Back to Journals » Journal of Inflammation Research » Volume 16
Advances in the Study of Plant-Derived Vesicle-Like Nanoparticles in Inflammatory Diseases
Authors Tan X , Xu Y , Zhou S , Pan M , Cao Y, Cai X, Zhao Q, Zhao K
Received 25 May 2023
Accepted for publication 20 September 2023
Published 29 September 2023 Volume 2023:16 Pages 4363—4372
DOI https://doi.org/10.2147/JIR.S421124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xuejun Tan,1 Yukun Xu,1 Sirui Zhou,1 Mingyue Pan,1 Yue Cao,1 Xiuping Cai,2 Qing Zhao,1,2,* Kewei Zhao1,2,*
1The Third Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Guangzhou Key Laboratory of Chinese Medicine Research on Prevention and Treatment of Osteoporosis, The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kewei Zhao; Qing Zhao, Department of Laboratory Medicine, The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, 261 Longxi Avenue, Liwan District, Guangzhou, Guangdong, People’s Republic of China, Tel +86-13711355225 ; +86-18825144487, Email [email protected]; [email protected]
Abstract: All humans are universally affected by inflammatory diseases, and there is an urgent need to identify new anti-inflammatory drugs with good therapeutic benefits and minimal side effects to the organism. Recently, it has been found that plant-derived vesicle-like nanoparticles (PDVLNs) have good biocompatibility, with their active ingredients exhibiting good therapeutic effects on inflammation. They can also be used as drug carriers for targeted delivery of anti-inflammatory drugs. Therefore, PDVLNs represent a popular research area for novel anti-inflammatory drugs. This paper details the origin, biological functions, isolation and purification, and identification of PDVLNs, as well as the therapeutic effects of their intrinsic bioactive components on inflammatory diseases. It also introduces their targets as drug carriers to facilitate the development and application of PDVLNs anti-inflammatory drugs.
Keywords: plant, vesicle-like nanoparticles, inflammation, inflammatory diseases
Introduction
Epidemiological studies have revealed a significant increase in the incidence of inflammatory diseases over the past 20 years,1 and the number of patients taking anti-inflammatory drugs has also been rising.2 Inflammatory diseases often occur in multiple systems and are systemic disorders with symptoms that persist over time and may recur. In clinical practice, patients are often comorbid with a variety of diseases, including cardiovascular, metabolic, and skeletal disorders, as well as cognitive deficits, further affecting quality of life and increasing mortality. This poses a great challenge for accurate diagnosis and treatment of the disease. Most of the drugs currently used to treat inflammatory diseases are synthetic drugs; however, they can all have serious side effects and long-term use can lead to multiple complications. Therefore, there is an urgent need to identify novel therapeutic agents for the treatment of inflammatory diseases.3 PDVLNs come from a wide range of sources, are safe and non-toxic, have low immunogenicity, can be mass-produced at low cost, and have good biocompatibility and environmentally friendly properties. Moreover, PDVLNs are directly edible, absorbed through the gastrointestinal tract, and play a huge role in species exchange between plant and mammalian cells, which makes PDVLNs attract a wide range of attention for therapeutic applications. Recent studies on the anti-inflammatory effects of PDVLNs, a class of drugs that effectively inhibit inflammation, have shown to possess anti-inflammatory bioactivities that are effective for treating many inflammatory diseases. In this review, we will describe in detail the isolation and purification, characterization, and biological functions of PDVLNs, as well as the therapeutic effects of their intrinsic bioactive components on inflammatory diseases, and their targeting as drug carriers in order to facilitate the development and application of anti-inflammatory drugs with PDVLNs.
Progress in the Study of Inflammatory Diseases
The inflammatory response is a protective immune response produced by the body in response to various harmful stimuli and represents a defense mechanism for the clearance of pathogens and maintenance of tissue homeostasis. Moreover, weak inflammatory responses can lead to persistent pathogen infection, whereas excessive inflammatory responses can cause chronic or systemic inflammatory disease.4,5 It is widely believed that inflammation constitutes part of the innate immune mechanism. If left unchecked, inflammation may evolve into acute or chronic inflammatory disease and can also represent the underlying cause of many chronic diseases. Therefore, the treatment of inflammatory diseases is an area of keen research interest in the medical field.
