid the complex background of the respiratory tract commensal microbiota and given the disadvantages of the current pathogenic diagnostic methods, a rapid and accurate alternative method is urgently needed. Metagenomic next-generation sequencing (mNGS) emerging as a promising technique for agent detection has rapidly shifted from basic research to clinical laboratories. The clinical application of metagenomics landmarks the revolutionary introduction of this pathogen-diagnostic technique, enabling the detection of a wide array of pathogens involving culture-independent, variant, rare, atypical, resistant, fastidious and previously undiscovered pathogens. It has demonstrated its ability to precisely detect rare pathogens when traditional methods fail and has provided new insights into the detection of unknown pathogens.2,4 However, reports on the application of mNGS combined with traditional methods remain scarce. In addition, mNGS furthers the concept of “precision diagnosis and treatment”, as it can be used for multidisciplinary infection diagnosis and management.5–7 However, to date, debate about the wax and wane of mNGS to what extent provide clinical benefits and controversy about the clinical value of mNGS in disease management still exists.8,9
Therefore, in our study, we first used mNGS technology to identify pathogens from patient BALF samples and compared the results to those obtained by traditional microbiological techniques, and then evaluated the diagnostic application and performance of mNGS and conventional culture approach. The efficacy of BALF mNGS in LRTIs was verified by comparing the results of mNGS, combined microbiological tests, and a composite reference standard. Finally, we assessed the emerging and progressive methods in patient diagnosis and management so as to better understand and deal with these infections.
Materials and Methods
Study Patients
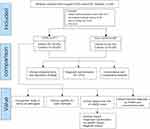
We retrospectively reviewed 122 patients suspected of having acute or chronic LRTIs who were admitted to Tianjin Medical University General Hospital, China from August 2020 to June 2021. Using our inclusion/ exclusion criteria (Figure 1), a total of 106 patients, including 46 males and 60 females, with an average age of 56 years (20–83 years) were enrolled for further detailed and elaborated assays and categorized into 2 groups defined as lower respiratory tract infections (LRTIs), non-Lower Respiratory Tract Infections (Non-LRTIs) according to a composite reference standard (final clinical diagnosis), including clinical signs and symptoms, microbiological evidences, imaging findings, together with clinical adjudication. All enrolled patients’ BALF samples were analysed using mNGS as well as by microbial culture in a pairwise manner. The comparative study between mNGS and traditional culture methodology is showed in Figure 1. At the same time, patients underwent other relevant examinations according to disease status. For traditional pathogen detection methods based on culture, microbes that are present in the normal flora of the skin or respiratory tract were not interpreted as pathogens. All procedures were carried out by well-trained physicians, and the study was approved by the Ethics Review Committee of Tianjin Medical University General Hospital. The study was performed in conformity with the relevant guidelines and regulations outlined in the Declaration of Helsinki.
The Process of Bronchoscopy and BALF Collection
Before bronchoalveolar lavage operation, routine clinical status assessment should be carried out to exclude bleeding and other risks, and the indications and contraindications of bronchoscopy should be strictly compared. Electrocardiograph and pulse oxygen saturation (SpO2) should be routinely monitored during the operation. Trained physicians used the virtual bronchoscope navigation system, endobronchial ultrasound system, and computed tomography imaging to precisely locate the site of the lesion according to a series of standard procedures. 1 ~ 2 mL 2% lidocaine was injected into the lavage lung segment through the biopsy hole, and local anesthesia was performed in the lavage lung segment. Then, the diseased site was rinsed several times with 37 °C or room temperature sterile saline, which was then recycled by appropriate negative pressure suction to obtain BALF with the aid of routine bronchoscopy or ultrathin bronchoscopy (Olympus, Tokyo, Japan). In order to identify possible contamination during the DNA extraction process from environmental sources or reagents, we collected saline control samples after injection through the bronchoscope using a sterile syringe. On account of the amount of DNA extracted from blank is extremely small, which is not sufficient for construction of sequencing library, the gathered saline control samples were then mixed with THP-1 cells for DNA extraction and nominated negative control 1. Equipped genomic DNA extracted from HeLa cells was designated as negative control 2 to determine potential contamination in the process of sequencing library construction. Standards are used as positive quality control, which is set to ensure that the experimental method is reliable and the reagent performance is excellent. These control samples were handled in parallel with the patient’s specimen. If the patient’s condition permitted, six to ten pieces of lung tissue were subsequently acquired for the histopathology examination. A portion of each specimen were sent to the clinical microbiology laboratory for traditional microbiology tests, while the remaining BALF samples were collected and stored at 4°C for mNGS analysis.
Traditional Microbiology Culture and Identification Methods
The conventional microbiology tests were performed using sputum and/or BALF samples culture of the bacteria or fungi in Columbia blood agar plates, Mac-Conkey agar plates, chocolate blood agar plates and anaerobic medium as necessary or in Sabouraud agar plates at 35 °C for a maximum period of 5 days. Bacterial or fungal identification was conducted by using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (bioMe´rieux, France) and complemented by the VITEK-II Compact automated microbiological system (bioMe´rieux, France). Using a plastic or wooden stick, a pure-pathogen growth colony was selected from a culture plate to be placed on a MALDI-TOF MS objective plate. One or many isolates were able to be tested at a time. The spot was overlaid with 1–2 μL of matrix and dried. The plate was deposited in the ionization chamber of the mass spectrometer. Charged particles were then separated in a high voltage field, and the time of flight of the particles was measured. Microbe identification was attained via the comparison of the obtained mass spectra with a reference database by the software.10 If no organisms were matched after comparison against the database of mass spectra by the Myla software, instead the pure colony was prepared to an appropriate turbidity, and then the microbe was identified using the VITEK-II Compact automated microbiological system.
