Back to Journals » Vascular Health and Risk Management » Volume 12
Advances in the management of heart failure: the role of ivabradine
Authors Müller-Werdan U, Stöckl G, Werdan K
Received 30 May 2016
Accepted for publication 12 September 2016
Published 17 November 2016 Volume 2016:12 Pages 453—470
DOI https://doi.org/10.2147/VHRM.S90383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Daniel Duprez
Ursula Müller-Werdan,1,2 Georg Stöckl,3 Karl Werdan4
1Charité – Universitätsmedizin Berlin, 2Protestant Geriatric Centre, Berlin, 3Department of Medical Affairs, Servier Deutschland GmbH, Munich, 4Department of Medicine III, University Hospital Halle (Saale), Martin-Luther-University Halle‑Wittenberg, Halle (Saale), Germany
Abstract: A high resting heart rate (≥70–75 b.p.m.) is a risk factor for patients with heart failure (HF) with reduced ejection fraction (EF), probably in the sense of accelerated atherosclerosis, with an increased morbidity and mortality. Beta-blockers not only reduce heart rate but also have negative inotropic and blood pressure-lowering effects, and therefore, in many patients, they cannot be given in the recommended dose. Ivabradine specifically inhibits the pacemaker current (funny current, If) of the sinoatrial node cells, resulting in therapeutic heart rate lowering without any negative inotropic and blood pressure-lowering effect. According to the European Society of Cardiology guidelines, ivabradine should be considered to reduce the risk of HF hospitalization and cardiovascular death in symptomatic patients with a reduced left ventricular EF ≤35% and sinus rhythm ≥70 b.p.m. despite treatment with an evidence-based dose of beta-blocker or a dose below the recommended dose (recommendation class “IIa” = weight of evidence/opinion is in favor of usefulness/efficacy: “should be considered”; level of evidence “B” = data derived from a single randomized clinical trial or large nonrandomized studies). Using a heart rate cutoff of ≥ 75 b.p.m., as licensed by the European Medicines Agency, treatment with ivabradine 5–7.5 mg b.i.d. reduces cardiovascular mortality by 17%, HF mortality by 39% and HF hospitalization rate by 30%. A high resting heart rate is not only a risk factor in HF with reduced EF but also at least a risk marker in HF with preserved EF, in acute HF and also in special forms of HF. In this review, we discuss the proven role of ivabradine in the validated indication “HF with reduced EF” together with interesting preliminary findings, and the potential role of ivabradine in further, specific forms of HF.
Keywords: heart failure, endotoxin, If inhibitor, ivabradine, pacemaker current inhibitor, heart rate, heart rate variability
Introduction to the management issues in the treatment of heart failure (HF) – the role of ivabradine
During the past half-century, age-adjusted cardiovascular disease-related mortality has declined by about two-thirds in industrialized nations. However, HF is a notable exception with this respect: in the US, hospitalizations because of HF have risen steadily since 1975 up to one million discharges per year.1 In Europe, 1–2% of the population suffer from HF, with the prevalence rising to ≥ 10% among the population aged ≥ 70 years; age-standardized death rate is about 30/100,000 population, with the mean age at death of 83 years.2
HF is not a homogenous entity (Table 1) but consists3 of HF with reduced ejection fraction (HFrEF; LVEF < 40%; “systolic” HF), of HF with preserved ejection fraction (HFpEF4; LVEF ≥ 50%; “diastolic” HF) and – according to the 2016 version of the ESC HF guidelinew – HF with mid-range ejection fraction (HFmrEF; LVEF 40–49%) of either ischemic or nonischemic origin. All these forms can present as acute HF, as chronic stable HF or as decompensated chronic HF.
Evidence-based treatment of all these different forms of HF is shown in the European3 and the American5 HF guidelines.
For the pacemaker channel inhibitor ivabradine, the European HF guideline3 gives the following recommendations for the treatment of HFrEF:
- Ivabradine should be considered to reduce the risk of HF hospitalization and cardiovascular death in symptomatic patients with LVEF ≤ 35%, in sinus rhythm and a resting heart rate ≥ 70 b.p.m. despite treatment with an evidence-based dose of beta-blocker (or maximum tolerated dose below that), an ACE inhibitor (or ARB) and an MRA (or ARB) (IIa/B) (recommendation class “IIa” = weight of evidence/opinion is in favor of usefulness/efficacy: “should be considered”; level of evidence “B” = data derived from a single randomized clinical trial or large nonrandomized studies).
- Ivabradine should be considered to reduce the risk of HF hospitalization and cardiovascular death in symptomatic patients with LVEF ≤ 35%, in sinus rhythm and with a resting heart rate ≥ 70 b.p.m. who are unable to tolerate or have contraindications for a beta-blocker. Patients should also receive an ACE inhibitor (or ARB) and an MRA (or ARB) (IIb/C) (recommendation class “IIb” = usefulness/efficacy is less well established by evidence/opinion; level of evidence “C” = consensus of the opinions of the experts and/or small studies, retrospective studies and registries).
In Germany, ivabradine (ivabradine hydrochloride as Procoralan® [Servier, Neuilly-sur-Seine, France]; 5 mg/7.5 mg film-coated tablets; starting dose 5 mg b.i.d., target dose 7.5 mg b.i.d.) is licensed for patients with chronic HF (NYHA II–IV) with systolic dysfunction who have sinus rhythm with a heart rate ≥ 75 b.p.m. It is used in combination with standard therapy including beta-blockers, or in patients who cannot be treated with beta-blockers.
The cardiovascular risk factor “resting heart rate”
Resting heart rate as a risk marker and risk factor
Among mammals, the number of heartbeats per lifetime is relatively constant (7.3 ± 5.6 × 108 heartbeats/lifetime) which is explained by the dependency of lifetime on the rules of energetics.6 However, it was only when ivabradine, a selective heart rate-reducing agent, was launched in cardiovascular medicine that interest regained in the role of an inadequately high heart rate as a simple-to-measure risk marker of morbidity and mortality.7
Heart rate is an important determinant of the cardiac oxygen equilibrium, with high heart rates leading to increased myocardial oxygen demand and impaired oxygen supply by shortening of diastolic time during the cardiac cycle.8 An elevated heart rate causes shortening of the duration of the whole cardiac cycle, predominantly at the cost of diastolic duration because systolic time remains fairly stable. The association of heart rate and diastolic duration is not linear, showing disproportionate shortening of diastolic time with rising heart rate. In contrast, low heart rates induce prolongation of diastolic duration, thereby improving coronary blood flow and oxygen supply, as perfusion of coronary arteries occurs mainly in diastole.9
For the sake of reproducibility, resting heart rate in patients should be measured in a standardized manner, with a resting phase of 5 (to 10) min before measurement, with at least two pulse measurements in sitting position with a duration of at least 30 s.
Increased resting heart rate indeed is a risk factor, a sign of sympathetic hyperactivity and/or reduced parasympathetic tone, with many detrimental consequences of a disturbed autonomic balance:7,10–17 acceleration of coronary atherosclerosis,18 plaque rupture and coronary heart disease, impairment of arterial compliance and distensibility, increase in the risk of coronary thrombosis, subclinical inflammation and reactive oxygen species (ROS) generation, myocardial ischemia, induction of left ventricular (LV) dysfunction and life-threatening ventricular arrhythmias. The main connecting link between high heart rate and morbidity and mortality is supposed to be the endothelial damage and dysfunction triggered by high heart rate, representing some kind of accelerated atherosclerosis.10,11,18
Many of these heart rate-induced impairments of vessels and heart can be improved by treatment with the heart rate-reducing agent ivabradine.10,11,13–17,19 Even life span prolongation in mice was reported by ivabradine-induced heart rate reduction.20 Though a good correlation exists between ivabradine-reduced heart rate reduction and beneficial actions, findings for some heart rate-independent effects of ivabradine were also reported.11,21–25
Resting heart rate as a risk factor of HF
A high resting heart rate is of prognostic relevance not only in middle-aged and elderly men and women with no apparent heart disease12 but also in a broad spectrum of cardiovascular diseases,7 including chronic HFrEF26–30 and acute/decompensated HFrEF31 as well as chronic HFpEF32–34 and acute/decompensated HFpEF.31
For the largest patient group, those with chronic HFrEF, Table 2 presents the prognostic relevance of a high resting heart rate (≥75/min vs. < 75/min) with respect to mortality and hospitalization because of HF, both in a large RCT (SHIFT study) and in a registry of ambulatory care.
  | Table 2 Prognostic relevance of resting heart rate in chronic systolic heart failure in sinus rhythm Notes: SHIFT study:27 data are given from patients of the placebo group (sinus rhythm ≥ 70/min; LVEF ≤ 35%; NYHA II–IV) under standard heart failure treatment including beta-blocker and excluding ivabradine. Of the total patients, 64.3% had a resting heart rate ≥ 75/min, with a higher value of the primary end point and the secondary end point “mortality” than those with a resting heart rate < 75/min. Registry data:30 data are given from patients from outpatient clinics (sinus rhythm; LVEF ≤ 35%; NYHA II–IV), with standard heart failure treatment including beta-blocker and excluding ivabradine. Of the total patients, 53% had a resting heart rate ≥ 75/min, with a higher value of the primary end point and the secondary end point “mortality” than those with a resting heart rate < 75/min. Abbreviations: LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RR, relative risk. |
In chronic HF patients of the CHARM study, baseline resting heart rate in those with sinus rhythm is associated with increased mortality, with every 10-b.p.m. increase associated with respective increases of 8% in all-cause mortality, in both HFrEF and HFpEF; however, this association was not observed in the 15% of patients with atrial fibrillation at baseline.28,35
A high heart rate also indicates an unfavorable prognosis in specific forms of acutely life-threatening disease states with impaired heart function (explained in the “Specific forms of HF” section and Table 1).