Mechanisms of the Inflammatory Response
The pathogenesis of inflammatory diseases is complex. Under normal conditions, the protective and tissue-damaging capacities of the inflammatory cascade response are typically maintained in a balanced state and regulated by inflammatory cytokines.6,7 However, chronic inflammation usually manifests as the massive destruction of damaged tissues resulting from the inflammatory response and little protection.8 If left unchecked, inflammation may present as a variety of inflammatory diseases, including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, immune inflammatory diseases, and tumor transformation.9–12 Although inflammation can develop into a variety of different inflammatory diseases, they share a common response mechanism. The specific mechanisms are as follows: 1) the recognition of noxious stimuli by cell surface membrane receptors; 2) activation of inflammatory pathways; 3) release of inflammatory markers; and 4) recruitment of inflammatory cells. The mechanisms of the inflammatory response are complex, uniform, and require continuous exploration by researchers to better cope with the development of inflammatory diseases.13
Classification of Inflammatory Diseases
There are various classifications of inflammatory diseases, which can be classified in detail according to the different durations and locations of onset. Acute and chronic inflammatory diseases can be classified according to the duration of onset. According to the site of origin, these diseases can be divided into inflammatory diseases of the cardiovascular system, brain, respiratory tract, gastrointestinal tract, and skin. With the unhealthy diet and irregular lifestyle associated with modern life, inflammatory diseases are becoming increasingly more common; however, the drugs commonly used in clinical treatment are accompanied by a greater number of side effects. Therefore, it is especially important to select the right medication for inflammatory diseases that is safe and without side effects.6
New Anti-Inflammatory Drugs
The anti-inflammatory effects of western drugs and herbs have their own advantages and disadvantages. Currently, for some severe and critical illnesses caused by inflammatory factors, the anti-inflammatory drugs commonly used clinically have anti-inflammatory effects; however, they are specific for treatment, are associated with certain serious adverse effects, and possess inaccurate efficacy. Since the results of anti-inflammatory and basic supportive treatment alone do not achieve the desired therapeutic effect, there is an urgent need to identify a new anti-inflammatory drug with good therapeutic effects and low side effects. In recent years, plants and extracted phytoactive ingredients have become an important source for the development of novel anti-inflammatory drugs.9 With the continuous research of PDVLNs, some scholars have found that PDVLNs inherit the properties of effective bioactive ingredients and can exert the anti-inflammatory properties of anti-inflammatory plants, representing an anti-inflammatory drug with greater biological properties and advantages compared to Chinese and Western medicines. The next section will elaborate on the research progress made to date regarding PDVLNs and further introduce their applicability for the treatment of inflammatory diseases.
Research Progress of PDVLNs
Extracellular vesicles (EVs) are phospholipid bilayer structures produced by mother cells under the influence of different factors, including inflammation, hypoxia, oxidative stress, senescence and apoptosis.14 EVs are termed exosomes, ectosomes, apoptotic bodies, oncosomes, microvesicles, microparticles, etc., depending on their origin and diameter.15–17 In 2007, researchers discovered for the first time, that plant cells can also secrete active substances similar to the morphological structure of animal-derived extracellular vesicles, known as PDVLNs.18,19 After performing in-depth studies, the particle size of PDVLNs was distributed in 30–1000 nm, and the structural characteristics were similar to those of animal-derived VLNs, all of which have lipid bilayer membrane structure and membrane surface proteins. In addition, these PDVLNs contained cytoplasmic components, such as mRNA, miRNA, proteins and some active components unique to plants.20 PDVLNs also have unique properties, with different PDVLNs possessing the physiological functions of their plants of origin. PDVLNs are widely available, safe, non-toxic, have low immunogenicity, can be mass-produced at low cost, have good biocompatibility and environmentally friendly properties. Moreover, PDVLNs are directly edible, absorbed through the gastrointestinal tract, and play a huge role in species exchange between plant and mammalian cells. Collectively, these traits make PDVLNs of wide interest for therapeutic applications.21–23
Isolation and Purification of PDVLNs
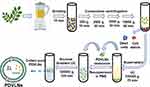