Other Conventional Laboratory-Based Pathogen Detection Testing
Other conventional laboratory-based pathogen detection testing like sputum smear Gram staining, tracheoscope brush smear Gram staining, BALF smear Gram staining, galactomannan (GM) test of serum and/or BALF, 1-3-β-D-glucan test, Aspergillus antibody test, and Cryptococcus capsular polysaccharide antigen (CrAg) test were measured for patients with consideration of fungal infection. Besides, acid-resistant staining, enzyme-linked immunospot assay (T-SPOT), BALF Xpert Mycobacterium tuberculosis /Rifampicin resistance (Xpert) assay, and tuberculosis antibody testing were performed only for patients with highly suspected tuberculosis. Chlamydia pneumoniae, Mycoplasma pneumoniae, Coxiella burnetii, Legionella pneumophila, adenovirus, respiratory syncytial virus, influenza A and B, parainfluenza viruses, human cytomegalovirus (CMV), Epstein–Barr virus (EBV) and so on serological antibody detection were executed only for patients with highly suspected atypical agent infection or viral infection. Lung biopsy specimens were sent to the histopathology laboratory for examination and according to requirement to process with KOH testing, Ziehl-Neelsen acid-fast, hematoxylin and eosin, and hexamine silver staining by smear microscopy.
mNGS Methodology
DNA Extraction
After 5–10 mL BALF was collected from patients and delivered to the commercial laboratory, a 0.5 mL specimen together with 1 g 0.5 mm magnetic beads were added to an autoclaved 1.5-mL microcentrifuge tube, which was then attached to a horizontal platform on a vortex mixer and blended vigorously at 2800–3200 rpm for half an hour. After agitation, 300μL of the processed sample was separated and transferred into another new sterile 1.5-mL microcentrifuge tube, and the Omega Biotech Mag-Bind® Universal Pathogen 96 kit (M4029, Omega Bio-Tek, Inc., USA) was used to extract the DNA from the BALF as per the manufacturer’s recommendation. Then by using a Qubit dsDNA assay kit (Life Technologies, USA), the concentration and quality of extracted DNA were determined. The extracted DNA was stored at −80°C until further operation.
Metagenome Sequencing
One microgram of the above extracted DNA per sample was used as an input material for sequencing library construction using a NexteraXT DNA sample preparation kit (Illumina, USA). After a succession of operations, such as DNA fragmentation was executed using endonuclease, DNA end-repair was performed by means of DNA Polymerase and Polynucleotide Kinase, splice connection was operated using DNA Ligase and PCR enrichment was conducted using high-fidelity DNA Polymerase, the DNA library was successfully constructed. At the same time, negative control 2 (prepared genomic DNA from HeLa cells) was used as to identify the possible contamination. After quality assessment, the libraries were sequenced on the ILLUMINASEQ sequencing platform, and paired-end raw reads were produced with a read length of 150 bp.
Data Processing and Bioinformatic Analysis
After obtaining raw sequencing data, high-quality sequencing data were generated by filtering out adapter contamination, repeated reads, low-quality reads (refers to the basic characteristics that do not meet the following indexes: Q30 base quantity ratio >80%, joint contamination ratio no more than 1%, effective sequence length no less than 50 bp, effective comparison rate of data should be greater than 70%) using fastp 0.19.511 (–detect_adapter_for_pe -W 4 -q 15 -u 40) with other parameters keeping as default settings. Fastqc 0.11.512 with default settings was used to assess the quality of processed reads. Bowtie tool13 was used to map to the reference human genome (GRCh38) to identify human sequence data, and matching reads were removed. Summary of metagenomic sequencing data was provided in Additional Tables.
Taxonomic Classification
Taxonomy of the remaining reads was classified using kraken2 (–paired) with kraken2 bacterial and archaeal databases and default settings.14
Criteria for a Positive mNGS Result
Tests results for the infectious microbes (involving bacteria, fungi or viruses) were considered positive if they met any of the following thresholds in mNGS: (i) culture and/or histopathological examination indicated positivity for bacteria, viruses or fungi, where invasive fungal disease was defined according to the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) criteria;15 (ii) at least 50 unique reads from a single species of bacteria, viruses or fungi; for pathogen with unique reads less than 50, because of the difficulty of wall-breaking and DNA extraction especially for Nocardia and fungi could still be diagnosed as infectious pathogens when the clinical situation was consistent; (iii) Mycobacteria: Mycobacterium tuberculosis (MTB) was considered positive when at least one unique read was mapped to either the species or genus level due to the difficulty of DNA extraction and low possibility for contamination;8,16 (iv) Nontuberculous mycobacteria (NTM) were defined as positive while the mapping read number (genus or species level) was in the top 10 in the bacteria list because of the low yield rate17 and the balance of hospital-to-laboratory environmental contamination.18 Mixed infection was set up if two or more contributory infectious pathogens were identified.
Statistical Analysis
The Shapiro–Wilk test was used to determine whether the quantitative data conformed to a normal distribution. Continuous variables were compared using the Student’s t-test or the Mann–Whitney U-test as appropriate, categorical variables were compared using the Pearson chi-squared (χ2) test, Fisher’s exact test or the McNemar test for discrete variables where appropriate. 2×2 contingency tables were established to determine the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The results are presented with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS version 26 software (IBM Corporation). A result with P < 0.05 was considered statistically significant.