Ivabradine – mode of action and pharmacology
If as a target of beta-blockers and ivabradine
HCN channels in the sinoatrial node
Target protein for physiological heart rate variation and pharmacologically induced therapeutic heart rate modification is the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel as tetramer in four isoforms. The HCN channels constitute the pacemaker current If in the sinoatrial node cells of the heart (Figure 1). In the human sinoatrial node, HCN1 (exclusively), HCN2 and HCN4 proteins are expressed.37
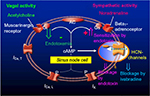  | Figure 1 Effects of endotoxin on pacemaker current If (HCN channels) and adrenergic pacemaker current If stimulation in human atrial cardiomyocytes. Notes: Endotoxin not only inhibits If but also intensifies beta-adrenoceptor-mediated stimulation of If.36 If inhibition by endotoxin is not an unspecific effect, as the L-type calcium current is not inhibited by endotoxin. ⇑ indicates stimulation and ⇓ indicates inhibition. Abbreviations: AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; Gi, inhibitory G protein; Gs, stimulatory G protein; HCN, hyperpolarization-activated cyclic nucleotide-gated; ICa,T, T-type calcium current; ICa,L, L-type calcium current; If, “funny” current; IK, potassium current. |
The pacemaker current If and regulation of cardiac automaticity
The pacemaker current If (Figure 1) generates spontaneous depolarizations toward the initiation threshold for an action potential in the sinoatrial node cells.38 Velocity of this spontaneous depolarization can be enhanced by sympathetic activity, mediated by norepinephrine binding to the beta1-adrenoceptors of these cells, thereby triggering a rise in intracellular cyclic adenosine monophosphate (cAMP) level. In contrast, velocity of this spontaneous depolarization can be slowed by the vagal activity, mediated by binding of acetylcholine to muscarinergic receptors. By binding to the beta1-adrenoceptors of the sinoatrial node cells, beta-blockers attenuate the endogenous sympathetic chronotropic effect. However, beta-blockers of course do bind not only to the beta1-adrenoceptors of the sinoatrial node cells but also to beta-adrenoceptors of many other cells. The consequence is that beta-blockers do have not only negative chronotropic but also negative inotropic effects on the heart, and they exert blockage of many well-known beta-adrenoceptor-mediated effects in various organs. Furthermore, beta-blockade may interact in an unwanted mode with alpha-adrenoceptor stimulation on the coronary circulation.39
However, it has to be mentioned that the If current mediated by HCN channels is not the only relevant determinant of cardiac rhythm generation.38 It is now well established that other ion currents, for example, triggered by L- and T-type calcium channels,40 and also by potassium channels, contribute to diastolic depolarization and action potential generation.41 Apart from membrane voltage-gated ion channels (“membrane clock”), other mechanisms have been identified as important components in rhythm control, namely, spontaneous rhythmic calcium release from the SR. This process activates a current generated by the sodium–calcium exchanger, which contributes to diastolic depolarization (“calcium clock”), ultimately triggering action potential firing.42 Although there is debate whether one of these “clock” mechanisms is the key initiator of diastolic depolarization and cardiac pacemaking,43 a close interplay between both concepts (“coupled clock”) seems to be crucial for proper rhythm generation in pacemaker cells.44
Interestingly in this context, it has recently been shown that ivabradine not only selectively blocks If in sinoatrial nodal pacemaker cells but is also associated with other unspecific effects on the calcium-dependent components of the “coupled clock” pacemaker mechanisms, for example, reduced SR calcium loading and a prolongation of the period of spontaneous local calcium releases from SR stores. Thus, ivabradine indirectly suppresses intracellular calcium cycling, which also contributes to the bradycardic effects of the drug, indicating once more the relevance of a coordinated cross talk between the two pacemaking “clocks”.45,46
Ivabradine and the pacemaker current If
In therapeutic concentrations (starting dose 5 mg b.i.d., target dose 7.5 mg b.i.d.), the If inhibitor ivabradine binds to HCN channel proteins in the sinoatrial node, thereby reducing the slope of If , with consecutive lowering of heart rate in patients with sinus rhythm. With respect to the heart, the action is specific for the sinoatrial node, with no other effects, either on intraatrial, atrioventricular (PQ interval) or intraventricular conduction time or on myocardial contractility or ventricular repolarization (QTc interval). Also, blood pressure is not altered.
Ivabradine and heart rate variability
HRV is considered as a measure of autonomic nervous system function, and a reduced HRV has been identified as an independent predictor of cardiac events and death in the general population without apparent heart disease,47 and also in those with chronic cardiac disease such as systolic HF.48 These results support the concept of autonomic imbalance with sympathetic overactivity as an important feature in HF.
Heart rate in general49 and also selective heart rate reduction by ivabradine are inversely correlated to HRV, as shown in a study with conscious rats.50 Improvement of HRV with ivabradine was blocked in this model by administration of the beta-blocker propranolol or the muscarinic antagonist atropine, indicating the critical importance of the two components of the autonomic nervous system.
Though acute administration of ivabradine in a short-term animal study was associated with a reflex increase of sympathetic nerve activity,51 there clearly seem to be beneficial long-term effects in clinical practice, as demonstrated by the 24-h Holter ECG substudy (n = 602 patients) of the SHIFT. A significant improvement in time domain HRV indices and various parameters of parasympathetic activity was demonstrated in the well-treated (including ACE inhibitors and beta-blockers) systolic HF patients after 8 months of additional treatment with ivabradine compared to placebo.52 Possible explanations for these positive effects of ivabradine on HRV include the following: prolonged diastole with enhanced cardiac blood supply and ventricular filling; and positive effects on structural ventricular remodeling and reduced sympathetic influence with concomitantly enhanced vagal tone, resulting in an improvement of the autonomic imbalance in sympatho-vagal regulation. These findings could be confirmed in a smaller patient cohort (n = 48) with nonischemic dilated cardiomyopathy, also demonstrating beneficial effects on a number of HRV indices with additional ivabradine treatment for 8 weeks.53
Pharmacology of ivabradine
Treatment should be started with 5 mg ivabradine b.i.d. (in patients > 75 years: 2.5 mg b.i.d.). After 3–4 weeks, dosage can be increased in the still symptomatic patients to the maximum dose of 7.5 mg b.i.d. In case of lowering of the heart rate < 50 b.p.m. or in case of symptomatic bradycardia under treatment, dosage should be reduced down to a minimum of 2.5 mg b.i.d. Treatment must be stopped if – despite dose reduction – heart rate remains < 50 b.p.m. or symptomatic bradycardia persists.
The enteral absorption of ivabradine hydrochloride taken as Procoralan tablet after oral ingestion is quick and nearly complete. In the fastened state, highest plasma levels will be achieved after 1 h. Bioavailability is about 40% (first-pass effect). Seventy percent of ivabradine in blood is bound to plasma proteins. The drug has an effective half-time of 11 h and is given twice daily. In patients under daily oral treatment with 5 mg b.i.d., peak concentration of 20 ng/mL and steady-state concentration of 10 ng/mL of ivabradine can be measured.54–56
The intravenous route57,58 is still experimental and not approved for clinical use.
HCN channels and inflammation
Endotoxin directly blocks If and indirectly sensitizes If to beta-adrenoceptor agonists
Several diseases involving the heart show an inflammatory component due to systemic inflammation, such as septic shock and multiorgan dysfunction syndrome (MODS). Further on, also bacterial and endotoxin translocation from the gut into the systemic circulation due to hypotension and low cardiac output – as in severe HF and cardiogenic shock – can aggravate cardiac impairment by cardiodepressive and antiarrhythmic effects of endotoxin (lipopolysaccharide ) and inflammatory mediators. And indeed, in cardiogenic shock complicating myocardial infarction, the proinflammatory cytokine interleukin-6 is a better predictor of unfavorable outcome than BNP.59
Though cardiodepression is in the focus of cardiac impairment, endotoxin also interferes with the rhythm: in human atrial cardiomyocytes which also bear HCN channels as do sinoatrial node cells, endotoxin was found to significantly impair If by suppressing the current at membrane potentials positive to –80 mV and slowing down current activation, but without effecting maximal current conductance. In a model of a spontaneously active sinoatrial cell, endotoxin-induced If impairment reduced the responsiveness of the model cell to fluctuations of autonomic input, thereby reducing heart rate variability between 38% and 62%.36 This endotoxin effect is not mediated by any of the intracellular modulatory pathways affecting HCN channels, but is instead due to a direct interaction with the channel.60–62 In addition to this direct inhibitory effect of endotoxin on If , endotoxin in human atrial cardiomyocytes also sensitizes If to beta-adrenergic stimulation, thereby increasing heart rate.36 In mice in vivo, endotoxin does not lower but increase heart rate,63 as can also be seen in humans. However, when autonomous nervous system in these mice is blocked by propranolol and atropine, endotoxin induces a significant reduction of heart rate.63 Thus, both effects can be demonstrated in the mice, while under in vivo conditions, the positive chronotropic effect of endotoxin by sensitization of If to the positive chronotropic beta-adrenoceptor stimulation prevails the direct negative chronotropic effect on If.