Due to the heterogeneity of vesicle-like nanoparticles (VLNs) in terms of size, origin, content, and function, there is currently no standard and universal technique for the separation of VLNs, which greatly limits the depth of research on their functional aspects. Most isolation techniques cannot completely separate VLNs from lipoproteins with similar physical properties and from the VLNs of non-in vivo pathways.24,25 The principle of ultracentrifugation is to separate the components according to the physical properties of the particles in the solution, the density, and viscosity of the solvent in order (Figure 1). This has the advantages of simple operation, high purity of VLNs, and can be used for the study of VLN proteins, DNA, and immune function, as well as the extraction of a large volume of samples without the use of reagents.26 However, the extraction process using this method is time-consuming and is associated with a low yield. Density gradient centrifugation generally forms sucrose concentration gradient bands by ultracentrifugation, whereas VLNs are mainly concentrated in the density bands of 1.13–1.20 g/mL.25 The polymer precipitation method is based on the principle of changing the solubility and dispersion of VLNs by adding polymers, and finally obtaining VLNs by centrifugation at a lower speed. This method is easy to operate, does not require ultracentrifugation, has a high yield, can retain the biological activity of VLNs to the greatest extent, and is suitable for RNA analysis of VLNs. However, this method is prone to introduce impurities, such as precipitation reagents and lipoproteins, which may affect the downstream analysis and is not suitable for the study of VLN proteins. Ultrafiltration and size-exclusion chromatography are methods used to achieve separation based on the existence of size differences between exosomes and VLNs. Ultrafiltration represents a new method for the extraction of VLNs due to its rapidity and medium purity without affecting the biological activity of VLNs; however, the adsorption and pressure of the membrane may lead to VLN deformation and rupture, resulting in losses and thereby affecting the results of the analysis. VLN purity separated by size exclusion chromatography is high, while preserving integrity and biological activity. The disadvantages include that the separation process is time-consuming, unsuitable for large-scale production, and expensive. Immunoaffinity chromatography can isolate VLNs and their subgroups with high purity, but requires the selection of appropriate markers, which may cause bias when used to isolate total VLNs due to differences in marker expression between subgroups.
In summary, the commonly used separation methods for VLNs include ultracentrifugation, polymer precipitation, ultrafiltration, size-exclusion chromatography, and immunoaffinity chromatography, among others.27 When performing VLN separation, the most suitable method should be selected by taking into account the sample volume, purity, cost, instrumentation, time, and purpose of the study.25 If a standard method for isolation and purification of PDVLNs is available, the biological efficacy of PDVLNs in anti-inflammation will also be more stable for better and more rapid clinical application in the future.
Characterization and Identification of PDVLNs
With the booming PDVLN nanotechnology, there is a higher demand for accurate and diverse characterization techniques. It is important to design and employ multiple characterization techniques to discriminate between the different subgroups of VLNs, especially for the design of therapeutic and drug nanocarriers for PDVLNs. Methods that have traditionally been technically used to characterize the size of VLNs include flow cytometry methods, dynamic light scattering (DLS), and transmission electron microscopy (TEM). Of these, flow cytometry is the most widely used. Flow cytometry allows for the rapid quantitative analysis of cells or cell-sized particles in suspensions. However, the ability to accurately determine the size of such particles is severely limited by the lower limit of the particle size of flow cytometers, which is around 300 nm. The size and zeta potential of dispersed PDVLNs can be measured and evaluated by DLS.28 The main difference between DLS and NTA is the concentration range. When the concentration is too low, NTA can fulfill the detection task very satisfactorily, while DLS can only detect samples with higher concentration. It has been shown that an ultrastructural analysis of the subcellular state of PDVLNs can be performed using transmission electron microscopy.29,30 Electron microscopy can be very intuitive to see the topography and size of the sample, but it is too costly and time-consuming to ensure the integrity of the sample after preparation. Compared with other technologies, NTA technology is simpler in sample processing, better able to ensure the original state of exosomes, and it is able to comprehensively characterize particles with a wide range of particle size distributions in suspensions, with the advantages of high resolution, fast detection speed, high accuracy and so on. Therefore, NTA is considered the gold standard for the identification of VLNs.
