Back to Journals » International Journal of Nanomedicine » Volume 17
Advances in Nanoliposomes for the Diagnosis and Treatment of Liver Cancer
Authors Li Y, Zhang R , Xu Z, Wang Z
Received 14 November 2021
Accepted for publication 26 January 2022
Published 26 February 2022 Volume 2022:17 Pages 909—925
DOI https://doi.org/10.2147/IJN.S349426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Yitong Li,1 Ruihang Zhang,2 Zhen Xu,1 Zhicheng Wang1
1NHC Key Laboratory of Radiobiology (Jilin University), School of Public Health, Jilin University, Changchun, 130021, Jilin, People’s Republic of China; 2Second Clinical Medical College, Henan University of Traditional Chinese Medicine, Zhengzhou, 450052, Henan, People’s Republic of China
Correspondence: Zhicheng Wang, NHC Key Laboratory of Radiobiology (Jilin University), School of Public Health, Jilin University, 1163 Xinmin Street, Changchun, 130021, Jilin, People’s Republic of China, Tel +86 13843131059, Fax +86 431185619443, Email [email protected]
Abstract: The mortality rate of liver cancer is gradually increasing worldwide due to the increasing risk factors such as fatty liver, diabetes, and alcoholic cirrhosis. The diagnostic methods of liver cancer include ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), among others. The treatment of liver cancer includes surgical resection, transplantation, ablation, and chemoembolization; however, treatment still faces multiple challenges due to its insidious development, high rate of recurrence after surgical resection, and high failure rate of transplantation. The emergence of liposomes has provided new insights into the treatment of liver cancer. Due to their excellent carrier properties and maneuverability, liposomes can be used to perform a variety of functions such as aiding in imaging diagnoses, combinatorial therapies, and integrating disease diagnosis and treatment. In this paper, we further discuss such advantages.
Keywords: liver targeting, nanotherapeutics, nanocarriers, multimodal imaging, nanomedicine, diagnostic treatment integration
Graphical Abstract:
Introduction
Liver cancer is the leading cause of cancer death, with an annual increase in incidence.1 Hepatocellular carcinoma (HCC) represents ~90% of primary liver cancers and constitutes a major global health problem.2 The risk factors for HCC include chronic hepatitis B and hepatitis C, alcohol addiction, metabolic liver disease (particularly nonalcoholic fatty liver disease) and exposure to dietary toxins, such as aflatoxins and aristolochic acid.3 The staging of HCC is particularly complicated due to the varying presence of concomitant liver dysfunction. The staging of HCC is generally performed using the Okuda staging system, the Cancer of the Liver Italian Program, the Hong Kong Liver Cancer (HKLC) staging system, and the Barcelona Clinic Liver Cancer (BCLC) staging system, which is the most popular.4 The current approach to HCC treatment depends on the stage at which the patient is diagnosed.5 The BCLC staging system recommends that only patients classified as BCLC stage 0 or A be administered surgical resection or liver transplantation. In contrast, patients with BCLC stage B and BCLC stage C are recommended for transarterial chemoembolization (TACE) or transarterial radioembolization (TARE).6 BCLC stage D, known as the end-stage, is currently treated symptomatically using the best supportive care.5
Although several studies have demonstrated the rationality of the BCLC system, the BCLC standard is also controversial. A meta-analysis of 2619 Asian HCC patients showed that surgical resection provided better overall survival than TACE in BCLC stage B patients, without any significant increase in postoperative complications or 30-day mortality.7
The Derwent Innovation Platform collects data from a global sample of patents related to HCC treatment technologies, which show future trends in the treatment of HCC. The platform explores novel molecular therapies, localized and visible local therapies, and combinations of immunotherapy with targeted therapies or other conventional treatments.8
In addition to surgical resection, liver transplantation, and percutaneous ablation, clinical studies have also recently evaluated liposomes and lipid nanoparticles as therapies for liver cancer. Liposomes are spontaneously-forming and spherical fragments consisting of a lipid bilayer and a hydrophilic core.9 Research on liposome technology has progressed from conventional vesicles (first-generation liposomes) to second-generation liposomes, in which long-circulating liposomes are obtained by modulating the lipid composition, size, and charge of the vesicle.10 Second-generation liposomes have been designed as tumor-targeting drug delivery vehicles for the precise delivery of active drugs into tumor cells.11 The unique properties of liposomes, including their biocompatibility, biodegradability, amphiphilic nature, low toxicity, non-ionicity, sustained release, and ability to be actively targeted, have provided many biomedical opportunities, especially for drug delivery.12 Liposomes can encapsulate drugs to prevent degradation by the immune system, and are highly biocompatible and effectively targeted. As a chemotherapeutic drug delivery system and gene or immunotherapy tools, liposomes enhance the safety of vector systems, the expression of therapeutic proteins, and the silencing of disease-causing genes.