Ivabradine blocks If even in the presence of endotoxin
Even in the presence of endotoxin, ivabradine can further decelerate pacemaker activity of human atrial cardiomyocytes and thereby effectively reduce heart rate.64
Clinical efficacy, safety and tolerability of ivabradine in HF
Ivabradine is approved for treatment of patients with chronic HFrEF, based on the data of the SHIFT study.16 All other forms of HF remain potential indications for the future.65
Needless to say that therapy with the pacemaker channel inhibitor ivabradine is restricted to patients with sinus rhythm. This is an important limitation, as atrial fibrillation is frequent in patients with HFrEF, ranging from 5% in mild to 10–26% in moderate and up to 50% in severe HF.66,67
Chronic HFrEF
The SHIFT
The SHIFT (“Systolic Heart failure treatment with the If inhibitor ivabradine Trial)16 is the landmark trial for ivabradine treatment of patients with chronic HFrEF: 6,558 patients with stable (≥4 weeks), chronic symptomatic HF under standard medication, an ejection fraction of ≤ 35%, a previous admission to hospital for worsening HF within the previous 12 months and sinus rhythm ≥ 70 b.p.m. were additionally treated using standard regimen with either placebo or ivabradine. Ivabradine regimen was started with 5 mg b.i.d., and the dose was either maintained if resting heart rate was between 50 b.p.m. and 60 b.p.m. or increased to 7.5 mg b.i.d. unless the resting heart rate was 60 b.p.m. or lower. Demographic data were as follows: mean age 60 years, 77% male; mean heart rate 80 b.p.m.; mean RRsyst 122 mm Hg; mean LVEF 19%, NYHA II/III 49%/50%, ischemic origin 68%; treatment with beta-blockers 90%, ACE inhibitors 79%, ARB 14%, diuretics 84%, MRAs 61% and cardiac glycosides 14%. Excluded were patients with HF due to congenital heart disease or primary severe valvular disease. After a median follow-up for analysis of 22.9 months (interquartile range 18–28 months) with a mean ivabradine dosage of 6.4 ± 1.6 mg b.i.d. at day 28 and 6.5 ± 1.6 mg b.i.d. at 1 year, the primary end point – composite of cardiovascular death or hospital admission for worsening HF – was achieved in 29% in the placebo group, but only in 24% in the ivabradine group, representing a significant relative reduction of 18% (HR 0.82; 95% CI 0.75–0.90; p < 0.0001). The effects were driven mainly by hospital admissions for worsening HF (21% vs. 16%; HR 0.74; 95% CI 0.66–0.83; p < 00001), while cardiovascular death was not significantly different (15% vs. 14%; HR 0.91; 95% CI 0.80–1.03; p = 0.128). However, deaths from HF were significantly reduced (5% vs. 3%; HR 0.74; 95% CI 0.58–0.94; p = 0.014). Five percent of ivabradine patients had symptomatic bradycardia compared with 1% of the placebo group (p < 0.0001). Visual side effects (phosphenes) occurred in 3% of patients on ivabradine and in 1% of patients on placebo (p < 0.0001).
In view of the positive SHIFT data, the neutral data of the BEAUTIFUL trial29,68 with 10,917 patients are a bit surprising. No beneficial effect of ivabradine in these patients with stable coronary artery disease and LV dysfunction (mean LVEF 32%) was seen with respect to cardiovascular death and to admission to hospital for acute myocardial infarction, and for new onset or worsening HF, either in the total population (heart rate ≥60 b.p.m.) or in the prespecified subpopulation (49%) with a heart rate ≥70%. In the SHIFT, 100% of the patients had symptomatic HF (NYHA II: 49%; NYHA III: 50%; NYHA IV: 2%), and in the BEAUTIFUL trial, 84% had symptomatic HF (NYHA I: 15%; NYHA II: 61%; NYHA III: 23%). From this comparison, it becomes evident that patients in the SHIFT had more severe symptomatic HF than those in the BEAUTIFUL trial. Also, another aspect might add to the discrepancy: in view of the SHIFT analysis, one could also speculate that patients with nonischemic HF might benefit even more (primary composite end point: HR 0.72; 95% CI 0.60–0.85) than patients with ischemic HF (HR 0.87; 95% CI 0.78–0.97; test for interaction, p = 0.59).
And finally, a word of caution may be allowed, though concerning patients without HF. In patients with stable coronary artery disease and sinus rhythm ≥70 b.p.m., but without clinical HF, ivabradine is of symptomatic but not of prognostic value, as shown in the SIGNIFY trial.19 In the prespecified SIGNIFY subgroup of patients with angina Canadian Cardiovascular Society grade ≥2, even a higher incidence of the primary end point – cardiovascular death or nonfatal myocardial infarction – was observed (HR 1.18; 95% CI 1.03–1.35; p = 0.02).
The EMA decision for a cutoff of ≥ 75 b.p.m.
Based on the positive data of the SHIFT, the European Medicines Agency (EMA) approved ivabradine for treatment of chronic heart failure. Ivabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction in patients in sinus rhythm and whose heart rate is >/= 75 b.p.m., in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. Therapy approval is given for patients with sinus rhythm ≥ 75 b.p.m. This – at a first glance – was a bit surprising, as the beneficial effects of SHIFT have been obtained in patients with a heart rate ≥ 70 b.p.m. This decision by the EMA is based on a retrospective analysis from the SHIFT study.27 When analyzing the benefit of ivabradine medication in SHIFT in patients with an initial heart rate ≥ 75 b.p.m., it becomes evident (Table 3) that those patients had not only a reduced rate of HF hospitalizations (30%) and deaths from HF (39%) but also a significant reduction of cardiovascular death rate by 17%. In contrast, the SHIFT patients with an initial heart rate < 75 b.p.m. did not benefit significantly regarding outcomes.27
  | Table 3 Therapeutic effects of heart rate reduction by ivabradine in heart failure patients with systolic dysfunction and sinus rhythm ≥ 75/min – data from the SHIFT Note: Data from the SHIFT study.27 Abbreviations: EF, ejection fraction; f, heart rate; NYHA, New York Heart Association. |
The INTENSIFY study: proof of concept in daily clinical practice
The INTENSIFY study is a prospective, open-label, multicenter, nonintervention study.69 A group of 1,956 HFrEF patients with an initial sinus rhythm (85 ± 11 b.p.m.) were treated in German outpatient clinics with ivabradine (44.1% of patients received 5 mg b.i.d., 52.4% received 7.5 mg b.i.d. and 3.5% received 2.5 mg b.i.d.).
After 4 months of treatment, heart rate has fallen down to 67 ± 8.9 b.p.m. In parallel with this heart rate reduction, the proportion of patients with signs of decompensation fell from 22.7% initially to 5.4%, and the proportion of BNP levels > 400 pg/mL dropped from 53.9% to 26.7%. This coincided with a reduction in NYHA class from 9.6% (I), 51.1% (II), 37.2% (III) and 2.1% (IV) initially to 24.0% (I), 60.5% (II), 14.8% (III) and 0.7% (IV), respectively.
After 4 months, the physicians rated the effectiveness of ivabradine as very good in 54.9% of patients and good in 41.5%. Tolerability was rated by the physicians as very good in 68.2% and good in 31.0%. At least one adverse event occurred in 2.9% of patients treated with ivabradine, with 1.4% being cardiac events, 0.5% related to the nervous system and 0.5% being eye events. Bradycardia (0.3%) was mainly seen in patients with an initial heart rate < 75 b.p.m. In 4.4% of the patients, the study drug was discontinued for different reasons (patient’s request: 50%, insufficient efficacy: 14.1%, intolerance: 20.5%, lack of compliance: 15.4%, other reasons: 29.5%). During this 4-month period, 0.3% of patients died, reflecting a low-risk chronic systolic heart failure outpatient cohort. In summary, the INTENSIFY study documented over a 4-month period of treatment that ivabradine – in 77.8% given in addition to a beta-blocker – can effectively reduce heart rate and symptoms in patients with HFrEF under daily clinical practice.69 A similar heart rate reduction and safety profile of ivabradine were also reported in the ADDITIONS study70 on 2,330 patients with stable angina pectoris. Needless to say that nonintervention trials such as the INTENSIFY study as well as the ADDITIONS study have well-known limitations: they were not blinded, they were not placebo controlled and both studies had a relatively short follow-up of 4 months.