A composition analysis of lipids, nucleic acids and proteins of PDVLNs is considered to be important characterization criterion for the quality control of PDVLNs.31,32 Plant and mammalian cell-derived VLNs enjoy a common technique for characterizing their chemical composition. Lipids represent an important component of the molecular structure of the PDVLN lipid bilayer, and the main analytical techniques used consist of chromatography-tandem mass spectrometry and magnetic resonance (NMR).33 Due to the limitation of NMR on sample volume and sensitivity, the lipid analysis of PDVLNs continues to be frequently performed by chromatography-tandem mass spectrometry. Developing an understanding of lipid composition and how each lipid component regulates PDVLNs will help investigators develop effective treatment strategies for PDVLNs.34 PDVLN proteins were detected by protein immunoblotting and enzyme-linked immunosorbent assay. SDS-PAGE and Western blot are widely used in protein analysis.35,36 In addition, the qualitative and quantitative analysis of PDVLNs proteins is important for the screening of various disease surface markers. PDVLNs contain both a large amount of protein and lipid components, as well as different forms of RNA and DNA, which are primarily dominated by RNA. Precipitation and centrifugation columns, amplification, and sequencing techniques are commonly used to extract and detect nucleic acids from PDVLNs.20,37,38
Biological Functions of PDVLNs
PDVLNs possess the following excellent biological functions: 1) good biocompatibility; 2) non-toxicity and low immunogenicity; 3) specific targeting; 4) extended drug cycle and duration of action; 5) mass production; and 6) crossing the blood-brain barrier.20,39,40 Understanding the biological functions of PDVLNs can better facilitate the research of PDVLNs in therapeutic applications. For example, a major obstacle in the treatment of neuroinflammatory diseases is the absence of an effective carrier that can transport drugs across the blood-brain barrier. However, PDVLNs are characterized by low immunogenicity, innate stability, specific targeting and crossing the blood-brain barrier, which could be an ideal drug delivery vehicle for neuroinflammatory diseases and provide a novel therapeutic modality for neuroinflammatory diseases. Among these, specific targeting is a major characteristic, and many groups have investigated PDVLN targeting. VLNs of mammalian origin are rapidly metabolized from the circulation after in vivo administration, which is not conducive to drug retention in the body.41 Moreover, PDVLNs target the colon and can be taken up by macrophages and intestinal stem cells, cross the intestinal mucosal barrier, and effectively remain in the intestine, ensuring targeted drug delivery to the intestine and increasing the local drug concentration.42 The specific targeting of PDVLNs can be effectively demonstrated by the following findings, in which the anti-inflammatory drug methotrexate (MTX) binds to grapefruit-derived nanovesicles (GDN); GNDs can specifically target colonic tissues, act as intestinal immunomodulators to maintain intestinal macrophage homeostasis, fully utilize the effect of MTX, and improve its therapeutic effect on colitis in mice.43
PDVLNs and Inflammatory Diseases
Edible plant-derived VLNs (eg, grapefruit, tomatoes, blueberries, and shiitake mushrooms) have been reported in the literature to possess various anti-inflammatory properties.44 The specific function of PDVLNs in inflammatory diseases depends primarily on their internal functional components, including RNA, lipids, proteins, and other metabolites. PDVLNs whose intrinsic substances exert anti-inflammatory, antiviral, anti-fibrotic, and anti-tumor effects are involved in the defense response to pathogenic invasion (Figure 2).15 The therapeutic effects of the different substances intrinsic to PDVLNs on inflammatory diseases will be described in detail below.
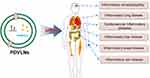 |
Figure 2 PDVLNs have application for a variety of inflammatory diseases. |
Therapeutic Effects of Nucleic Acids in PDVLNs on Inflammatory Diseases
miRNAs have been detected in most plant extracts, suggesting that edible medicinal plant preparations may deliver miRNAs to mammals.45 miRNAs in PDVLNs have also been reported to play a regulatory role in the inflammatory response. In a study of oral ginger-derived nanoparticles (GDNPs), 27 miRNAs were found to be highly expressed in GDNPs, and could resist lipopolysaccharide (LPS)-induced inflammation by down-regulating the expression of NF-κB, IL-6, IL-8, and TNF-α. These findings indicated that nucleic acids in GDNPs have potential for the treatment of inflammatory diseases.20 Moreover, in 2018, a research team also studied the new active ingredient of ginger, exosome-like nanoparticles, and found that microRNAs in ginger exosome-like nanoparticles (GELN) can affect the intestinal flora and improve the barrier function of the intestine, thereby reducing DSS-induced colitis in mice.26 It has also been found that ginger-derived ELNs can down-regulate the expression of the pro-inflammatory factors, TNF-α, IL-1β, IL-6, and promote the expression of the anti-inflammatory factors, IL-10 and IL-12, in a colitis model. Moreover, the microRNAs of these factors are known to play a major role in the uptake by the intestinal flora of mice, altering the intestinal flora by regulating the level of mRNA expression of Lactobacillus composition and thus reduce colitis in mice.46,47 Ginger-derived miRNAs of VLNs can be used to treat colitis in mice, as well as inhibit the proliferation of the periodontal pathogen Porphyromonas gingivalis and treat chronic periodontitis.