Due to their extensive advantages, liposomes have been studied for drug delivery to tumor tissues via two main targeting approaches: passive targeting and active targeting. The most common active targeting strategy for liposome agents is the selective binding to cancer cell surfaces that express specific receptors.13 Frequently used ligands for targeting HCC cells include small molecules (eg, folic acid receptor), peptides (eg, the VDAC1-based peptide, R-Tf-D-LP4), proteins (eg, Asialoglycoprotein receptor), and nanoantibodies, among others.14–16 In addition to nanocarriers, radioisotopes and drugs have also been modified as targeting ligands for molecular imaging and radioimmunotherapy.17 Liposomes can maintain high concentrations in tumor microenvironments through the enhanced permeability and retention (EPR) effect. In tumorous tissues, the absence of vasculature-supportive tissues initiates the formation of leaky vessels and pores (100 nm to 2 μm in diameter), and the poor lymphatic system offers great opportunity to aggregation of medicine.18 Many contained drugs can trigger release in multiple forms. Artificial external stimuli such as light and heat therapy can promote the release of drugs from liposomes. Unique features of the tumor microenvironment can also act as endogenous stimuli (pH, redox potential, or unique enzymatic activities), while external stimuli (heat or light) can also be applied at the target location to trigger the controlled release of the active pharmaceutical ingredient (APIs). In liposome carrier systems, triggered release is generally based on membrane instability due to local defects in the bilayer, thus, resulting in the release of liposome-encapsulated drugs.19 The drug can be targeted to the lesion with minimal side effects and minimal damage to healthy organs.
For diagnostics, advanced imaging contrast agents increase the differences in signal intensity between diseased and normal tissues. Liposomal multimodal imaging used for drug delivery can also be used as a diagnostic tool to monitor the distribution of drugs in vivo. In terms of therapeutics, liposomes can be modified and adapted for combination therapies, immunotherapies, gene therapies, and other conventional therapies. Thus, such innovations offer opportunities to cure HCC. Despite being an ideal tool for liver cancer treatment, liposome-based therapies also face urgent challenges that must be resolved before their clinical application, such as the stability of carriers and their short residence times in tissues.
The application of liposomes for liver cancer treatment has great potential. In this review, we discuss the current liver cancer treatments and basic information on liposomes. We summarize recent achievements in liposome-based imaging diagnostics and multiple therapies (Chemotherapy, gene therapy and immunotherapy) for HCC. Particular emphasis is placed on the integration of diagnostics and treatment. Finally, we provide perspectives on additional applications of liposome-based technologies.