Intravenous ivabradine in advanced systolic HF
De Ferrari et al57 looked for the hemodynamic effects (Table 4) of intravenously given ivabradine in ten NYHA class III patients (50 ± 12 years) with advanced HFrEF (mean LVEF 21 ± 7%) and sinus rhythm with a mean heart rate of 93 ± 8 b.p.m. Ivabradine regimen was started by an infusion of 0.1 mg/kg over 90 min, followed by 0.05–0.075 mg/kg in the subsequent 90 min. At 4 h, the time point of largest hemodynamic changes achieved, ivabradine significantly had reduced heart rate at the maximum of 27% (24% and 30% among patients treated (n = 6) and not treated (n = 4) with beta-blockers, respectively), without decreasing cardiac index, but leading to a significant increase in SV and LV systolic work. Heart rate remained significantly reduced up to 24 h, when heart rate was still significantly 11 b.p.m. lower than baseline. No significant effect of ivabradine was found on PR, QRS or QT intervals or on laboratory findings.57
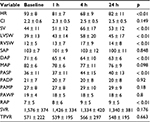  | Table 4 Hemodynamic effects of intravenously applied ivabradine in patients with advanced heart failure Note: The p values are for the prespecified contrasts between baseline and 3, 4, 6, 8, and 24 h of Table 2 of De Ferrari GM, Mazzuero A, Agnesina L, et al. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail. 2008;10(6):550–555.57 Abbreviations: CI, cardiac index (L ×min–1×m–2); DAP, diastolic arterial pressure (mm Hg); HR, heart rate (min–1); LVSW, left ventricular systolic work (g); MAP, mean arterial pressure (mm Hg); PADP, pulmonary arterial diastolic pressure (mm Hg); PAMP, pulmonary arterial mean pressure (mm Hg); PASP, pulmonary arterial systolic pressure (mm Hg); PAWP, pulmonary artery wedge pressure (mm Hg); RAP, right atrial pressure (mm Hg); RVSW, right ventricular systolic work (g); SAP, systolic arterial pressure (mm Hg); SV, stroke volume (mL); SVR, systemic vascular resistance (dyn×s×cm–5); TPVR, total pulmonary vascular resistance (dyn×s×cm–5); h, hours. |
Chronic HFpEF
Prognosis
Despite somewhat lower than that of HFrEF, mortality of HFpEF ranges from 10% to 30% annually.71 The majority of deaths in HFpEF are of cardiovascular origin (sudden death and HF), comprising 51–60% of deaths in epidemiological studies and ~70% in clinical trials. Non-cardiovascular deaths constitute a higher proportion of deaths in HFpEF than in HFrEF.71 HFpEF with atrial fibrillation represents a more advanced disease than HFpEF with sinus rhythm.72
Relevance of resting heart rate
Relevant for morbidity and mortality
Also in HFpEF, a high heart rate indicates unfavorable morbidity (HF hospitalization rates33) and also unfavorable all-cause, cardiovascular and HF mortality.32,34
Heart rate as a therapeutic target
In HFpEF, neither for ACE-inhibitors/ARB4 or beta-blockers3,73–77 nor for MRAs,3,78–80 a beneficial therapeutic effect has been shown. A positive exception could be effective treatment by beta-blockers of HFpEF risk factors such as hypertension, thereby delaying the progression of HFpEF.81
With respect to ivabradine, no RCT with a primary composite end point “cardiovascular death or hospital admission for worsening HF” does exist for HFpEF patients.4
Ivabradine and exercise tolerance in HFpEF
Patients with HFpEF suffer from exertional dyspnea and exercise intolerance. Ivabradine seems to be able to improve exercise-induced symptoms. Seventy-one patients with HFpEF (NYHA II/III; EF ≥50%; age 67 ± 9 years; 55% under beta-blocker treatment) in sinus rhythm (resting heart rate 72 ± 7 b.p.m.), reduced exercise capacity (< 80% of normal range) and increased LV filling pressure during exertion were treated for 7 days with either 5 mg ivabradine twice daily or placebo.82 After 7 days, only the ivabradine group demonstrated a significant reduction in resting heart rate (62 ± 8 b.p.m. compared to baseline heart rate of 72 ± 7 b.p.m; p = 0.001), combined with a significant improvement in exercise capacity (from 4.2 ± 1.8 METs at baseline to 5.7 ± 1.9 METs, p = 0.001) as well as in peak oxygen uptake (from 14.0 ± 6.1 mL/min/kg to 17.0 ± 3.3 mL/min/kg; p = 0.001). The change in METs was greater in the treated patients than in controls (∆ 1.5 ± 1.2 METs vs. ∆ 0.4 ± 1.2 METs, p = 0.001) as was the change in peak volume of oxygen uptake (∆ 3.0 ± 3.6 mL/min/kg vs. ∆ 0.4 ± 2.7 mL/min/kg, p = 0.003). In parallel, the ivabradine group showed an improvement in resting LV lusitropic function, while the placebo group did not, with the intergroup difference being significant (p = 0.01). During exercise, LV filling pressure was reduced by ivabradine.
These positive short-time (7 days) effects of the randomized study82 have been extended to 12 months by an observational study83 with 48 HFpEF patients (NYHA II/III; 55 ± 10 years), treated with ivabradine (5 mg twice daily; in case if heart rate did not decrease < 75 b.p.m., 7.5 mg twice daily). After 1 month, resting heart rate had fallen from 85 ± 5 b.p.m. to 68 ± 4 b.p.m., exercise duration had increased from 6.1 ± 1.9 min to 7.1 ± 1.3 min, in parallel with an increase in peak oxygen uptake from 16 mL/min/kg to 18 mL/min/kg, and an improvement in diastolic dysfunction. These improvements remained stable thereafter for the full observation period of 12 months.
Very similar positive findings of ivabradine in HFpEF patients were described up to 36 months by several groups.84–86 Switching from beta-blockers to ivabradine in patients with coronary artery disease and elevated LV filling pressure (E/e′ > 8) may cause a reduction in LV filling pressure and an improved SV response to exercise.84 On the other hand, Pal et al87 did not see these positive effects in a randomized, crossover study. They compared selective heart rate reduction by ivabradine (7.5 mg twice daily) with placebo for 2 weeks each in 22 symptomatic patients with HFpEF who had objective evidence of exercise limitation (peak oxygen consumption at maximal exercise < 80% of normal range) in comparison with 22 similarly treated matched asymptomatic hypertensive volunteers. Ivabradine selectively reduced peak heart rate compared with placebo in the HFpEF (107 b.p.m. vs. 129 b.p.m.; p < 0.0001) and hypertensive (127 b.p.m. vs. 145 b.p.m.; p = 0.003) cohorts. However, submaximal exercise capacity was not enhanced but reduced by ivabradine vs. placebo, and the same was true for the change in peak oxygen consumption (–2.1 mL/min/kg vs. 0.9 mL/min/kg). The authors87 state that the reasons underlying the discrepant results of their own and those of Kosmala et al82 are unclear, but may be due to the different age of the study patients – 74.6 ± 5.9 years87 vs. 66.5 ± 8.5 years.82 Older patients have an advanced chronotropic incompetence and a diminished SV reserve (a largely fixed SV) and are therefore more sensitive to heart rate reduction. The larger mean resting heart rate reduction from 77 b.p.m. to 57 b.p.m. achieved with 7.5 mg ivabradine twice daily in these older patients87 in comparison to the reduction of only 10 b.p.m. by ivabradine 5 mg twice daily in the younger patient group82 might support this hypothesis.
Taken together, there is some evidence that ivabradine can improve exercise capacity and attenuate diastolic dysfunction in HFpEF patients, but further supporting evidence is needed.88,89
Acute/decompensated chronic HF
Limited study results
Also in patients with acute/decompensated HF,90–92 ivabradine has been applied by oral route.93,94 Sargento et al94 studied ten consecutive patients with acute/decompensated HFrEF under full standard treatment, an LVEF < 40% (mean 31.2 ± 9.2%), RRsyst > 90 mm Hg and sinus rhythm > 70 b.p.m. (admission: mean 88.3 ± 11.1 b.p.m.; immediately before ivabradine addition: 82.8 ± 13.9 b.p.m.). Ivabradine was given orally (5 mg b.i.d.; in patients > 75 years, a lower dose of 2.5 mg b.i.d. could be considered), with 60% of patients having started this therapy by the second day. Preexisting mean serum creatinine was 1.48 ± 0.6 mg/dL, and mean GFRMDRD was 62.9 ± 29.8 mL/min/1.73 m2. The mean dose of ivabradine at discharge was 9.5 ± 2.8 mg/day. Ivabradine treatment resulted in a reduction in heart rate by 10.7 ± 7.2 b.p.m. 24 h after starting treatment and by 16.3 ± 8.2 b.p.m. at discharge. RRsyst was significantly decreased 24 h after initiation of ivabradine (from 113.2 ± 17.4 mm Hg to 105.6 ± 13.6 mm Hg; p = 0.008), but diastolic and mean blood pressure were not. The surrogate marker Nt-ProBNP at baseline and heart rate correlated significantly (p = 0.013). At discharge, the NYHA class had fallen by one and two levels in 70% and 30% of patients, respectively. The subgroup whose NYHA class fell by two levels had lower heart rate values at discharge (69.7 ± 7.6 b.p.m. vs. 59.0 ± 3.6 b.p.m.; p = 0.033). None of the patients suspended ivabradine. There were no reports of hemodynamic deterioration because of symptomatic bradycardia or other adverse events.94
Lowering catecholamine-induced tachycardia by ivabradine
In those patients with acute/decompensated heart necessitating inotropes according to guidelines recommendations,3 ivabradine via the oral route has effectively been tried to attenuate a detrimental heart rate increase triggered by inotropes used such as dobutamine.95,96
Specific forms of HF
Sinus tachycardia in cardiac transplant patients
Cardiac transplant patients often have a relatively high resting heart rate (> 90 b.p.m.) due to graft denervation. Several studies described an effective heart rate reduction in cardiac transplant patients with a beta-blocker, with diltiazem or with ivabradine.97–99 However, there is no clear consensus about what the normal range of heart rate should be following heart transplantation and whether therapeutic heart rate reduction might be of prognostic relevance.99
Chronic or prolonged severe tachycardia in cardiac transplant patients – especially in combination with reduced pump function – can trigger cardiac decompensation and shock. Beta-blockers for control of tachycardia are only helpful when contractility of the heart is not severely impaired; otherwise, they bear the risk of further deterioration of heart function. In this situation, ivabradine might be helpful to control sinus tachycardia. Zwicker et al100 report successful treatment with ivabradine of a heart transplant patient who developed cardiogenic shock due to long-acting tachycardia (sinus tachycardia of 120/min), with further deterioration of cardiac function after administration of the short-acting beta-blocker esmolol under invasive hemodynamic monitoring. Heart rate control by administration of increasing doses of ivabradine supported recovery from cardiogenic shock and led to an improvement in the patient’s clinical condition as well as LV function during follow-up.100
Acute peripartum cardiomyopathy (PPCM)
High initial resting heart rate in a patient with PPCM indicates poor prognosis.101 The proposed therapeutic strategy consists of treatment with standard HF medication.102 In the absence of complete recovery and when beta-blocker uptitration is not possible and sinus rhythm is > 75 b.p.m., ivabradine can be given. Ivabradine should be tapered, when beta-blocker uptitration is possible and/or heart rate is < 60 b.p.m. After complete and sustained recovery of LV structure and function, ivabradine medication should be continued when sinus rhythm is > 75 b.p.m. despite beta-blocker uptitration.102 An impressive improvement of clinical symptoms and cardiac function by additional ivabradine treatment has been described in 20 patients in a substudy of the PPCM registry.103
Tachycardia in cardiogenic shock complicating myocardial infarction
Patients with cardiogenic shock complicating myocardial infarction (post-AMI CS) usually show a high heart rate, often aggravated by catecholamine therapy. A case report104 and a small randomized trial105 documented the heart rate-lowering effect of ivabradine in post-AMI CS patients and also reported some beneficial therapeutic effects.