The sRNAs in Dandelion decoction also have anti-inflammatory effects. It has been shown that a lipid complex exists in the human gastrointestinal tract that takes up sRNA and delivers it to organs and tissues throughout the body. sRNA-6, a new active ingredient in dandelion decoction-derived ELNs, can be taken up by lipid complexes in the gastrointestinal tract and exhibits a significant ameliorating effect on polyinosinic acid-induced lung inflammation in mice. This finding suggests that herbal exosome-like nanoparticles may serve as a representative of precision medicine.48
Honey, a natural substance derived from plant flowers, has been found to alleviate chronic inflammation and liver damage in rats fed a high-fat diet. Honey-derived VLNs (H-VLNS) significantly inhibited NLRP3 inflammatory vesicle activity in primary macrophages, mainly by MIR-4057 in H-VLNS, suggesting that miRNAs of H-VLNS have intrinsic anti-inflammatory activity.49
Grapefruit-derived nanovectors (GNV) can efficiently deliver a variety of substances, including drugs, DNA expression vectors, siRNA and antibodies, without toxicity in mouse model studies. When researchers combined GNV with polyethyleneimine (PEI), the combination of the two (PGNV) was effective at delivering miRNA from the nasal cavity to the brain. In addition, the miR17 carried by GNV could treat brain inflammation-related diseases, such as brain tumors in mice.50
Bitter melon-derived extracellular vesicles (BMEVs) also have intrinsic anti-inflammatory functions, significantly reducing NLRP3 expression. BMEV RNA has also been shown to mediate anti-inflammatory bioactivity.51
Therapeutic Effects of Lipids in PDVLNs on Inflammatory Diseases
Currently, lipids are the preferred carriers for drug delivery and play an important role in PDVLNs. Moreover, several research teams have identified the therapeutic effects of lipids in PDVLNs on inflammatory diseases.
Grapefruit-derived nanovesicles (GDNs) exert anti-inflammatory effects via their lipids following their successful uptake by intestinal macrophages, which upregulated the expression of HO-1 and IL-10 and inhibit the production of IL-6, IL-1β, and TNF-α.43 A previous study demonstrated that GDNs could be selectively taken up by intestinal macrophages and ameliorate DSS-induced colitis in mice, which could be developed for oral small molecule drugs to reduce the inflammatory response to human disease.52 It was also found that the lipids in G-ELN are active biomolecules that can inhibit the activity of NLRP3 inflammatory vesicles, identifying G-ELN as a novel effective drug to inhibit the composition and activation of NLRP3 inflammatory vesicles.53
It has also been shown that lipid-mediated broccoli-derived nanoparticles (BDN) can be selectively taken up by dendritic cells and maintain intestinal immune homeostasis, thereby preventing and potentially even being able to treat intestine-related inflammatory diseases.54
In addition, studies have shown that ginger-derived exosome-like nanoparticles (GELNs) also have anti-inflammatory properties. The expression of GELN lipids can be used to treat chronic periodontitis.55 Indeed, it was previously demonstrated that GELN inhibited the attachment of Pseudomonas gingivalis to oral epithelial cells, decreased the level of IL-1β, IL-6, IL-8, and TNF-α expression, as well as reduced the recruitment of macrophages, leukocytes, and CD3 cells in the oral tissue microenvironment. The above findings indicate that GELN can be used as a potential therapeutic agent for the prevention or treatment of chronic periodontitis.
Other Components of PDVLNs for Inflammatory Diseases
There are also many unexplored bioactive molecules in PDVLNs, which perhaps also have some therapeutic effect on inflammatory diseases (eg, proteins). PDVLN protein-mediated inflammatory diseases have been poorly studied to date and should be further elucidated in the future.