Current Status of Liver Cancer Treatment
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and is a malignant tumor that originates from hepatocytes.20 HCC is the fifth most common cancer in men and the ninth most common cancer in women, with approximately 500,000 and 200,000 new cases per year globally, respectively. HCC also often presents with complex pathogenesis and structural mutations in proto-oncogenes due to the addition of exogenous pathogenic factors such as viruses, excessive alcohol consumption, obesity, and aflatoxins.21,22 In addition, several epidemiological surveys and experimental studies have shown that the risk of death from HCC was significantly associated with mass size, the number of masses, macrovascular invasion, and intrahepatic metastases; thus, suggesting that hepatocarcinogenesis is influenced by genetic factors.23–25
The commonly used staging criteria for HCC include the BCLC staging system and the recently proposed HKLCstaging system. HKLC is derived from studies in Asian cohorts, where advanced HCC is the focus of clinical studies.2,26,27 In early-stage tumors, ablation is a first-line treatment comparable to hepatic resection due to the lower mortality rate.28 Ablation and transcatheter chemoembolization are alternative treatments that act locally.2 HCC, which often occurs in the context of cirrhosis, also suffers from postoperative recurrence, which can be resolved by transplantation. However, recurrence of HCC still occurs in 10–20% of such patients.29,30
Non-surgical treatment of HCC (local intra-arterial therapy, systemic multikinase inhibitor therapy, and immunotherapy) has been an emerging field of development in recent years.31 TACE blocks the blood supply vessels with an embolic agent (usually iodine oil), thus, causing ischemic necrosis of the tumor, with relative preservation of the rest of the liver parenchyma due to the dual blood supply.2,32 TACE is often contraindicated in cirrhotic decompensation, renal failure, main portal vein obstruction, and extensive tumor burden.33 Systemic therapy for advanced HCC mainly consists of targeted agents and immune checkpoint inhibitors.34
Liposomes
Nanoparticles are widely fabricated using liposomes, ie lipid nanoparticles (LNPs). Liposomes are typically between 50 and 200 nm and primarily consist of phospholipids and cholesterol. Phospholipids self-assemble into spherical lipid bilayers through their lipophilic tails, and can be used to encapsulate drugs into liposomes. Because liposomes protect the encapsulated cargo from degradation by the immune system, they have the advantage of biocompatibility and enhanced targeting efficiencies.35 Liposomes can be divided into monolayer vesicles and multilayer vesicles depending on the number of bilayers. In addition, monolayered liposomes are divided into small and large monolayered vesicles.36
The mechanisms of liposome-targeted therapy include active targeting and passive targeting. Passive targeting relies on the enhanced permeability and retention (EPR) effect, also known as the high permeability long retention effect, which is a unique phenomenon in the vascular system of solid tumors that is based on specific anatomical structures and pathophysiology. Examples of EPR include leaky vessels with holes; abundant vascular transmitters such as bradykinin, nitric oxide (NO), carbon monoxide (CO), and vascular endothelial growth factor; and poor lymphatic drainage. Thus, such features lead to nanoparticle deposition in tumor tissues.37 However, the existence and importance of the EPR effect have recently been hotly debated, and the heterogeneity of EPR between different tumors has called its application into question.38 Therefore, the effectiveness of nanomedicine can be improved with advances in active targeting strategies. Active targeting of nanomaterials usually targets substances overexpressed in tumors, and can be classified as liver-cell targeting or tumor endothelial vascular targeting, among other types of targeting depending on the site (Table 1 and Figure 1).39
 |
Table 1 Active Targeting of HCC Through VCAM-1, GA-R, ASGPR as Well as Others Receptors |
Use of Liposomes
Liposomes for Diagnostics
HCC usually occurs with chronic liver disease or cirrhosis. In a randomized controlled trial of 18,816 Hepatitis B virus (HBV)-infected patients in China, it was shown that serum alpha-fetoprotein (AFP) testing and abdominal ultrasound (US) at 6-month intervals decreased mortality from HCC by 37%. Thus, early detection of patients at high risk of HCC increases the ability for treatment and improves survival rates.56 Traditional diagnostic tools for HCC are based on cytology or histology, and lesion tissue is obtained by biopsy. With advances in computed tomography (CT) and magnetic resonance imaging (MRI), it is now possible to reliably diagnose HCC without biopsies.3 The American Association for the Study of Liver Diseases (AASLD) recommends the use of multiphasic CT or multiphasic MRI for the diagnostic evaluation of HCC. According to the Liver Imaging Reporting and Data System (LI-RADS), masses larger than 10 mm on multiphase examinations are assigned category codes reflecting their relative probability of being benign, HCC, or other liver malignancies. For example LI-RADS 1 and LI-RADS 2 are used to indicate definitely and probably benign tumors, respectively.26 The coding system has played an important role in the early diagnosis of liver cancer.