In the prospective randomized trial,105, 58 patients with post-AMI CS in sinus rhythm and after primary percutaneous coronary intervention were treated either with standard medical treatment (28 patients) or with standard medical treatment plus ivabradine (30 patients; starting with 2.5 mg b.i.d.) given orally or – in mechanically ventilated patients – by nasogastric route. Heart rate in the ivabradine group fell from 97.2 ± 6.8 b.p.m. initially to 86.9 ± 4.8 b.p.m. after 1 week, to 79.8 ± 5.5 b.p.m. after 4 weeks and to 65.7 ± 9.8 b.p.m. after 6 months. In the control group, the respective values were 94.6 ± 6.0 b.p.m. initially (intergroup difference: not significant), 90.5 ± 4.6 b.p.m. after 1 week (intergroup difference: not significant), 87.0 ± 6.9 b.p.m. after 4 weeks (intergroup difference: p < 0.005) and 81.9 ± 7.5 b.p.m. after 6 months (intergroup difference: p < 0.001). Better improvement in the ivabradine group occurred with respect to blood pressure stabilization, LVEF, LV diastolic dysfunction and NT-ProBNP. Two patients died in the ivabradine group, and four patients died in the control group. Patients in the ivabradine group did not experience adverse events.
Septic shock and multiple organ dysfunction syndrome (MODS)
Heart rate as a risk marker
An inadequately high resting heart rate – as a component of autonomic dysfunction – is a well-known phenomenon in patients with septic shock106 and in critically ill patients with MODS107 in general. High heart rate in MODS patients is of prognostic relevance: in a study with 89 patients with MODS of septic and of non-septic origin108 (APACHE II score ≥ 20), median baseline heart rate was 83 b.p.m. in 28-day survivors and 92 b.p.m. in 28-day non-survivors (p = 0.048; aHR 2.3 for initial heart rate ≥ 90/< 90 b.p.m.).
Heart rate reduction by the short-acting beta-blocker esmolol
In a trial by Morelli et al,109 154 patients with septic shock and a heart rate of at least 95 b.p.m. were randomized either to a 96-h infusion with esmolol (25–2,000 mg/h) or to placebo. Esmolol reduced heart rate by 28 b.p.m. (control: –6 b.p.m.), and all esmolol patients did achieve the prespecified heart rate corridor of 80–94 b.p.m., which was the primary end point. In the control group, 28-day mortality was 80.5%, and in the esmolol group, 49.4% (aHR 0.39; 95% CI 0.26–0.59; p < 0.001).
Heart rate reduction by ivabradine
In the MODIFY trial (protocol110), we prospectively randomized 70 patients with MODS (APACHE II score ≥ 20) of septic and non-septic origin with an elevated heart rate ≥90 b.p.m. to either a standard therapy or a standard therapy plus a 4-day treatment with ivabradine given by the enteral route (up to 7.5 mg b.i.d.). Primary end point was proportion of patients with a reduction of heart rate by at least 10 b.p.m. In the ivabradine group, initial heart rate fell after the 4-day treatment from an initial value of > 100 b.p.m. to < 90 b.p.m.; 28-day mortality was not significantly different in both groups (Werdan et al, in preparation).
Heart rate, a risk marker or risk factor?
Future trials should answer the question whether the high heart rate as a component of septic cardiomyopathy106 and MODS108 is indeed a risk factor and not only a risk marker, and whether lowering of a high resting heart rate can indeed lower mortality in these patients.
Bradycardia in out-of-hospital cardiac arrest (OHCA) patients indicates favorable prognosis
Bradycardia during targeted temperature management (TTM) of patients with Return-Of-Spontaneous-Circulation (ROSC) is a physiologic response to lower body temperature and has been associated with favorable outcome in smaller observational studies. This finding was confirmed in a large multicenter cohort of comatose ROSC patients with OHCA treated with TTM at 33°C (n = 447) and at 36°C (n = 430).111 These findings therefore define spontaneous bradycardia during TTM as a novel, early marker in ROSC patients after OHCA. Whether active heart rate reduction, for example, by beta-blocker or ivabradine, might improve prognosis remains to be tested.
Beta-blocker and ivabradine in combination
The SHIFT was designed to demonstrate prognostic effects of ivabradine in already well-treated HF patients on standard medication (including beta-blockers), but 11% of the study cohort did not receive beta-blockers due to intolerance or contraindications. Subgroup analyses revealed that ivabradine treatment without concomitant beta-blockers in these patients achieved a significant outcome benefit with a relative reduction of the primary end point of 32%, compared to placebo.112 Nevertheless, the drug is often used in combination with beta-blockers due to complementarity of action, which is described in the following paragraphs. Ivabradine has only little interactions with other cardiovascular drugs and can therefore be given together with the guideline-recommended standard medication for HF such as ACE inhibitors, beta-blockers, ARBs, MRAs, diuretics and cardiac glycosides.
Ivabradine fills the beta-blocker gap
In the attempt to achieve therapeutic heart rate reduction in HFrEF patients, a synergistic approach of beta-blocker and ivabradine is often necessary: many patients do tolerate only suboptimal but not the recommended full doses of a beta-blocker because of objective or subjective side effects. Therefore, an additional heart rate-reducing agent is necessary to achieve the goal. In the SHIFT,112 89% of the patients had beta-blockers, but only 26% of them were at the target dose, and only 56% had at least ≥ 50% of the target dose, irrespective of the specific beta-blocker used in this trial (46% carvedilol, 25% bisoprolol, 14% metoprolol succinate, 10% metoprolol tartrate, 3% nebivolol, 2% other). The reasons for this suboptimal beta-blocker dosing were hypotension (44%), fatigue (32%), dyspnea (24%), dizziness (13%), bradycardia (6%) and others (9%). The reasons for nonprescription of beta-blocker in the 11% of the SHIFT patients despite strong guideline recommendation were COPD (37%), hypotension (17%), asthma (10%), cardiac decompensation (7%), dizziness or bradycardia (7%), fatigue (5%), Raynaud syndrome or peripheral arterial disease (5%) and others (13%). It is interesting to see that these absolute and relative contraindications to achieve full beta-blocker dosing are seen not only in the SHIFT with chronic HF patients16 but also in other large ivabradine RCTs testing patients with stable coronary artery disease with LV dysfunction (BEAUTIFUL trial)68 and without clinical HF (SIGNIFY trial).19 These beta-blocker contraindications are also prohibiting “real-life” noninterventional ivabradine trials with patients with HFrEF69 as well with chronic coronary artery disease.70 Therefore, there is a clear gap in achieving full therapeutic heart rate reduction with beta-blockers alone in many HF patients. This gap can be filled by combination with ivabradine, a drug with a remarkably low side-effect profile. In the SHIFT,16 significantly higher adverse event rates in the ivabradine group in comparison to the control group were only seen for symptomatic bradycardia (5% vs. 1%, drug withdrawal in 1% vs. < 0.02%), asymptomatic bradycardia (6% vs. 1%, drug withdrawal in 1% vs. < 1%), atrial fibrillation (9% vs. 8%, drug withdrawal in 4% vs. 3%, n.s.), phosphenes (3% vs. 1%, drug withdrawal in < 1% vs. < 1%, n.s.) and blurred vision (1% vs. < 1%, drug withdrawal in < 1% vs. 1%, n.s.). In the noninterventional INTENSIFY study69 with addition of ivabradine for 4 months to standard HF medication, 2.9% of the 1,956 HFrEF patients reported at least one adverse event, most commonly affecting the heart (1.4%), the nervous system (0.5%) and the eyes (0.5%); bradycardia was detected in this study in 0.3% of patients (n = 5), and seen more common in the group with the baseline heart rate < 75 b.p.m.
Role of beta-blocker dose
It has been known for many years that the reduction in mortality of HFrEF patients by beta-blocker treatment shows stronger correlation with the achieved heart rate reduction than with the beta-blocker dosage used. The SHIFT study has now confirmed and extended this correlation in HFrEF patients with sinus rhythm ≥ 70 b.p.m. for ivabradine as well as for the combination of beta-blocker and ivabradine. The magnitude of heart rate reduction achieved by beta-blocker plus ivabradine, rather than beta-blocker background dose, determines subsequent effect on outcome.112 The effect of ivabradine on the primary composite end point of cardiovascular death or HF hospitalization is independent from the type of beta-blocker given.113 Patients prescribed a combination of carvedilol – the most often used beta-blocker in the SHIFT study – and ivabradine had lower rates of primary composite end point (HR 0.80; 95% CI 0.68–0.94), HF hospitalization (HR 0.73; 95% CI 0.6–0.86) and cardiovascular hospitalization (HR 0.80; 95% CI 0.69–0.92) than patients under carvedilol and placebo.113 The dose of carvedilol had no detectable effect, and there were no unexpected safety issues.113
Exercise tolerance: the CARVIVA trial
Not only prognosis but also exercise tolerance can be improved by the combination of beta-blocker and ivabradine in comparison to beta-blocker alone: in the CARVIVA HF trial114 with 131 HF patients (NYHA II/II, LVEF 27 ± 4.9%), ivabradine as well as the combination of carvedilol plus ivabradine after 12 weeks of treatment improved exercise tolerance and QoL, but carvedilol alone did not, though in all three patient groups, heart rate was reduced, which was strongest in the combination group.
This can be explained by dominant negative effects of an increasing blockade of beta1-adrenoceptors with higher doses of beta1-selective beta-blockers. Such effects prevent upregulation of heart rate and cardiac output during physical exercise and result in reduced exercise capacity, which is additionally impaired by negative inotropic properties of beta-blockers. In contrast, treatment with ivabradine alone or in combination with low- or medium-dose beta-blockers allows adequate rise in heart rate with increasing sympathetic activation (induced by physical exercise), as ivabradine lacks any inhibitory effects on beta1-adrenoceptors and maintains cardiac conduction and contractility.115 Combination therapy therefore enables additive heart rate reduction without the negative effects that would be generated by high doses of an equivalent beta-blocker monotherapy.