PDVLNs Can Be Used as Drug Carriers to Treat Inflammatory Diseases
VLNs can be used as therapeutic vectors and targets for the prevention and treatment of inflammatory diseases.56 Among the new vector systems introduced in recent years, PDVLNs have great potential as drug delivery systems (DDS). Since PDVLNs are mostly edible and can be used as carriers for delivering specific drugs without toxicity and side effects, They can cross the blood-brain barrier and enter the brain through nasal administration, but cannot pass from mother to fetus through the placenta.57 they have become a popular area of research.15
Compared to existing drug delivery systems, edible plant-derived exosome-like nanoparticles have relatively high internalization rates, low immunogenicity, a lack of toxicity or side effects, do not cause inflammatory reactions or necrosis, are gastrointestinally stable, tissue-specific and targeted, can be mass-produced, and are excellent candidates for drug delivery vehicles.34,39 For example, grapefruit-derived ELNs can deliver active substances (eg, chemotherapeutic agents, RNA, DNA, and proteins) to different types of cells, and intravenous administration of grapefruit-derived ELNs loaded with folic acid to pregnant mice significantly increased the targeting efficiency of folic acid to folic acid receptor cells without affecting the fetus through the placental barrier.18,22
Plant exosomes have been found to have drug delivery capabilities targeting the intestinal tract, displaying great potential for the treatment of intestinal diseases. This plant-derived exosome-targeted delivery system can stably and efficiently load chemical or nucleic acid drugs and deliver them to sites of intestinal inflammation, thereby reducing inflammation or inhibiting gene expression (Figure 3).58 This new method of drug delivery has been shown to be effective for the treatment of intestinal inflammation.59 When faced with inflammatory bowel disease, drug-targeting systems using plant-derived exosomes as carriers can offer a new therapeutic approach for the targeted delivery of inflammatory drugs.60–62
In studies of edible ginger-derived nanoparticles (GDNVs) against inflammatory bowel disease and colon associated cancers, a novel, natural, non-toxic delivery system was shown to target inflammation in the intestinal mucosa and block destructive factors while promoting healing.63 It was additionally observed that nanoparticles made from ginger-derived lipids can be used as a delivery platform for the therapeutic drug, doxorubicin, with good biocompatibility. This finding indicates that GDNV is a better delivery vehicle than commercially available drugs.47 Another study showed that ginger-derived exosome-like nanoparticles can be efficiently loaded with doxorubicin and have better pH-dependent drug release properties compared to commercially available doxorubicin liposomes, targeting colon cancer cells, and significantly improving the antitumor efficiency of chemotherapeutic drugs.47,64
Conclusion
Inflammation is a fundamental pathological process and a defensive response to external infections. Each year, countless patients suffer from inflammatory diseases. Therefore, the treatment of inflammatory diseases currently represents an area of keen research interest in the medical field. Although many drugs have been developed for the treatment of inflammatory diseases, their targeted therapeutic effects still need to be further enhanced, and PDVLNs can deliver drugs to the site of inflammation to achieve precise therapeutic effects due to their unique targeting properties. Therefore, PDVLNs for inflammatory diseases have become a major research topic, opening up a new field for the study of therapeutic drugs for inflammatory diseases. However, there is currently no standardized procedure for the isolation and purification of PDVLNs, which may lead to slight differences in their anti-inflammatory efficacy It is believed that with the passage of time and the progress of research, the continuous improvement of the quality evaluation system of PDVLNs will promote the progress of research on plant-derived extracellular vesicles in inflammatory diseases. The above studies suggest that PDVLNs exert anti-inflammatory effects through their intrinsic active substances, suggesting that studying the bioactive components of PDVLNs can provide some insight into the treatment of inflammatory diseases with PDVLNs. The intrinsic bioactive components of PDVLNs can be used to load drugs into PDVLNs, which may pose a risk of interaction between intrinsic bioactive components and exogenous drugs. Therefore, extensive and in-depth studies are still needed for the loading of drugs into PDVLNs without any potential interactions. Although the quality evaluation system of PDVLNs is not perfect, it is believed that their own targeting and bioactivity can bring progress in the treatment of inflammatory diseases, and their specific mechanism of action may be the focus of future research. Although there is still a long way to go in terms of the clinical application of PDVLNs for inflammatory diseases, it is believed that with the continuous in-depth research and enthusiasm of researchers, the clinical application of PDVLNs will be adapted in the near future.