MRI
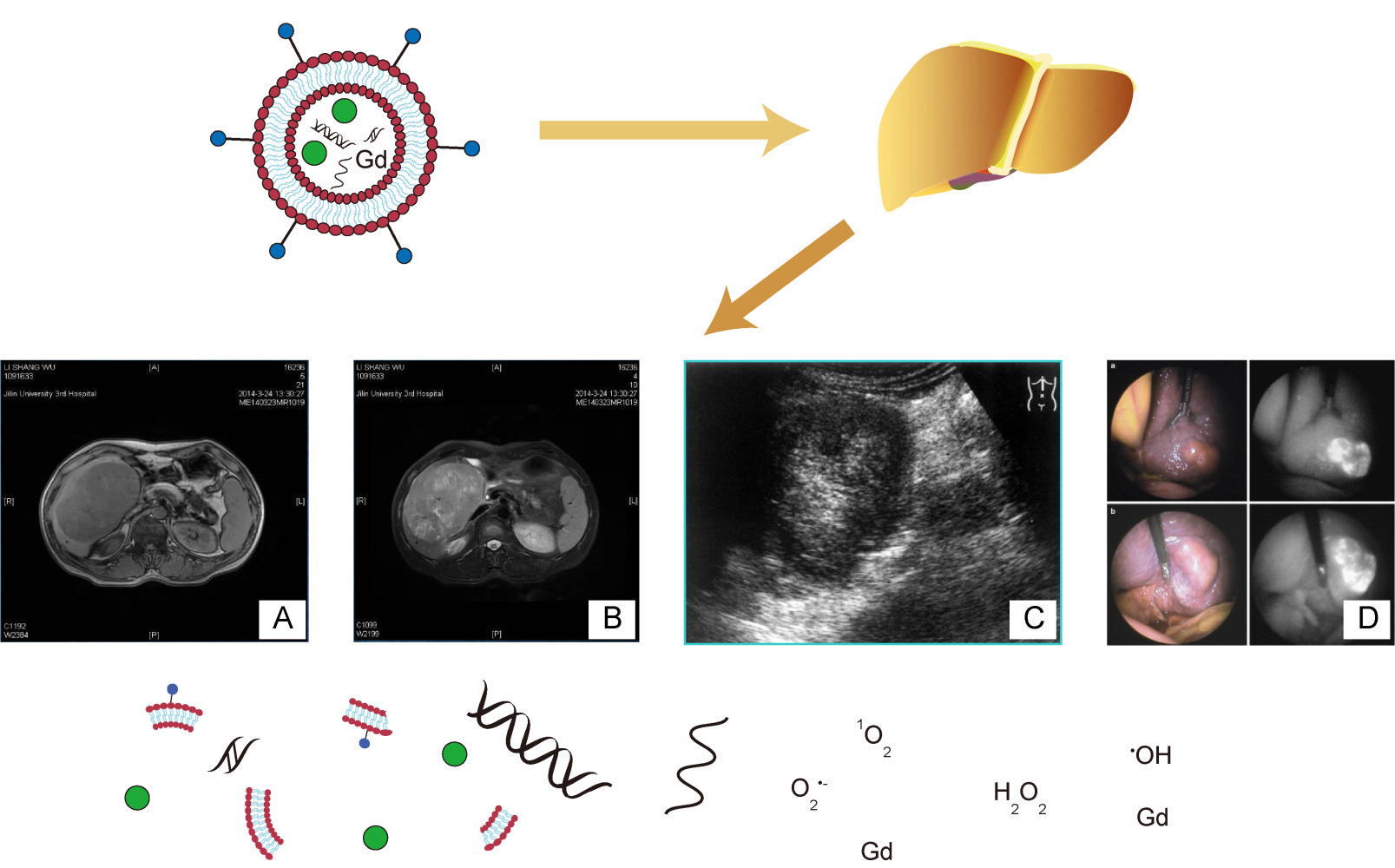
MRI has become one of the most powerful diagnostic tools in biomedicine, largely due to its non-invasive nature, sub-millimetre spatial resolution, high anatomical contrast, and excellent soft-tissue differentiation. In many cases, however, gadolinium-based contrast agents (CA) are required to enhance the differences in signals between diseased areas and normal tissue. Liposomes are biocompatible platforms based on nanocarriers. There are two main forms of binding of CA to liposomes: encapsulation of water-soluble reagents of gadolinium chelates in liposome cavities or modification of liposome bilayers with gadolinium chelates.25 One research group prepared a rare-earth-doped nanoparticle (Gd-REs@Lips) and imaged patients with primary liver cancer (HCC), showing that the nanoparticle used as a T2-weighted imaging contrast agent increased the differences in signal intensities between HCC tissue and the surrounding normal liver tissue on MRI; thus, enabling the accurate detection of liver cancer.57 Another advantage of gadolinium-based liposomes over conventional gadolinium-based contrast agents is their non-toxicity. Gadolinium deposition has been shown to disrupt intracellular lysosomal function, and cause oxidative damage and fibrous deposition.58 However, Simeckova et al evaluated gadolinium-based liposome complexes containing 1, 2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (PE-DTPA) chelated with Gd+3, and reverse transcription-polymerase chain reaction (RT-PCR) assays revealed that such complexes did not increase the expression of stress-related genes, including early growth response-1 (EGR1), activating transcription factor 3 (ATF3), growth differentiation factor 15 (GDF15), and fibroblast growth factor 21 (FGF21). The complex also failed to increase the production of inflammatory factors and exhibited good biocompatibility.59
CT also requires the use of contrast agents to aid in disease diagnosis. Fouillet et al used iodomelanol-containing liposomes to enhance liver contrast and found that it increased the diagnostic rate to more than 60% higher than the pre-push control values when detecting liver tumors in rats.60
Multimodal Imaging
Multimodal imaging is a novel imaging technique that integrates two or more imaging modalities, and is often used to combine anatomical information with high-resolution anatomical information with molecular-level biological information. It allows simultaneous high spatial resolution, soft tissue contrast and high sensitivity to molecular level biological information. At the same time it overcomes the limitations of individual imaging modalities in the full description of the disease and produces accurate images. For example, PET/CT complements the low resolution of PET and the inability of CT to perform functional imaging.61
Radiolabelled liposomes have been used as diagnostic tools to monitor distribution of drugs in vivo and in real time, which optimizes the therapeutic efficacy of liposomal drug delivery. It has been reported that a [111In]-liposome platform was used as a drug delivery vehicle to encapsulate a novel 18F-labeled carboplatin drug derivative ([18F]-FCP), which was used as a bimolecular imaging tool. Dual-modality imaging is performed using 18F positron emission tomography (PET) and 111In single-photon emission CT (SPECT) when both the vehicle and the drug are labelled. PET/SPECT imaging can greatly enhance the tracking of the movement of encapsulated drugs in vivo.62