“The heart rate goal”
The findings presented argue for a reduction of heart rate to < 60/min or at least for a reduction of 10 b.p.m. in patients with HFrEF and sinus rhythm of ≥ 75 b.p.m., either by beta-blocker alone or by the combination of beta-blocker plus ivabradine. For treatment, the lower heart rate limit is either 50 b.p.m. or symptomatic bradycardia. As many HFrEF patients under beta-blocker have a heart rate ≥ 75 b.p.m., there is a need for a combination therapy of beta-blocker plus ivabradine in these patients. For patients with chronic stable angina pectoris, a combination preparation (Implicor®) is now on the market for use, with a fixed combination of metoprolol tartrate and ivabradine (25/5 mg, 50/5 mg, 25/7.5 mg, 50/7.5 mg). In the opinion of the authors, similarly, a combination product of beta-blocker and ivabradine would also be desirable for the indication of chronic systolic HF.
Patient focus perspectives: quality of life (QoL)
QoL: a stepchild of HF therapy!
Reduction in mortality is important in chronic HF, but it is only a poor reflection of how HF patients experience their situation and what impact treatment may have on a patient’s day-to-day life.116 QoL – as reflected by symptoms and the impact of disease on social, emotional and occupational function – may be even more important than longevity. HF as a chronic disease impairs patients’ QoL117 to a similar extent as end-stage renal disease in a patient on chronic dialysis.118 Table 5 shows representative QoL data from elderly HF patients (Table 5, A) in comparison to community-dwelling elderly (Table 5, B).117
  | Table 5 Health-related quality of life in elderly heart failure patients (A) in comparison to community-dwelling elderly (B), and impact of heart failure medication – ivabradine (C, D) vs. beta-blocker (E, F) – on health-related quality of life Notes: Columns 1–4: comparison of quality of life – as assessed by the Medical Outcome. Study 36-item General Health Survey (RAND-36) – in 781 patients with heart failure with 781 community-dwelling elderly. Modified from Lesman-Leegte et al.117 Columns 5–11: quality of life improvement – as documented by the SF-36 questionnaire – by ivabradine and beta-blockers in comparison in heart failure patients. aCenter for Epidemiological Studies-Depression scale ≥ 16.117 bCenter for Epidemiological Studies-Depression scale.117 The bolding is to show those medical outcomes with a significant improvement by ivabradine, but no significant improvement by beta-blocker. Data in columns 1–4 are from Lesman-Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15(1):17–23.117 and columns 5–11 are from Riccioni G, Masciocco L, Benvenuto A, et al. Ivabradine improves quality of life in subjects with chronic heart failure compared to treatment with β-blockers: results of a multicentric observational APULIA study. Pharmacology. 2013;92(5–6):276–280.119 Abbreviations: IVA, ivabradine; SD, standard deviation. |
Guideline-recommended HF medication is clearly focused on prolongation of life, but much less on improving QoL:119,120 ACE inhibitors and ARBs,120 beta-blockers114,119–121 and also the aldosterone antagonist eplerenone122 are neutral, or only modestly improve or at best delay the progressive worsening of QoL.
QoL improvement by ivabradine
Ivabradine, on the other hand, seems clearly to improve health-related QoL. In SHIFT, low health-related QoL is associated with an increased rate of cardiovascular death or hospital admission for HF.116 Reduction of heart rate with ivabradine is associated with improved health-related QoL, as assessed by the Kansas City Cardiomyopathy Questionnaire. The magnitude of heart rate reduction is related to the extent of improvement in health-related QoL.116 And also in the nonintervention INTENSIFY study, the mean value of the European quality of life-5 dimensions QOL index increased from 0.64 ± 0.28 to 0.79 ± 0.21 after a 4-month period of additional ivabradine treatment with standard HF treatment including beta-blockers.69
QoL: beta-blockers and ivabradine in comparison
A direct comparison of the effect of treatment with beta-blocker vs. ivabradine on QoL is presented in the APULIA study with 221 patients with typical symptoms and signs of HFrEF (< 50%) (NHYA II–IV; mean LVEF: 44 ± 5% in ivabradine group and 43 ± 6% in beta-blocker group; initial heart rate 72 ± 5 b.p.m. in ivabradine group and 72 ± 4 b.p.m. in beta-blocker group).119 According to the new HF classification of the ESC,3 these patients would now be classified as patients with HFmrEF. In addition to HF standard treatment without beta-blockers, 110 patients with contraindications to beta-blocker treatment received for 1 month ivabradine 5 mg b.i.d., and 111 patients received beta-blockers (50 patients bisoprolol 1.25 m b.i.d.; 51 patients carvedilol 6.25 mg b.i.d.). Ivabradine treatment as well as beta-blocker treatment (Table 5, C–F) was associated with a significant improvement of physical functioning (p 0.001 vs. p 0.01), physical role (p 0.001 vs. p 0.01), general health (p 0.001 vs. p 0.03) and mental health (p 0.001 vs. p 0.01). But only ivabradine, not beta-blockers achieved a significant improvement in body pain (p 0.001 vs. p 0.5), vitality (p 0.01 vs. p 0.44), social functioning (p 0.01 vs. p 0.92), emotional role (p 0.01 vs. p 0.85), physical component summaries (p 0.01 vs. p 0.52) and mental component summaries (p 0.01 vs. p 0.80) (Table 5, C–F). Thus, an improvement in global physical and in global mental activity was only achieved by ivabradine treatment, but not by beta-blocker treatment. With respect to improvement of health-related QoL, the sum of data clearly favors ivabradine over beta-blockers.
Ivabradine in the old patients
HF is a syndrome that predominantly affects the elderly (> 65 years), the old (> 75 years) and also the very old (> 85 years) patients. In these patient groups, drug efficacy and drug safety may be influenced by altered drug metabolism, by polypharmacy with drug interactions and by lower drug adherence. In view of this, the low side-effect profile of ivabradine proven in RCTs as well as in clinical practice (explained in the “Ivabradine fills the beta-blocker gap” section) is a great advantage.
The elderly patients in the SHIFT
In the SHIFT, an age group analysis was carried out with 6,505 patients, categorized into the age groups “< 53 years” (n = 1,522), “53 to < 60 years” (n = 1,521), “60 to < 69 years” (n = 1,750) and “≥ 69 years” (n = 1,712).123 With advancing age, the percentage of women increased from 16% to 34%, as did the percentage of NYHA II/III from 45% to 59%, as well as the incidence of comorbidity, and creatinine clearance fell from 87 mL/min/1.73 m2 to 63 mL/min/1.73 m2. RRsyst rose with age from 118 mm Hg to 124 mm Hg, but heart rate fell from 81 b.p.m. to 79 b.p.m. The use of HF medications declined with increasing age for beta-blockers from 93% to 85% and for those with the beta-blocker target dose from 27% to 18%, for MRAs from 68% to 54% and for cardiac glycosides from 30% to 17%, while the use of ACE inhibitors (~ 80%) and ARB (~ 15 %) remained fairly constant.
As expected, both age and heart rate separately contributed significantly to outcome. For each b.p.m. increase in heart rate, the relative risk of the primary end point (cardiovascular death and HF hospitalization) increased in the groups by 3.6%, 3.1%, 3.2% and 2.2%, respectively. Ivabradine uptitration reduced heart rate similarly in all age groups, by 11 b.p.m.
The primary end point was reduced by ivabradine in all age groups, ranging from 38% (HR 0.62; 95% CI 0.50–0.78; p < 0.001) in the youngest patients < 53 years to 16% (HR 0.84; 95% CI 0.71–0.99; p = 0.035) in the oldest. Adverse events as bradycardia and phosphenes did not increase with increasing age, and in the Holter substudy, there were no episodes of severe bradycardia and no clinically relevant pauses with ivabradine in any age group.
The elderly patients in the INTENSIFY nonintervention study
The results of the INTENSIFY study (explained in the “The INTENSIFY study: proof of concept in daily clinical practice” section) confirm the efficacy and safety of ivabradine across the age spectrum of patients with chronic HFrEF in daily practice:69 the mean age of the INTENSIFY patients was 67 ± 11.7 years, with 750 patients being < 65 years, 967 being between 65 years and 80 years and 224 being > 80 years. Comparable effectiveness (alterations in NYHA classification and signs of decompensation), improvement in health-related QoL and tolerability were seen in all three age groups in this study.69
In the sum of data, ivabradine sustains its efficacy, low side-effect profile and patient adherence also in the old patients with HFrEF, and it is also safe when taking the comorbidity of the old patients into account.
Conclusion: the place of ivabradine in HF therapy
Especially in the elderly patients, HF is only one of a number of disease entities present. Therefore, medication for HF has to be considered within the scope of the total approach of disease management in the individual elderly patients. Cardiac drugs used should not interfere with other drugs and should not further impair but even improve health-related QoL which often is reduced because of comorbidities.
In the patients with HF, the ten most common conditions other than hypertension and ischemic heart disease are anemia, arrhythmias, cognitive dysfunction, depression, diabetes, musculoskeletal disorders, renal dysfunction, respiratory diseases, sleep disorders and thyroid disease.124
A multidisciplinary position statement identified five key steps (ARISE-HF) that could potentially improve clinical outcomes if applied in a systematic manner:124
- Acknowledge multimorbidity as a clinical syndrome that is associated with poor health outcomes
- Routinely profile (using a standardized protocol – adapted to the local health care system) all patients hospitalized with HF to determine the extent of concurrent multimorbidity
- Identify individualized priorities and person-centered goals on the extent of concurrent multimorbidity
- Support individualized, home-based, multidisciplinary, case management to supplement standard HF management, and
- Evaluate health outcomes well beyond acute hospitalization and encompass all-cause events and a person-centered perspective in affected individuals.
With respect to our patients with HF, this means that our medication should not only prevent HF-related death and hospitalization but also improve QoL, which often is severely impaired due to the symptoms of HF. Further, our medication should not further deteriorate QoL by severe adverse events. And, we should know which target – symptomatic and/or prognostic? – we have to achieve.