Results
Patient Characteristics and Laboratory Findings
Among the 106 patients, there were 77 patients with LRTI and 29 patients without LRTI. The clinical characteristics of patients with and without LRTI in some dimensions were different (Tables 1 and 2). The rate of patients with fever was significantly higher in the LRTI group than in the non-LRTI group (P = 0.013). The incidence of a history of lung disease and the rate of antibiotic use were much higher in the LRTI group than in the non-LRTI group (28.6% vs 10.3%; 84.4% vs 34.5%). The level of the inflammation indicator C-reactive protein (CRP) was significantly higher in the LRTI group than in the non-LRTI group (P = 0.006). Whereas the frequency and degree of leukocytosis, the percentage of neutrophils and procalcitonin were comparable for patients with LRIT and those with non-LRTI and thus could not be used reliably to distinguish between the two conditions. The percentage of peripheral blood lymphocytes did not demonstrate a significant difference between the 2 groups. In contrast, the percentage of lymphocytes in BALF was significantly higher in the LRTI group than in the non-LRTI group (P = 0.004).
 |
Table 1 Enrolled Patient Demographics and Clinical Manifestation |
 |
Table 2 Laboratory Findings of Patients |
Comparison of Diagnostic Performance of Bronchoalveolar Lavage Fluid mNGS and Culture for Differentiating LRTI from Non-LRTI
The comparison of mNGS and BALF culture for the LRTI and non-LRTI groups are illustrated in Figure 2A. Taking the composite reference standard as a gold standard, mNGS had a sensitivity of 76.6% (95% confidence interval [CI], 65.6–85.5%), which was superior to that of culture (76.6% vs 18.2%; P < 0.01); specificity of 79.3% (95% CI, 60.3–92.0%), which was not significantly different from that of culture (79.3% vs 89.7%; P = 0.38); NPV of 56.1% (95% CI,39.7–71.5%) and PPV of 90.8% (95% CI, 81.0–96.5%) respectively, with the negative likelihood ratio and positive likelihood ratio being 0.30 and 3.70. The NPV and PPV achieved using BALF culture were 29.2 (95% CI, 20.1–39.8%) and 82.4% (95% CI, 56.6–96.2%) respectively, with the negative likelihood ratio and positive likelihood ratio being 0.91 and 1.77 (Table 3). Furthermore, for cases where specific pathogens were clinically suspected, the sensitivities for detecting MTB, Cryptococcus, and Aspergillus by mNGS were 75.0% (12/16), 50.0% (3/6), and 83.3% (5/6), respectively. However, for MTB and Cryptococcus infection, conventional microbiological methods Xpert and CrAg were both observed to have a relatively higher yield rate than mNGS, although the difference was not significant due to the small sample size (87.5% vs 75.0%, P > 0.05; 100.0% vs 50.0%, P > 0.05, respectively) (Figure 2B).
 |
Table 3 Performance of mNGS and Conventional Culture Testing in Diagnosis of LRTIs |
Concordance Between mNGS and BALF Culture for Pathogen Detection
In our results, mNGS and BALF culture were both positive in 16 of 106 (15.1%) cases and were both negative in 40 of 106 (37.7%) cases. Forty-nine samples were positive by mNGS only (46.2%) and 1 was positive by BALF culture only (0.9%). For double-positive samples, the 2 results were completely matched at the genus level in 9 of 106 cases and totally mismatched in 1 of 106 cases (Figure 2C). The remaining 6 cases were found to be “partly matched”, indicative of at least 1 overlap of pathogens when polymicrobial results were observed in the mNGS and BALF culture tests (Additional Table 1).
Adjudication Classification of mNGS Results
Pathogens detected by mNGS in the LRTI group were divided into 4 categories: (1) definite, BALF mNGS result is consistent with results from microbiologic tests (sputum or BALF culture, PCR testing, and pathological examination) conducted within one week of BALF collection; (2) probable, BALF mNGS-based pathogen is likely the cause of LRTI based on clinical, radiologic, or laboratory findings; (3) possible, BALF mNGS-based pathogen has pathogenic potential and is concordant with clinical presentation but an alternative explanation is more likely; and (4) unlikely, BALF mNGS-based pathogen has pathogenic potential but is discordant with clinical presentation. Among the 77 LRTI cases, only 17 (22.1%) cases were detected definite or probable pathogens by sputum and BALF culture, and the number up to 31 (40.3%) cases when culture combined with other microbiological tests. However, BALF mNGS detected as many as 50 (65.0%) cases with definite or probable pathogens (Figure 3). Hence, as a pioneering tool, microbial mNGS vastly improved the aetiological diagnosis of LRTIs.
Analysis of “False-Positive”a in the Non-LRTIs Group and “mNGS unlikely”b in the LRTIs Group
Possible reasons for the “mNGS False-Positive” in the non-LRTIs group and “mNGS unlikely”b in the LRTIs group results, include colonization, contamination and overinterpretation (Table 4). In samples NO.1 and NO.37, Tropheryma whipplei was detected. But the mNGS results were both paradoxical with clinical signs and symptoms. In NO.23 specimen with a false-positive result by the application of mNGS (Prevotella melaninogenica), the traditional methods detected Rhizobium radiobacter. In this case, the patient was finally diagnosed with lung adenocarcinoma, and the condition improved after chemotherapy. Therefore, it was a false-positive result both in the culture and the mNGS. In the non-LRTIs Group, for case NO.25 Pseudomonas putida was isolated using both culture and mNGS, but the situation of the patient was well and with no signs of inflammatory infection except for a moderately elevated CRP level and antibiotic drugs were not administered, likely colonization was for the explanation.