Guan et al fabricated gold nanorods@liposome core-shell nanoparticles (Au@liposome-ICG) containing indocyanine green (ICG). ICG is the most commonly used near-infrared (NIR) fluorescent dye, however, several factors limit its potential application as a bimodal contrast agent, including its aggregation, rapid clearance, fluorescence burst, and low efficiency in converting laser energy to heat. Gold nanorods (AuNR) are effective photoacoustic probes due to their low toxicity, good biocompatibility, and easy exudation into the tumor region. With Au@liposome-ICG-mediated photoacoustic fluorescence dual-modality imaging, enhanced photoacoustic tomography (PAT) signals can be obtained for preoperative diagnoses, the use of stable fluorescence signals for intraoperative manipulation, and the safe resection of tumors.63
High-intensity focused ultrasound (HIFU) uses a high-energy focusing device to focus multiple low-energy ultrasound beams on the target tumor to induce irreversible coagulative necrosis. HIFU requires multiple imaging techniques for synergistic detection. Wang et al constructed a multifunctional bio-targeting booster consisting of Bifidobacterium longum, ICG, and perfluorohexane (PFH) co-loaded cationic lipid nanoparticles (CL-ICG-PFH-NPs). The booster used the negative surface charge of B. longum to guide the cationic lipid nanoparticles to the tumor area by electrostatic adsorption, and achieved HIFU synergy and multimodality imaging using photoacoustic imaging (PA), fluorescence imaging (FL), and ultrasound imaging (US) in vitro. Thus, the biologically-targeted potentiator not only provided information for the early diagnosis of tumors, but also improved HIFU therapy by enabling multimodal imaging and monitoring during HIFU ablation.64
Liposomes for Therapeutic Usage
Liposome Applications in Chemotherapy
There are many types of cells in the HCC tumor microenvironment, including HCC cells and other stromal cells, vascular endothelial cells, immune cells, and fibroblasts. Such cells and the extracellular matrices they produce have a great impact on tumor development and the response to antitumor therapy.65 HCC cells constitute the primary component of HCC and overexpress antigens and receptors, including ASGP-R, GA-R and transferrin receptor (TfR). Such overexpression is a characteristic that distinguishes HCC cells from other cells, and can be used as targets for liposomes used for HCC therapy.66–68
There is substantial evidence to suggest that HCC may originate from cancer stem cells (CSCs). CSCs have the ability to self-renew and differentiate, which can initiate tumor formation, promote tumor growth, produce distant metastases, and cause recurrence following treatment. Thus, targeting CSCs may efficiently inhibit hepatocellular carcinoma growth and recurrence at the source.69 Hepatic macrophages, the largest population of innate immune cells in the liver, consist of Kupffer cells (KCs) from the fetal yolk sac and infiltrated bone marrow-derived monocytes/macrophages, which are important target cells for the treatment of HCC.70 Hepatic macrophages suppress antitumor immunity, and high levels of macrophage infiltration is predictive of poor prognosis in HCC patients. Thus, targeting KCs may help maintain hepatic antitumor immunity and inhibit tumor growth.71–73 Chemotherapy is a primary systemic antitumor therapy, and the inability to distinguish between normal and malignant cells is its main drawback as it can lead to adverse systemic effects. Therefore, chemotherapy is often used for the treatment of HCC with local therapies, such as TACE.74 Thus, nanoliposome delivery to target chemotherapies has great prospects. Such delivery mechanisms can improve the targeting of treatments and the concentration of chemotherapeutic drugs in tumor tissues, thus, improving the efficacy of such treatments while reducing adverse effects.