Acknowledgments
This work was supported by Guangdong Engineering Research Center of Chinese herbal vesicles, the National Natural Science Foundation of China [grant numbers 81973633], National Natural Science Foundation of China [grant numbers 82174119], Key Laboratory Construction Project of Guangzhou Science and Technology Bureau [grant numbers 202102100007], Youth Innovative Talents Project of Guangdong Province (grant numbers 2020KQNCX015), and Medical Science and Technology Research Fund Project of Guangdong Province (grant numbers A2021339); . Science and Technology Projects in Liwan District, Guangzhou [Grant Number: 202201009 and 20230710], Young Talent Support Project of Guangzhou Association for Science and Technology [Grant Number: QT2023036], Special focus areas for general Universities in Guangdong Province [Grant Number: 2022ZDZX2016].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ji J, Sundquist J, Sundquist K. Gender-specific incidence of autoimmune diseases from national registers. J Autoimmun. 2016;69:102–106. doi:10.1016/j.jaut.2016.03.003
2. Schein CH. Repurposing approved drugs on the pathway to novel therapies. Med Res Rev. 2020;40(2):586–605. doi:10.1002/med.21627
3. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8(12):1605. doi:10.3390/cells8121605
4. Daskalaki MG, Tsatsanis C, Kampranis SC. Histone methylation and acetylation in macrophages as a mechanism for regulation of inflammatory responses. J Cell Physiol. 2018;233(9):6495–6507. doi:10.1002/jcp.26497
5. Visan I. Inflammasomes drive tau pathology. Nat Immunol. 2020;21(1):8. doi:10.1038/s41590-019-0572-1
6. Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu Rev Physiol. 2017;79(1):567–592. doi:10.1146/annurev-physiol-022516-034348
7. Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–589. doi:10.1093/eurheartj/eht367
8. Chung HJ, Lee HS, Shin JS, et al. Modulation of acute and chronic inflammatory processes by a traditional medicine preparation GCSB-5 both in vitro and in vivo animal models. J Ethnopharmacol. 2010;130(3):450–459. doi:10.1016/j.jep.2010.05.020
9. Patil KR, Mahajan UB, Unger BS, et al. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int J Mol Sci. 2019;20(18):4367. doi:10.3390/ijms20184367
10. Simmons DL. What makes a good anti-inflammatory drug target? Drug Discov Today. 2006;11(5–6):210–219. doi:10.1016/s1359-6446(05)03721-9
11. Debnath S, Ghosh S, Hazra B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl(4)-induced hepatotoxicity in rats. Food Chem Toxicol. 2013;59:485–491. doi:10.1016/j.fct.2013.06.036
12. Fangkrathok N, Junlatat J, Sripanidkulchai B. In vivo and in vitro anti-inflammatory activity of Lentinus polychrous extract. J Ethnopharmacol. 2013;147(3):631–637. doi:10.1016/j.jep.2013.03.055
13. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi:10.18632/oncotarget.23208
14. Simeone P, Bologna G, Lanuti P, et al. Extracellular vesicles as signaling mediators and disease biomarkers across biological barriers. Int J Mol Sci. 2020;21(7):2514. doi:10.3390/ijms21072514
15. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326
16. Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360(6393):1126–1129. doi:10.1126/science.aar4142
17. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
18. Cui Y, Gao J, He Y, Jiang L. Plant extracellular vesicles. Protoplasma. 2020;257(1):3–12. doi:10.1007/s00709-019-01435-6
19. An Q, Huckelhoven R, Kogel KH, van Bel AJ. Do plant cells secrete exosomes derived from multivesicular bodies? Cell Microbiol. 2007;8(6):1009–1019. doi:10.1111/j.1462-5822.2006.00683.x
20. Cai Y, Zhang L, Zhang Y, Lu R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics. 2022;14(4):822. doi:10.3390/pharmaceutics14040822
21. Raimondo S, Giavaresi G, Lorico A, Alessandro R. Extracellular vesicles as biological shuttles for targeted therapies. Int J Mol Sci. 2019;20(8):1848. doi:10.3390/ijms20081848
22. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi:10.1038/ncomms2886
23. Feng J, Xiu Q, Huang Y, et al. Plant derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv Mater. 2023;35(24):e2207826. doi:10.1002/adma.202207826
24. Zhang Y, Bi J, Huang J, et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi:10.2147/IJN.S264498
25. Yang XX, Sun C, Wang L, Guo XL. New insight into isolation, identification techniques and medical applications of exosomes. J Control Release. 2019;308:119–129. doi:10.1016/j.jconrel.2019.07.021
26. Coughlan C, Bruce KD, Burgy O, et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol. 2020;88(1). doi:10.1002/cpcb.110
27. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