 |
Table 4 Analysis of “False-Positive”a (n = 6) in the Non-LRTIs and “mNGS unlikely”b (n = 3) in the LRTIs Results |
Comparison of mNGS and Culture Testing by Pathogens
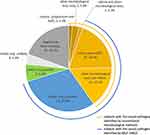
In 77 specimens, in addition to viruses, diverse bacteria (Mycobacterium tuberculosis complex [MTBC], NTM, Haemophilus influenzae, Haemophilus parainfluenzae, Tropheryma whipplei, Stenotrophomonas maltophilia, Legionella pneumophila, Chlamydophila psittaci, Streptococcus mitis and Acinetobacter radioresistens) and fungi (Cryptococcus neoformans, Pneumocystis jirovecii, and Schizophyllum commune) were identified by mNGS, but not by culture; however, the Enterobacter cloacae complex was detected only by culture. Among the 121 microbes isolated, the most commonly detected pathogen by mNGS was MTB (12/121), followed by Streptococcus pneumoniae (11/121), viruses (9/121) and Pseudomonas aeruginosa (8/121) (Figure 4). In our study, due to the special requirements of some strains and the restrictions of culturing conditions, MTBC and Cryptococcus neoformans were not detected by culture, however, 2/16 (12.5%), 7/16 (43.8%), 5/16 (31.3%), 7/16 (43.8%), and 1/16 (6.3%) MTB microorganisms were supported by PCR testing, pathological examination, acid-resistant staining, enzyme-linked immunospot assay (T-SPOT), and tuberculosis antibody testing respectively, mNGS illustrated the same diagnostic efficiency with the gene Xpert, accounting for 75.0% and 87.5% (P = 0.50). (Figure 2B and Additional Table 2). Meanwhile we observed that mNGS has no obvious diagnostic advantage for cryptococcal infection. Of the 6 patients with primary diagnosed pulmonary cryptococcosis, mNGS, pathological examination, and PCR testing identified Cryptococcus neoformans in 3, 2, and 1 patient respectively (Additional Table 2), another with NO.87 patient was supported by imaging evidence. In contrast, the results of the CrAg test were positive in all 6 patients (Additional Table 2 and Figure 2B). In conclusion, mNGS can detect both common and rare pathogens even without any prior hypothesis, and for some special strains, it can greatly increase the detection rate of causative pathogens in combination with other conventional laboratory-based microbiology testing. Therefore, we should also consider the results of conventional detection methods when selecting mNGS. An Additional Table provides more details about these 77 LRTI cases (see Additional Table 2).
The Ability of mNGS and Culture Testing in Polymicrobial Infection
Based on our results, mNGS demonstrated huge potential ability in polymicrobial infection, as is shown in Table 5. Of the 77 enrolled patients, 23 (29.9%) cases were detected to have polymicrobial infections using mNGS. In contrast, only 4 (5.2%) cases were detected co-pathogens infection by all conventional microbiological test. In order to detect more pathogens, we often combine many different types of microbiology methods, but with mNGS we could fulfill the destination in a single assay.
 |
Table 5 Results Obtained in the Analysis of LRTIs (n = 77) Specimens of Patients |
LRTI with No Identified Pathogen
In all 15 cases that were finally diagnosed as LRTI, neither traditional microbiology methods nor mNGS identified the presence of any infectious microorganism. The histopathology or imaging examination results and the clinical diagnosis of these 15 cases are shown in Table 6.
 |
Table 6 Patients with LRTI with No Identified Pathogen by Two Methods |
Clinical Impact and Role of mNGS Results
Adjustment of the Clinical Treatment Strategy After Acquiring the Identities of Pathogens Detected by mNGS
In our study, we finally attempted to assess the clinical impact of the assay’s findings. mNGS led to an overall beneficial effect on treatment in 57.1% (44/77) of patients. (Table 7). The beneficial effects were categorized as either the initiation of targeted treatment (n = 34) (Table 8) or the successful treatment adjustment (n = 10). The successful treatment adjustment involved antibiotic treatment adjustment without de-escalation (n = 6), antibiotic downgrade (n = 1) and antibiotic discontinuation (n = 3). mNGS also helped to identify the aetiology or confirm appropriate treatment in 15.6% of patients (12/77). mNGS presented no clinical benefit because of failure in identifying pathogens or guiding targeted antimicrobial treatment in slightly less than 30% of the patients. Overall, using mNGS, clinicians can be more precise in their care and management of patients.
 |
Table 7 Clinical Impact and Role of mNGS Results |
 |
Table 8 Cases Initiate Targeted Antimicrobial Treatment After Acquiring the Results of mNGS (n = 34) |
Discussion
mNGS has achieved considerable progress over the past few decades and has been increasingly used for contributory pathogen detection in clinical samples.7,16,19,20 In this study, we systematically compared identification by mNGS and BALF culture in a pairwise manner and uncovered mNGS to be advantageous in several dimensions. Firstly, one of the supreme appealing advantages currently is that mNGS can detect rare and fastidious pathogens, solving the shortcomings of traditional culture methods. Our study confirmed the overwhelming priority of mNGS in pathogen detection and identification in LRTIs. Previous studies have detected Pigeon paramyxovirus type 1 from BALF,4 Leishmania from cerebrospinal fluid,2 Parvimonas micra from synovial fluid,21 and Chlamydia psittaci from lung biopsy tissues,22 etc. In our study, MTBC, Tropheryma whipplei, Legionella pneumophila, Cryptococcus neoformans, Pneumocystis jirovecii, Schizophyllum commune, and Viruses were identified using mNGS, but not culture. With mNGS, more microorganisms encompass bacteria (66 vs 11), fungi (15 vs 4) and viruses were identified than culture (Figure 4). In the present study, 22.1%, 40.3% of cases were detected as definite or probable pathogens by culture and all conventional microbiological tests, respectively. However, mNGS can detect most of the pathogens in LRTIs (65.0%), which surpasses the sum of the percentages of the former two (Figure 3). This result was similar to that of previous research.19 Moreover, the positive detection rate of mNGS was enormously higher than that of BALF culture (76.6%vs 18.2%, P < 0.01), whereas the specificity was not significantly different from that of BALF culture (79.3% vs 89.7%; P = 0.38). Overall, its excellent performance plays in it can span the pathogen-detection spectrum, with high accuracy in LRTI diagnostics.