Targeting Hepatocytes
Multiple cellular hepatoma targets are described in Table 1. It is well documented that the asialoglycoprotein receptor (ASGPR) is highly expressed in well-differentiated HCC cells and that it specifically recognizes glycoproteins, especially D-galactose or N-acetylgalactosamine (galactosamine). Li et al constructed novel ASGPR-targeting poly (polyethylene glycol paclitaxel) (PTX) nanoliposomes that were loaded with PTX with Tn-modified nanoliposomes (Tn-Lipo-PTX), and GalNAc (Tn antigen) as a ligand to target the paclitaxel to tumors. That vehicle improved drug efficacy and tumor site accumulation, while reducing drug toxicity.75 Zhang et al constructed a Lupeol-loaded liposome system (Gal-lupeol-L system) using lactoferrin as the target ligand to deliver Lupeol to ASGPR-expressing HCC cells. In vivo and in vitro experiments revealed the good tumor-suppressive effects of that liposome system.76 Additionally, Wang et al modified adriamycin (DOX) liposomes using ester bond-linked shearable polyethylene glycol lipids and galactosyl lipids. The polyethylene glycol-lipid-modified liposomes have been shown to prolong the circulation time and reduce uptake by the mononuclear phagocyte system. Thus, exhibiting a higher safety profile than free drugs.77
HBV virus infection is a risk factor for HCC and contains a highly specific amino acid sequence (HBVpreS1) in its envelope protein that imparts extreme affinity for the liver.78 It was later shown that the specific target of HBV on the hepatocyte membrane is the sodium-taurocholate co-transporting polypeptide (NTCP/SLC10A1).79 A myristoylated peptide, Myrcludex B, was developed and bound with high affinity and specificity to the NTCP on hepatocyte membranes, preventing the binding of viral particles to their target cells.80 Zhan was the first to prepare HBV preS1-derived lipopeptide-functionalized liposomes targeting hepatocytes, and showed that HBVpreS/2-48myr conjugated to PEGylated liposomes (HBVP-Lip) could specifically deliver loaded drugs to hepatocyte. Thus, those findings opened new possibilities for liver-specific drug delivery systems, gene delivery systems, and bioimaging systems.81 However, there are limits to the size of the nanoformulations with entry to the liver being limited when the diameter is higher than the average diameter of the endothelial window of the hepatic sinusoids in healthy humans (approximately 100 nm).82 To further optimize, Witzigmann et al prepared hepatotropic liposome particles to specifically target NTCP, and found using gamma scintigraphy and fluorescence microscopy that active NTCP could mediate the endocytosis of hepatocytes.83
Targeting Other Cells
Based on the dual action of liposomes and magnetic thermotherapy, thermosensitive magnetoliposomes (TMs) render tumor treatment more effective and safer. An anti-CD90+ Ab-modified TM loaded with 17-allylamino-17-demethoxygeldanamycin (17-AAG) (CD90@17-AAG/TMs) was reported to effectively target and kill CD90+ liver cancer stem cells (LCSC) and reduce the chance of HCC recurrence.84 KCs account for 35% of the non-parenchymal cells of the liver and 80–90% of the monocyte-macrophage system.85 They also secrete and synthesize a variety of bioactive substances.86 The activation of KCs and the overexpression of inflammatory factors such as tumor necrosis factor-α (TNF-α) are the main initiators of pro-inflammatory/sustained imbalance.