Having this in mind, ivabradine is a good candidate for a HF drug: its QoL profile is above average, and its side-effect profile is below average. Many HFrEF patients under standard medical treatment inclusive of beta-blocker have a resting heart rate of ≥75 b.p.m. (Table 2) and therefore might benefit from additional ivabradine treatment (Table 3). In these patients, a reduction of heart rate should be achieved by additional ivabradine to ≤ 60 b.p.m. or at least a reduction by > 10 b.p.m. within 4 weeks. If this goal can be achieved, then we can tell the patient that his cardiovascular mortality risk within the next 2.5 years will be lowered by 17%, his HF mortality risk will be lowered by 39%, his HF hospitalization risk will be lowered by 30% and his health-related QoL will probably improve.
Disclosure
U. Müller-Werdan received a honorarium for a lecture from Servier. G. Stöckl is an employee of Servier (Medical Affairs). K. Werdan has been engaged in ivabradine clinical trials fully or partly supported by Servier, has received honoraria for lectures and manuscripts from Servier, was a member of the German ivabradine advisory board of Servier and received research grants for experimental and clinical ivabradine research from Servier. The authors report no other conflicts of interest in this work.
References
Braunwald E. Heart Failure. JACC Heart Fail. 2013;1(1):1–20. | ||
Laribi S, Aouba A, Nikolaou M, et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail. 2012;14(3):234–239. | ||
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. | ||
Tannenbaum S, Sayer GT. Advances in the pathophysiology and treatment of heart failure with preserved ejection fraction. Curr Opin Cardiol. 2015;30(3):250–258. | ||
Yancy CW, Jesup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. | ||
Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30(4):1104–1106. | ||
Fox K, Borer JS, Camm AJ, et al; Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. | ||
Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153(8):1589–1601. | ||
Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308(1):236–240. | ||
Custodis F, Baumhäkel M, Schlimmer N, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117(18):2377–2387. | ||
Drouin A, Gendron ME, Thorin E, Gillis MA, Mahlberg-Gaudin F, Tardif JC. Chronic heart rate reduction by ivabradine prevents endothelial dysfunction in dyslipidaemic mice. Br J Pharmacol. 2008;154(4):749–757. | ||
Johansen CD, Olsen RH, Pedersen LR, et al. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J. 2013;34(23): 1732–1739. | ||
Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109(13):1674–1679. | ||
Reil JC, Tardif JC, Ford I, et al. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62(21):1977–1985. | ||
Reil JC, Hohl M, Reil GH, et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34(36):2839–2849. | ||
Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomized placebo-controlled study. Lancet. 2010;376(9744):875–885. | ||
Tardif JC, O’Meara E, Komajda M, et al; SHIFT Investigators. Effects of selective heart rate reduction with ivabradine on left ventricular remodeling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32(20):2507–2515. | ||
Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226(4671):180–182. | ||
Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R; SIGNIFY Investigators. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371(12):1091–1099. | ||
Gent S, Kleinbongard P, Damman P, Neuhäuser M, Heusch G. Heart rate reduction and longevity in mice. Basic Res Cardiol. 2015;110(2):2. | ||
Heusch G. Pleiotropic action(s) of the bradycardic agent ivabradine: cardiovascular protection beyond heart rate reduction. Br J Pharmacol. 2008;155(7):970–971. | ||
Heusch G, Skyschally A, Gres P, van Caster P, Schilawa D, Schulz R. Improvement of regional myocardial blood flow and function and reduction of infarct size with ivabradine: protection beyond heart rate reduction. Eur Heart J. 2008;29(18):2265–2275. | ||
Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G. Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol. 2015;172(17):4380–4390. | ||
Rienzo M, Melka J, Bize A, et al. Ivabradine improves left ventricular function during chronic hypertension in conscious pigs. Hypertension. 2015;65(1):122–129. | ||
Skalidis E, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215(1):160–165. | ||
Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomized placebo-controlled trial. Lancet. 2010;376(9744):886–894. | ||
Böhm M, Borer J, Ford I, et al. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol. 2013;102(1):11–22. | ||
Castagno D, Skali H, Takeuchi M, et al; CHARM Investigators. Association of heart rate and outcome in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) program. J Am Coll Cardiol. 2012;59(20):1785–1795. | ||
Fox K, Ford I, Steg G, Tendera M, Robertson M, Ferrari R; BEAUTIFUL Investigators. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomized controlled trial. Lancet. 2008;372(9641):817–821. | ||
Franke J, Wolter JS, Meme L, et al. Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed? Clin Res Cardiol. 2013;102(1):23–31. | ||
Logeart D, Seronde MF, Degroote P, et al. Raised heart rate at discharge after acute heart failure is an independent predictor of one-year mortality. Eur Heart J. 2012;32(Suppl 1):485. | ||
Böhm M, Perez A-C, Jhund PS, et al; I-Preserve Committee and Investigators. Relationship between heart rate and mortality and morbidity in the irbesartan patients with heart failure and preserved systolic function trial (I-Preserve). Eur J Heart Fail. 2014;16(7):778–787. | ||
Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol. 2012;155(2):249–256. | ||
Takada T, Sakata Y, Miyata S, et al; CHART-2 Investigators. Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the CHART-2 Study. Eur J Heart Fail. 2014;16(3):309–316. | ||
Howlett JG. Does slow and steady win the race? J Am Coll Cardiol. 2012;59(20):1796–1798. | ||
Zorn-Pauly K, Pelzmann B, Lang P, et al. Endotoxin impairs the human pacemaker current IF. Shock. 2007;28(6):655–661. | ||
Li N, Csepe TA, Hansen BJ, et al. Molecular mapping of sinoatrial node HCN channel expression in the human heart. Circ Arrhythm Electrophysiol. 2015;8(5):1219–1227. | ||
DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106(3):434–446. | ||
Heusch G, Baumgart D, Camici P, et al. alpha-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101(6):689–694. | ||
Mesirca P, Torrente AG, Mangoni ME. Functional role of voltage gated Ca(2+) channels in heart automaticity. Front Physiol. 2015;6:19. | ||
Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007;45(2):189–207. | ||
Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88(12):1254–1258. | ||
Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47(2):157–170. | ||
Yaniv Y, Lakatta EG, Maltsev VA. From two competing oscillators to one coupled-clock pacemaker cell system. Front Physiol. 2015;6:28. | ||
Yaniv Y, Sirenko S, Ziman BD, Spurgeon HA, Maltsev VA, Lakatta EG. New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: bradycardic effects of ivabradine are linked to suppression of intracellular Ca(2)(+) cycling. J Mol Cell Cardiol. 2013;62:80–89. | ||
Yaniv Y, Lyashkov AE, Sirenko S, et al. Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells. J Mol Cell Cardiol. 2014;77:1–10. | ||
Tsuji H, Larson MG, Venditti FJ Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. | ||
Ponikowski P, Anker SD, Chua TP, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79(12):1645–1650. | ||
Kazmi SZ, Zhang ZH, Aziz W, et al. Inverse correlation between heart rate variability and heart rate demonstrated by linear and nonlinear analysis. PLoS One. 2016;11(6):e0157557. | ||
Mangin L, Swynghedauw B, Benis A, Thibault N, Lerebours G, Carré F. Relationships between heart rate and heart rate variability: study in conscious rats. J Cardiovasc Pharmacol. 1998;32(4):601–607. | ||
Dias da Silva VJ, Tobaldini E, Rocchetti M, et al. Modulation of sympathetic activity and heart rate variability by ivabradine. Cardiovasc Res. 2015;108(1):31–38. | ||
Böhm M, Borer JS, Camm J, et al. Twenty-four-hour heart rate lowering with ivabradine in chronic heart failure: insights from the SHIFT Holter substudy. Eur J Heart Fail. 2015;17(5):518–526. | ||
Kurtoglu E, Balta S, Karakus Y, et al. Ivabradine improves heart rate variability in patients with nonischemic dilated cardiomyopathy. Arq Bras Cardiol. 2014;103(4):308–314. | ||
Evans ND, Godfrey KR, Chapman MJ, Chappell MJ, Aarons L, Duffull SB. An identifiability analysis of a parent-metabolite pharmakokinetic model for ivabradine. J Pharmacokinet Pharmacodyn. 2001;28(1):93–105. | ||
Francois-Bouchard M, Simonin G, Bossant MJ, Boursier-Neyret C. Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;745(2):261–269. | ||
Ragueneau I, Laveille C, Jochemsen R, Resplandy G, Funck-Brentano C, Jaillon P. Pharmakokinetic-pharmacodynamic modeling of the effects of ivabradine, a direct sinus node inhibitor, on heart rate in healthy volunteers. Clin Pharmacol Ther. 1998;64(2):192–203. | ||
De Ferrari GM, Mazzuero A, Agnesina L, et al. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail. 2008;10(6):550–555. | ||
Steg P, Lopez-de-Sà E, Schiele F, et al; VIVIFY (eValuation of the IntraVenous If inhibitor ivabradine after ST segment elevation mYocardial infarction) Investigators. Safety of intravenous ivabradine in acute ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention: a randomized, placebo-controlled, double-blind, pilot study. Eur Heart J Acute Cardiovasc Care. 2013;2(3):270–279. | ||
Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. | ||
Klöckner U, Rueckschloss U, Grossmann C, et al. Differential reduction of HCN channel activity by various types of lipopolysaccharide J Mol Cell Cardiol. 2011;51(2):226–235. | ||
Klöckner U, Rueckschloss U, Grossmann C, et al. Inhibition of cardiac pacemaker channel hHCN2 depends on intercalation of lipopolysaccharide into channel-containing membrane microdomains. J Physiol. 2014;592(6):1199–1211. | ||
Barbuti A, DiFrancesco D. The “fun” side of sepsis. J Physiol. 2014;592(6):1171. | ||