mNGS another notable ascendency is a promising and robust technique for the diagnosis of infectious disease because a comprehensive spectrum of potential causative microorganisms —viruses, bacteria, fungi, and parasites — can be identified in a single assay.23 That is, when a patient is underwent concurrent infection with different microbes, mNGS could identify two or more pathogens directly from a patient sample without a need of additional testing or a prior knowledge of the likely type of infectious agent. An increasing number of reports have demonstrated that this technology can be successfully applied to solve medical diagnostic dilemmas about mixed infections and have shown its predominant strength over conventional tests like PCR, serological antibody, or culture.6,7,24 The fact that the respiratory tract is an open passageway with exposure to a wide variety of pathogens increases the possibility of mixed infections. A prior study6 reported that the sensitivity of mNGS in diagnosing mixed pulmonary infection was 97.2%, which was much higher than that of conventional test (97.2% vs 13.9%; P < 0.01). A retrospective research24 indicated that a total of 69 (69/140 = 49.29%) cases were positive for mixed infection by mNGS only, and combined with conventional test results, the positive ratio of the mixed infection increased to 63.57% (89/140), with the most common patterns being bacterial–fungal coinfection and bacterial–bacterial coinfection. In our study, the diagnostic rate of the mixed infections elevated from 29.9% (23/77) when using solely the mNGS method to 40.3% (31/77) when combining mNGS and conventional techniques (Table 5). Our study also displayed that the combination of mNGS and conventional tests is helpful to improve the diagnostic rate of the mixed infection. Similarly, the diversity of interactions between microorganisms also partially explains why some clinical treatments fail. Much remains unknown about LRTI caused by mixed infection, for example, which pathogen initiated the infection, which microorganism dominated in the co-infection, which mechanisms were at play, how the co-infection developed, what the risk factors of co-infections were. Further in-depth research on these problems can provide valuable guidance on rational clinical treatment of co-infection.
In theory, the application of mNGS is beneficial to antibiotic stewardship. For one thing, patients who remain undiagnosed nearly always require empiric broad-spectrum therapy, with an increased risk of adverse side effects and antimicrobial drug resistance. For another, cessation of antibiotic therapy if no pathogen is detected can reduce the abuse of antibiotics. Besides, patients who were detected definitive pathogens will receive pathogen-oriented therapy. Herein, we verify the theory in real-world settings and explore the aetiological diagnosis depending on mNGS and investigate to what extent it affects the clinical management of patients. A prospective study25 enrolled 57 immunocompetent (ICO) and 75 immunocompromised (ICH) pneumonia patients, and showed that mNGS led to an overall beneficial effect on treatment in 33.3% (19/57) of the ICO patients and 52.0% (39/75) of the ICH patients, involving antimicrobial treatment de-escalation (n = 12 in ICO, n = 18 in ICH) and targeted treatment initiation (n = 7 in ICO, n = 21 in ICH). Another a large-scale multicenter prospective study7 enrolling 159 patients showed that mNGS identified multiple types of pathogens requiring different targeted treatments in 9 cases and had an overall positive clinical effect on 64 cases (40.3%), including 5 cases empirical treatment continued (3.1%), 19 cases treatment adjusted without de-escalation (11.9%) and 40 cases antibiotic de-escalated (25.2%). Compared with the two former studies, our study has a relatively higher positive impact (44/77 = 57.1%). The possible explanation reason may be the different criteria for division positive impact and the complexity of the various diseases. But all three studies emphasize the positive role of mNGS results in managing patients. In addition, the rapid feedback of mNGS might guide clinical laboratories to improve culture conditions for fastidious organisms, expedite clinical decision making, and establish pathogen-oriented therapy. An interesting phenomenon was observed that in our clinical laboratory Nocardia, Cryptococcus, Haemophilus influenzae, and Streptococcus pneumoniae were initially negative by culture and were later isolated with prolonged incubation time or adjusting culturing conditions based on mNGS results. So mNGS makes up for the lack of understanding of some microorganisms in the past, and promotes the improvements in traditional culture methods for some specific strains. These benefits are additional positive effects that we have not referred to due to the indirect role of mNGS.