Zoledronate (ZOD) stops the progression of HCC, and Zhao et al used ZOD to prepare liposome-encapsulated zoledronate for intravenous administration and selectively induced the apoptosis of rat KCs.87 Animal experiments have shown that zoledronic acid liposomes help to concentrate ZOD in the target area, while reducing it in others, which reduces toxicity.88
Many chemotherapeutic agents such as arsenic trioxide (ATO) are toxic to cells, which has limited their applications. Jin et al prepared liposome-encapsulated arsenic-manganese complexes, denoted as LP@MnAsx, which enhanced therapeutic efficacy through pH-sensitive drug release, prolonged circulation time, and improved tumor accumulation. Manganese (Mn2+) ions were recently found to be effective T1 contrast agents.89 Arsenite (AsO33-), the ionic state of ATO, forms precipitates with Mn2+ in a neutral environment, while in an acidic environment, the compound can dissociate, releasing Mn2+ ions and arsenite. In turn, Mn2+ enables the real-time MRI detection of arsenite release in acidic tumor microenvironments, and therefore, a versatile drug delivery system was developed that was sensitive to microenvironmental pH value.90 Additionally, Mn2+ can also cause further damage to tumors by generating reactive oxygen species (ROS) through a Fenton-like reaction.91
Liposome Applications for Gene Therapy
Liposome applications for gene therapy can provide small interfering ribonucleic acid (siRNA), messenger RNA (mRNA), deoxyribonucleic acid (DNA), or gene editing complexes that provide a new approach to the treatment of liver cancer by silencing disease-causing genes, expressing therapeutic proteins, or correcting genetic defects (Figure 2).92
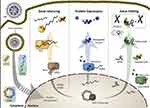 |
Figure 2 Mechanism of liposome combined gene therapy. Abbreviations: siRNA, small interfering RNA; mRNA, message RNA; cas9, clustered regularly interspaced short palindromic repeat-associated nuclease 9; crRNA, CRISPR RNA; tracrRNA, trans-acting crRNA. Notes: Encapsulating siRNA, mRNA, or DNA in liposomes. siRNA binds to certain enzymes and proteins in the cell to form an RNA-induced silencing complex (RISC) that binds to the target mRNA, cutting it off and degrading it. mRNA enters the cell and expresses proteins. cas9/tracrRNA/rNcrRNA targets DNA to cause double-stranded breaks (DSB) in DNA, resulting in gene editing. Reprinted from Advanced Drug Delivery Reviews, 159, Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver, 344-363, Copyright 2022, with permission from Elsevier.92 |
RNA interference (RNAi) is a means of regulating gene expression by siRNAs and microRNAs (miRNAs).93 siRNAs bind to the RNA-induced silencing complex (RISC), and once they enter the cytoplasm, they induce gene silencing by directing the specific cleavage of sequences of fully complementary paired mRNAs. Comparatively, miRNAs mediate the inhibition of translation and the termination of transcription of incompletely complementary mRNAs.94 miRNAs may also mediate mRNA degradation in cytoplasmic compartments, known as processing bodies (P-bodies), thus preventing protein synthesis.95 Primate synthesized siRNAs are unable to cross biological membranes by passive diffusion due to their high molecular weight and polyanionic nature; therefore, they require drug delivery strategies. Lipid nanoparticles containing siRNAs can silence genes, and thus, are precise therapeutic tools.96 Wanatabe’s team attempted to silence the gene expression of hepatitis C virus (HCV) in vivo using novel cationic liposome-encapsulated siRNA molecules. They used enzyme-linked immunosorbent assays (ELISA) to measure the expression of the HCV core protein in mouse liver and found that siRNA/lactosylated cationic liposome 5 (CL-LA5) complexes (siRNA/CL-LA5) specifically inhibited HCV protein expression in a dose-dependent manner. Interestingly, siRNA/CL-LA5 did not induce a highly biocompatible interferon (IFN) response in the liver, as conventional siRNAs did.97