Ebelt H, Geißler I, Ruccius S, et al. Direct inhibition, but indirect sensitization of pacemaker activity to sympathetic tone by the interaction of endotoxin with HCN-channels. Clin Exp Pharmacol Physiol. 2015;42(8):874–880. | ||
Scheruebel S, Koyani CN, Hallström S, et al. If blocking potency of ivabradine is preserved under elevated endotoxin levels in human atrial myocytes. J Mol Cell Cardiol. 2014;72:64–73. | ||
Bonadei I, Vizzardi E, Sciatti E, et al. Is there a role for ivabradine beyond its conventional use? Cardiovasc Ther. 2014;32(4):189–192. | ||
Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516–2525. | ||
Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D–8D. | ||
Fox K, Ford I, Steg G, Tendera M, Ferrari R; BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372(9641):807–816. | ||
Zugck C, Martinka P, Stöckl G. Ivabradine treatment in a chronic heart failure patient cohort: symptom reduction and improvement in quality of life in clinical practice. Adv Ther. 2014;31(9):961–974. | ||
Werdan K, Ebelt H, Nuding S, Höpfner F, Hack G, Müller-Werdan U. Ivabradine in combination with betablocker improves symptoms and quality of life in patients with stable angina pectoris: results from the ADDITIONS study. Clin Res Cardiol. 2012;101(5):365–373. | ||
Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15(6):604–613. | ||
Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(1):123–130. | ||
Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20(2):193–201. | ||
Conraads VM, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14(2):219–225. | ||
Edelmann F, Musial-Bright L, Gelbrich G, et al; CIBIS-ELD Investigators and Project Multicenter Trials in the Competence Network Heart Failure. Tolerability and feasibility of beta-blocker titration in HFpEF versus HFrEF: insights from the CIBIS-ELD trial. JACC Heart Fail. 2016;4(2):140–149. | ||
Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis. PLoS One. 2014;9(3):e90555. | ||
Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312(19):2008–2018. | ||
Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781–791. | ||
Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. | ||
Pfeffer MA, Claggett B, Assman SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42. | ||
Gu J, Fan YQ, Bian L, et al. Long-term prescription of beta-blocker delays the progression of heart failure with preserved ejection fraction in patients with hypertension: a retrospective observational study. Eur J Prev Cardiol. 2016;23(13):1421–1428. | ||
Kosmala W, Holland DJ, Rojekt A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62(15):1330–1338. | ||
Simantirakis EN, Nakou ES, Kallergis EM, et al. Long-term effect of If-channel inhibition on diastolic function and exercise capacity in heart failure patients with preserved ejection fraction. Int J Cardiol. 2015;187:9–11. | ||
Fischer-Rasokat U, Honold J, Lochmann D, et al. β-Blockers and ivabradine differentially affect cardiopulmonary function and left ventricular filling index. Clin Res Cardiol. 2016;105(6):527–534. | ||
Adamyan KG, Tumasyan LR, Chilingaryan AL, Tunyan LG. Efficacy of long-term ivabradine therapy on prognosis, left and right heart functional parameters in patients with chronic heart failure and preserved left ventricular systolic function. Eur Heart J. 2015;36(Suppl 1):666–667. | ||
De Masi De Luca G. Ivabradine and diastolic heart failure. J Am Coll Cardiol. 2012;59(13 Suppl 1) E1009. | ||
Pal N, Sivaswamy N, Mahmoud M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation. 2015;132(18):1719–1725. | ||
Kitzman DW. Conventional wisdom in heart failure treatment challenged again: does heart rate lowering worsen exercise intolerance in heart failure with preserved ejection fraction? Circulation. 2015;132(18):1687–1689. | ||
Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62(15):1339–1342. | ||
Logeart D, Isnard R, Resche-Rigon M, et al; Heart Failure of the French Society of Cardiology. Current aspects of the spectrum of acute heart failure syndromes in a real-life setting: the OFICA study. Eur J Heart Fail. 2013;15(4):465–476. | ||
Mebazaa A, Parissis J, Porcher R, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37(2):290–301. | ||
Senni M, Gavazzi A, Oliva F, et al; IN HF Outcome Investigators. In-hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. Results from IN-HF Outcome Registry. Int J Cardiol. 2014;173(2);163–169. | ||
Aroutunov AG, Aroutunov GP, Volkova AL, Bylova NA. Prognosis of heart rate control on decompensated heart failure patients. Heart Lung Circ. 2008;17(1):S5–S6. | ||
Sargento L, Satendra M, Longo S, Lousada N, dos Reis RP. Heart rate reduction with ivabradine in patients with acute decompensated systolic heart failure Am J Cardiovasc Drugs. 2014;14(3):229–235. | ||
Cavusoglu Y, Mert U, Nadir A, Mutlu F, Morrad B, Ulus T. Ivabradine treatment prevents dobutamine-induced increase in heart rate in patients with acute decompensated heart failure. J Cardiovasc Med (Hagerstown). 2015;16(9):603–609. | ||
Link A, Reil JC, Selejan S, Böhm M. Effect of ivabradine in dobutamine induced sinus tachycardia in a case of acute heart failure. Clin ResCardiol. 2009;98(8):513–515. | ||
Doesch AO, Celik S, Ehlermann P, et al. Heart rate reduction after heart transplantation with beta-blocker versus the selective If channel antagonist ivabradine. Transplantation. 2007:84(8):988–996. | ||
Doesch AO, Ammon K, Konstandin M, et al. Heart rate reduction for 12 months with ivabradine reduces left ventricular mass in cardiac allograft recipients. Transplantation. 2009;88(6):835–841. | ||
Sekar B, Critchley WR, Williams SG, Shaw SM. Should we consider heart rate reduction in cardiac transplant recipients? Clin Cardiol. 2013;36(2):68–73. | ||
Zwicker C, Becker M, Lepper W, Koch KC, Westenfeld R. Cardiogenic shock due to tachycardiomyopathy after heart transplantation: successful treatment with ivabradine. Cardiology. 2010;116(3):174–177. | ||
Libhaber E, Sliwa K, Bachelier K, Lamont K, Böhm M. Low systolic blood pressure and high resting heart rate as predictors of outcome in patients with peripartum cardiomyopathy. Int J Cardiol. 2015;190:376–382. | ||
Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J. Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J. 2015;36(18):1090–1097. | ||
Haghikia A, Tongers J, Berliner D, et al. Early ivabradine treatment in patients with acute peripartum cardiomyopathy: subanalysis of the German PPCM registry. Int J Cardiol. 2016;216:165–167. | ||
Bonadei I, Sciatti E, Vizzardi E, D’Aloia A, Metra M. Ivabradine during cardiogenic shock: a clinical case and review of the literature. Heart Lung. 2015;44(1):57–58. | ||
Barillà F, Pannarale G, Torromeo C, et al. Ivabradine in patients with ST-elevation myocardial infarction complicated by cardiogenic shock: a preliminary randomized prospective study. Clin Drug Investig. 2016;36(10):849–856. | ||
Werdan K, Nuding S, Müller-Werdan U. Potential Pathophysiological Mechanisms in Septic Cardiomyopathy: An Overview. Frontiers in Myocardia. Vol. 1. Edited by Vasilios E. Papaioannou. Sharjah, UAE: Bentham Science Publishers Ltd. 2015:3–40. | ||
Sander O, Welters ID, Foex P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33(1):81–88. | ||
Hoke RS, Müller-Werdan U, Lautenschäger C, Werdan K, Ebelt H. Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol. 2012;101(2):139–147. | ||
Morelli A, Ertmer C, Westphal M et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–1691. | ||
Nuding S, Ebelt E, Hoke RS, et al. Reducing elevated heart rate in patients with multiple organ dysfunction syndrome by the I(f) (funny channel current) inhibitor ivabradine MODI(f)Y Trial. Clin Res Cardiol. 2011;100(10):915–923. | ||
Thomsen JH, Nielsen N, Hassager C, et al. Bradycardia during targeted temperature management: an early marker of lower mortality and favorable neurologic outcome in comatose out-of-hospital cardiac arrest patients. Crit Care Med. 2016;44(2):308–318. | ||
Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose? Findings from the SHIFT (Systolic Heart failure treatment with the If inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59(22):1938–1945. | ||
Bocchi EA, Böhm M, Borer JS, et al; SHIFT investigators. Effect of combining ivabradine and β-blockers: focus on the use of carvedilol in the SHIFT population. Cardiology. 2015;131(4):218–224. | ||
Volterrani M, Cice G, Caminit G, et al. Effect of carvedilol, ivabradine or their combination on exercise capacity in patients with heart failure (the CARVIVA HF trial). Int J Cardiol. 2011;151(2):218–224. | ||
Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275(2):659–666. | ||
Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure results from the SHIFT study. Eur Heart J. 2011;32(19):2395–2404. | ||
Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15(1):17–23. | ||
Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–241. | ||
Riccioni G, Masciocco L, Benvenuto A, et al. Ivabradine improves quality of life in subjects with chronic heart failure compared to treatment with β-blockers: results of a multicentric observational APULIA study. Pharmacology. 2013;92(5–6):276–280. | ||
Dobre D, de Jongste MJL, Haaijer-Ruskamp FM, Sandermann R, van Veldhuisen DJ, Ranchor AV. The enigma of quality of life in patients with heart failure. Int J Cardiol. 2008;125(3):407–409. | ||
Dobre D, van Jaarsfeld CHM, deJongste MJL, Ruskamp FMH, Ranchor AV. The effect of beta-blocker therapy on quality of life in heart failure patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2007;16(2):152–159. | ||
Udelson JE, Feldman AM, Greenberg B, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonisms with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3(3):347–353. | ||
Tavazzi L, Swedberg K, Komajda M, et al; SHIFT Investigators. Efficacy and safety of ivabradine in chronic heart failure across the age spectrum: insights from the SHIFT study. Eur J Heart Fail. 2013;15(11):1296–1303. | ||
Stewart S, Riegel B, Boyd C, et al. Establishing a pragmatic framework to optimise health outcomes in heart failure and multimorbidity (ARISE-HF): a multidisciplinary position statement. Int J Cardiol. 2016;212:1–10. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