Although mNGS has vividly showcased incomparable advantages over the conventional microbiology approach mentioned above, currently still no methods can solve all problems. According to our results, 65.0% cases in LRTIs were detected as definite or probable pathogens by mNGS, nearly 20.0% (15/77) cases were negative by both tests. The cause of this result is likely multifactorial. First, in the pre-analysis phase, similar to other molecular diagnostic techniques, mNGS requires strict storage and transport conditions to decrease the possibility of nucleic acid degradation. It is commonly administered that a high-quality specimen is crucial for the accuracy of the results. In our study, BALF specimens were delivered to a commercial laboratory, not the clinical microbiology laboratory, which may have increased the turnaround time, resulting in partial potential pathogens omission. A previous study26 has manifested that optimized sequencing adaptors enable rapid and real-time metagenomic identification of pathogens for the Illumina NextSeq platform in an approximately 9.1 to 10.1-h sample-to-answer turnaround time. In the near future, with the advancement of mNGS technique, an increasing number of causative pathogens will be identified at earlier times. Second, insufficient DNA extraction in the analysis phase may lead to a low number of reads or insufficient genome coverage in the setting of greater human host background reads. Additional means such as enzymatic disruption of cell wall, increasing sequencing depth, and refining detection thresholds can potentially alleviate this problem. Third, in the post-analysis phase, the interpretation of test results needs to be combined with clinical practice, nucleic acids cannot be simply equated with pathogenic microbes. Moreover, we should attach great importance to the false-positive results. The contamination of laboratories and specimens and the interference of the human host background were the main problems. For instance, in case NO.23, we identified Prevotella melaninogenica, which is a gram-negative anaerobic bacterium that typically produces melanin on the blood plate is considered normal oral flora,27 but is closely related to acute endodontic infections28 and oral lichen planus.29 For another, of the 106 enrolled patients, Tropheryma whipplei was the sole pathogen isolated in 4 patients, comprising 15,428, 4311, 95 and 88 reads respectively, but the symptoms of only 2 patients were consistent with the final clinical diagnosis, the remaining two patients were regarded as positive depending on the criteria for a positive mNGS result but presented discordant clinical signs and symptoms. Tropheryma whipplei is a bacterium associated with Whipple’s disease, which commonly demonstrates as weight loss, arthralgia, diarrhea and is diagnosed by the histology of small bowel biopsies.30,31 Acute infections can cause endocarditis,32 gastroenteritis, bacteraemia, and pneumonia.33 Due to lack of microbiological test, diagnosis of Whipple’s disease is ordinarily difficult and should be combined with other laboratory findings, such as periodic acid-Schiff (PAS) staining.34 The currently recommended initial therapy for Tropheryma whipplei is ceftriaxone, alternative initial therapy is meropenem, and long-term therapy is co-trimoxazole, and doxycycline in combination with hydroxychloroquine as alternative long-term treatment.31 In our study, 1 patient was treated with meropenem due to an allergy to ceftriaxone. The other patient was recommended to stop using antibiotics when the sequencing result was obtained. False-positive sequencing results may lead to unnecessary antibiotic treatment and accrue negative impact on patients. Further consideration that the ruling criteria of mNGS positive may not be suitable for Tropheryma whipplei that need evidence from the accumulated data. Therefore, in order to circumvent these issues, it is commonly recommended that negative controls of sterile deionized water34 should be performed together with specimens as well as positive controls with each sequencing run, and stringent bioinformatics thresholds should be established to filter out laboratory contamination and reduce within-run spillover from high positive samples.7 Even if we are in the phase of exploring its utility confronted with plenty of unprecedentedly challenges, take advantage of its drawbacks innovatively will overturn the situation. Surprisingly novel research35 has established a pipeline to concurrently detect pathogens and cancer based on the application of clinical metagenomics. In general, in order to increase the accuracy of pathogen detection, the human reads are removed in the bioinformatic pipeline, but this pioneering and advanced study take efficiently advantage of the sequencing data simultaneous detection of infectious and non-infectious diseases that drives evolution of the diagnostic landscape.36 The multidimensional nature of metagenomics enable and put forward interdisciplinary collaboration and diagnostics, for example microbiology and oncology, prenatal screening and microbiology, and microbiology and immunology.36 With the advent of artificial intelligence (AI) and big data, it is anticipated that through integration and joint use of sequencing data and innovation optimization will promote better clinical applications of metagenomics.
With regard to microbiology and immunology, a previous study37 described that inhabitant microbes and the immune system have complicated mutual relationships, and disruption of these complex and dynamic interactions can cause disease and have extreme consequences for host health. As such, in non-HIV-infected patients with Pneumocystis jirovecii pneumonia is a great threat to immunosuppressed patients, and it is quite common for these immunocompromised patients to be prescribed preventive antibiotics. To date, with mNGS to detect a broad panel of pathogens in a single test and simultaneously to interrogate host responses has great potential utility in the diagnosis of infectious disease.19,38 Hence, in our study, we attempted to analyse the differences in immunity between the 2 groups. However, the percentage lymphocytes in BALF were significantly higher in the LRTI group than in the non-LRTI group, and no difference was calculated between the 2 groups in peripheral blood T lymphocytes, T helper (Th) lymphocytes, T suppressor (Ts) lymphocytes, B lymphocytes, or natural killer (NK) cells or the percentage of other cells in BALF (Table 2). Speculating different stages of the inflammatory response and different types of the inflammation, and some cases of mixed infection plus a small sample size may co-interact the results. Based on previous studies, the concept of host-directed therapy has been proposed in the treatment of infectious diseases, and recommended future treatment regimens for infectious diseases would converge with the concept of personalized medicine, providing the best possible combinations that are adjusted not only for the agent but also for the patient.39 Further basic studies regarding on the cellular and molecular mechanisms of LRTI immune imbalance and mechanisms by which methods pathogenic microorganisms invade or escape from LRTI immunity will offer us new insights into microbiology and immunology.