28. van der Pol E, Hoekstra AG, Sturk A, et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8(12):2596–2607. doi:10.1111/j.1538-7836.2010.04074.x
29. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
30. Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–14893. doi:10.1073/pnas.1408301111
31. Stanly C, Fiume I, Capasso G, Pocsfalvi G. Isolation of exosome-like vesicles from plants by ultracentrifugation on Sucrose/Deuterium Oxide (D2O) density cushions. Methods Mol Biol. 2016;1459:259–269. doi:10.1007/978-1-4939-3804-9_18
32. Woith E, Melzig MF. Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Int J Mol Sci. 2019;20(2):357. doi:10.3390/ijms20020357
33. Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4(6):910–921. doi:10.1039/c5bm00583c
34. Dad HA, Gu T-W, Zhu A-Q, Huang L-Q, Peng L-H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
35. Li X, Bao H, Wang Z, et al. Biogenesis and function of multivesicular bodies in plant immunity. Front Plant Sci. 2018;9:979. doi:10.3389/fpls.2018.00979
36. Fujita D, Arai T, Komori H, et al. Apple-derived nanoparticles modulate expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 cells. Mol Pharm. 2018;15(12):5772–5780. doi:10.1021/acs.molpharmaceut.8b00921
37. Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173(1):728–741. doi:10.1104/pp.16.01253
38. Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22(1):107–126. doi:10.1038/cr.2011.158
39. Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6(9):1312–1321. doi:10.1039/C7TB03207B
40. Sarvarian P, Samadi P, Gholipour E, et al. Application of emerging plant-derived nanoparticles as a novel approach for nano-drug delivery systems. Immunol Invest. 2022;51(4):1039–1059. doi:10.1080/08820139.2021.1891094
41. Smyth T, Kullberg M, Malik N, et al. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi:10.1016/j.jconrel.2014.12.013
42. Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8(2):90–100. doi:10.1038/nrgastro.2010.211
43. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
44. Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi:10.7717/peerj.5186
45. Xie W, Melzig MF. The stability of medicinal plant micrornas in the herb preparation process. Molecules. 2018;23(4):919. doi:10.3390/molecules23040919
46. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652 e638. doi:10.1016/j.chom.2018.10.001
47. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
48. Li X, Liang Z, Du J, et al. Herbal decoctosome is a novel form of medicine. Sci China Life Sci. 2019;62(3):333–348. doi:10.1007/s11427-018-9508-0
49. Chen X, Liu B, Li X, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles. 2021;10(4):e12069. doi:10.1002/jev2.12069
50. Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. doi:10.1158/0008-5472.CAN-14-3095
51. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnology. 2021;19(1):259. doi:10.1186/s12951-021-00995-1
52. Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4(2):e1134415. doi:10.1080/21688370.2015.1134415
53. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
54. Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–1515 e1503. doi:10.1053/j.gastro.2012.02.050
55. Sundaram K, Miller DP, Kumar A, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience. 2019;21:308–327. doi:10.1016/j.isci.2019.10.032
56. Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–364. doi:10.1038/nrrheum.2014.19
57. Alzahrani FA, Khan MI, Kameli N, Alsahafi E, Riza YM. Plant-derived extracellular vesicles and their exciting potential as the future of next-generation drug delivery. Biomolecules. 2023;13(5):839. doi:10.3390/biom13050839
58. Lautenschlager C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71:58–76. doi:10.1016/j.addr.2013.10.001
59. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
60. Lu L, Chen G, Qiu Y, et al. Nanoparticle-based oral delivery systems for colon targeting: principles and design strategies. Sci Bull. 2016;61(9):670–681. doi:10.1007/s11434-016-1056-4
61. Man F, Meng C, Liu Y, et al. The study of ginger-derived extracellular vesicles as a natural nanoscale drug carrier and their intestinal absorption in rats. AAPS Pharm Sci Tech. 2021;22(6):206. doi:10.1208/s12249-021-02087-7
62. Sriwastva MK, Deng ZB, Wang B, et al. Exosome‐like nanoparticles from Mulberry bark prevent DSS‐induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23(3):e53365. doi:10.15252/embr.202153365
63. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
64. Zhang M. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Futuremedicine. 2017;12(6). doi:10.2217/nnm-2017-0196
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.