Taken together, the respiratory tract, in fact is not a sterile environment and harbors microbial communities during both healthy and diseased states. Discriminating respiratory pathogens from background commensal microbiota is a central challenge for LRTI diagnostics and is particularly relevant for sensitive molecular assays. Our findings ascertained that mNGS detected microbes related to human diseases in 67.6% (45/63) of samples from LRTI patients who had received negative results from traditional pathogen detection, and identified at least one microbial species in 76.6% (59/77) of LRTI cases. In contrast, among 59 patients with pathogens identified by mNGS, conventional microbiological methods confirmed pathogenic infections in less than 50.0% (28/59) of cases. mNGS can yield outstanding accuracy in diagnosing LRTI, with outperformed sensitivity than culture (76.6% vs 18.2%, P < 0.01). Gratitude for this promising approach, eventually, 34 (44.2%) patients underwent the causative pathogen-directed therapy, 7 (9.1%) patients underwent an antibiotic adjustment or change, and 3 (3.9%) patients discontinued antibiotics. The outlined above elucidate the myriad of advantages of mNGS compared with traditional microbiology approaches and abundantly clear the technique for tailor-made diagnostics and customized treatment of LRTI. However, currently there is no unified standard on how to use mNGS indicators (build a cut-off value for the identification of pathogens in LRTIs using ROC curves of different pathogens, the sequencing reads, genomic coverage, and relative abundance of each organism) to differentiate legitimate pathogens from commensal microbiota or colonizers or contaminants, and adds complexity to the interpretation of metagenomic sequencing data. Future large-scale studies can help optimize host and microbe LRTI rule-out thresholds and further evaluate test performance before deployment in a clinical setting. In addition, the proliferation of available sequencing instruments and exponential reductions in sequencing costs over the ensuing decade will drive the rapid adoption of mNGS technology to be useful for administrating therapeutic decisions. Although many challenges and predicaments must be overcome for its use in clinical practice, it is foreseeable that mNGS will be a revolutionary technology for clinical microbiological diagnosis and bring affirmatively considerable prospects through multidisciplinary enhanced collaboration for patients and families in the near future.
There are several limitations associated with this study. First, in our study, DNA was detected while RNA was excluded and a relatively small volume of subjects, which may lead to biased conclusions that the detection of RNA viruses is sparse. Second, BALF samples were always collected after antibiotic therapy due to the patients’ complicated illnesses, thus, the incidence of bacterial and fungal detection by culture decreased, while mNGS detection was not affected, resulting in a lower sensitivity of the former. Third, our determination of the optimal thresholds for test interpretation may affect the sensitivity and specificity of mNGS. We acknowledge that there is no validated protocol for the determination of such a threshold, and as a result the decision to set this level of detection based on our clinical laboratory experience to assign certain pathogens as contaminants was arbitrary. Additional studies from other laboratory centers are necessary to determine the optimal thresholds and determination of contaminants. Finally, we did not further analyse multiple infections and assess the outcome of patients who received targeted antimicrobial treatment in our pilot study due to the contributory pathogens detected by mNGS so that to strongly portray the positive effect of mNGS in a real-world setting.
Conclusions
In summary, mNGS can be used to effectively identify pathogens of LRTI in patients, especially in those patients with negative cultures due to infections with fastidious pathogens or recent antibiotic administration. mNGS combined with conventional microbiological methods maximizes pathogen detection in the vast majority of patients with LRTI (approximately 70.0%) and advances the diagnostic performance, especially for diagnosing polymicrobial infection, thus demonstrating a positive role in the rational use of antibiotics. This pioneering technique exhibits high accuracy in diagnosing LRTI, but several challenges remain, and it should not be used as a standalone test.
Abbreviations
LRTIs, lower respiratory tract infections; mNGS, metagenomic next-generation sequencing; BALF, bronchoalveolar lavage fluid; PCR, polymerase chain reactions; Non-LRTIs, non-lower respiratory tract infections; MALDI-TOF, MS matrix-assisted laser desorption ionization time-of-flight mass spectrometry; CrAg, Cryptococcus capsular polysaccharide antigen; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; CMV, human cytomegalovirus; EBV, Epstein-Barr virus; EORTC/MSGERC, the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium; Xpert, xpert Mycobacterium tuberculosis /rifampicin resistance; CD3, cluster of differentiation 3 receptors; CD4, cluster of differentiation 4 receptors; CD8, cluster of differentiation 8 receptors; CD19, cluster of differentiation 19 receptors; CD16, cluster of differentiation 16 receptors; CD56, cluster of differentiation 56 receptors; Th, helper T; Ts, suppressor T; NK, natural killer; CRP, c-reactive protein; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; MTB, Mycobacterium tuberculosis; MTBC, Mycobacterium tuberculosis complex; NTM, nontuberculous mycobacteria; ANCA, anti-neutrophil cytoplasmic antibodies; GM, galactomannan; PAS, periodic acid-Schiff; ICO, immunocompetent; ICH, immunocompromised; qPCR, quantitative PCR; HIV, human immunodeficiency virus; TMP/SMZ, trimethoprim/sulfamethoxazole.
Data Sharing Statement
The dataset used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the Ethics Committee of Tianjin Medical University General Hospital (NO. IRB2022-WZ-048). Informed consent was waived because this was a retrospectively study. We obtained patients’ data from the Medical Records and Statistics Room. We analyzed the data anonymously. The use of the raw data was permitted by the Ethics Committee of Tianjin Medical University General Hospital.
Acknowledgments
We express our grateful appreciation to all the patients who donate their biological samples and all medical staff involved in the treatment and care of patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector; it was not supported by external funding.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. doi:10.1016/s1473-3099(18)30310-4
2. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
3. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548. doi:10.1056/NEJMra1403772
4. Zou X, Suo L, Wang Y, et al. Concurrent pigeon paramyxovirus-1 and Acinetobacter baumannii infection in a fatal case of pneumonia. Emerg Microbes Infect. 2022;11(1):968–977. doi:10.1080/22221751.2022.2054366
5. Zhao Z, Song J, Yang C, et al. Prevalence of fungal and bacterial co-infection in pulmonary fungal infections: a metagenomic next generation sequencing-based study. Front Cell Infect Microbiol. 2021;11:749905. doi:10.3389/fcimb.2021.749905
6. Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19(1):252. doi:10.1186/s12890-019-1022-4
7. Zhou H, Larkin PMK, Zhao D, et al. Clinical impact of metagenomic next-generation sequencing of bronchoalveolar lavage in the diagnosis and management of pneumonia: a multicenter prospective observational study. J Mol Diagn. 2021;23(10):1259–1268. doi:10.1016/j.jmoldx.2021.06.007
8. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
9. Hogan CA, Yang S, Garner OB, et al. Clinical impact of metagenomic next-generation sequencing of plasma cell-free DNA for the diagnosis of infectious diseases: a multicenter retrospective cohort study. Clin Infect Dis. 2021;72(2):239–245. doi:10.1093/cid/ciaa035
10. Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61(1):100–111. doi:10.1373/clinchem.2014.221770
11. Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi:10.1093/bioinformatics/bty560
12. Andrews S. Fast QC A quality control tool for high throughput sequence data. Cambridge, UK: Babraham Bioinformatics; 2013. Available from: www.bioinformatics.babraham.ac.uk/projects/fastqc/.
13. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi:10.1186/gb-2009-10-3-r25
14. Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20(1):257. doi:10.1186/s13059-019-1891-0
15. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi:10.1093/cid/ciz1008
16. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
17. Özçolpan O, Sürücüoğlu S, Özkütük N, Çavuşoğlu C. Distribution of nontuberculous mycobacteria isolated from clinical specimens and identified with DNA sequence analysis. Mikrobiyol Bul. 2015;49(4):484–493. doi:10.5578/mb.9698
18. van Ingen J, Kohl TA, Kranzer K, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis. 2017;17(10):1033–1041. doi:10.1016/s1473-3099(17)30324-9
19. Chen H, Yin Y, Gao H, et al. Clinical utility of in-house metagenomic next-generation sequencing for the diagnosis of lower respiratory tract infections and analysis of the host immune response. Clin Infect Dis. 2020;71(Suppl 4):S416–S426. doi:10.1093/cid/ciaa1516
20. Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi:10.1056/NEJMoa1803396
21. Huang Z, Zhang C, Li W, et al. Metagenomic next-generation sequencing contribution in identifying prosthetic joint infection due to Parvimonas micra: a case report. J Bone Jt Infect. 2019;4(1):50–55. doi:10.7150/jbji.30615
22. Gu L, Liu W, Ru M, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. 2020;20(1):65. doi:10.1186/s12890-020-1098-x
23. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. 2015;6(6):e01888–15. doi:10.1128/mBio.01888-15
24. Xie G, Zhao B, Wang X, et al. Exploring the clinical utility of metagenomic next-generation sequencing in the diagnosis of pulmonary infection. Infect Dis Ther. 2021;10(3):1419–1435. doi:10.1007/s40121-021-00476-w
25. Zhan Y, Xu T, He F, et al. Clinical evaluation of a metagenomics-based assay for pneumonia management. Front Microbiol. 2021;12:751073. doi:10.3389/fmicb.2021.751073
26. Zhang D, Zhang J, Du J, et al. Optimized sequencing adaptors enable rapid and real-time metagenomic identification of pathogens during runtime of sequencing. Clin Chem. 2022;68:826–836. doi:10.1093/clinchem/hvac024
27. Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi:10.1038/nature11234
28. Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics. 2012;13:345. doi:10.1186/1471-2164-13-345
29. Zheng SW, Xu P, Cai LT, et al. The presence of Prevotella melaninogenica within tissue and preliminary study on its role in the pathogenesis of oral lichen planus. Oral Dis. 2021;28:1580–1590. doi:10.1111/odi.13862
30. Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii”. Clin Microbiol Rev. 2001;14(3):561–583. doi:10.1128/CMR.14.3.561-583.2001
31. Marth T, Moos V, Müller C, Biagi F, Schneider T. Tropheryma whipplei infection and Whipple’s disease. Lancet Infect Dis. 2016;16(3):e13–e22. doi:10.1016/s1473-3099(15)00537-x
32. Sarvananthan S, Velissaris T, Miskolczi S, Yam T, Shah BN. Tropheryma whipplei endocarditis. Echocardiography. 2021;38(4):697–700. doi:10.1111/echo.15007
33. Fenollar F, Mediannikov O, Socolovschi C, et al. Tropheryma whipplei bacteremia during fever in rural West Africa. Clin Infect Dis. 2010;51(5):515–521. doi:10.1086/655677
34. Guo Y, Li L, Li Z, Sun L, Wang H. Tropheryma whipplei detection by nanopore sequencing in patients with interstitial lung disease. Front Microbiol. 2021;12:760696. doi:10.3389/fmicb.2021.760696
35. Guo Y, Li H, Chen H, et al. Metagenomic next-generation sequencing to identify pathogens and cancer in lung biopsy tissue. EBioMedicine. 2021;73:103639. doi:10.1016/j.ebiom.2021.103639
36. de Vries JJC. The multidimensional nature of metagenomics drives interdisciplinary diagnostics. EBioMedicine. 2021;74:103694. doi:10.1016/j.ebiom.2021.103694
37. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi:10.1126/science.1223490
38. Langelier C, Kalantar KL, Moazed F, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A. 2018;115(52):E12353–E12362. doi:10.1073/pnas.1809700115
39. Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018;17(1):35–56. doi:10.1038/nrd.2017.162
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.