Liver fibrosis genes also have an important role in hepatocarcinogenesis. The encapsulation of a procollagen α1 (I) (Col1a1) siRNA duplex (siCol1a1) into cationic C12-200 LNP (LNP-siCol1a1) has been reported, and the siCol1a1 was validated to have silencing efficacy against fibrosis genes, with a 90% dose-dependence. Thus, those data reflect the importance of anti-fibrotic therapy in inhibiting the progression of liver disease.93 Specific enhancement of gene expression through the targeted delivery of DNA can also be used for the treatment of HCC. Cationic lipid complexes consist of cationic lipids and neutral lipids or cholesterol. Negatively charged DNA is compressed and forms complexes with positively charged lipids, making it easier to transduce in rapidly-dividing cells.98 Wang’s team proposed a strategy to modify liposomes with the TfR, and delivering the acetylcholinesterase (AChE) gene by targeting the TfR on the surface of HCC cells for gene therapy.99 Compared to DNA-based therapies, mRNA has a greater potential success of targeted delivery and effect of gene. Because it does not require access to the nucleus. Unfortunately, however, mRNA is capable of inducing an immune response, which can be avoided by mRNA-based liposomes.100
Miao et al introduced alkyne and ester groups into the lipid tail, where the alkyne enhanced endosomal escape and systemic tolerance, and the ester promoted cellular uptake of the drug. Such a synergistic formulation was efficacious for gene delivery, with approximately 10-fold higher protein expression compared to unmodified lipids.101 In some experiments, the protein was infused directly into the tumor, thus, providing unexpected results. Kringle 1-5 (K1-5) is an excellent gene delivery candidate due to its physiological production, non-immunogenic nature, avoidance of transduction to other tumor cells, and strong and specific anti-angiogenic activity.91,102 Torimura et al prepared liposome-K1-5 cDNA complexes using K1-5-containing Cos-1 cells as the substrate and intravenously injected them into transplanted tumor-bearing mice, showing the inhibitory effect of liposome-K1-5 cDNA complexes on angiogenesis in tumor tissue.102–106
Liposome Applications for Immunotherapy
The immune microenvironment of HCC has suppressive tumor-associated macrophages (HCC-TAM) and liver sinusoidal endothelial cells (LSEC) functions as antigen-presenting cells that regulate the immune effects of the liver. In the physiological state, HCC-TAM and LSEC prevent immune responses by suppressing CD4+ and CD8+ T lymphocytes. Similar to LSEC, KCs and HDCs secrete suppressive cytokines and induce an increase in Treg cells.107 Furthermore, the HCC microenvironment is characterized by the high expression of immune checkpoint molecules. The combination of LipC6 bound CD8+ cells and eliminated the immune response leads to a significant decrease in the activity of the effectors of anti-tumor immune responses, which results in tumor immune evasion. In light of these findings, improving the tumor microenvironment is key for the treatment of HCC.108 Li et al have developed a liposome containing C6-ceramide (LipC6) and showed that it not only binds to the CD8+ T cell-mediated immune response to eliminate liver tumors in situ, but also promoted the polarization of TAMs to the M1 phenotype.109
Immunoliposomes are monoclonal antibodies or antibody fragments modified on the surface of liposomes that can actively target tumor tissue for specific immunotherapeutic purposes. During that process, liposomes act as adjuvants in addition to being the antigen carriers. Iwama et al encapsulated glypican-3 (GPC3)-derived cytotoxic T lymphocyte (CTL) peptides in liposomes (pGPC3-liposome), and investigated their antitumor potential. GPC3 behaved as a TAA in HCC tissue, exhibiting high expression. Thus, the results showed that pGPC3-liposomes effectively stimulated CTL in vivo and that liposomes were essential for the induction of CTL.110
The Integration of Diagnosis and Treatment
US
Ultrasound imaging (US) is widely used for diagnostic imaging due to its physical properties, low-cost, safety, and non-invasiveness. Additionally, US can induce multiple biological effects such as thermal, mechanical, and chemical effects, which can be used for therapeutic purposes (Figure 3).111
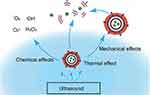 |
Figure 3 Ultrasound induces thermal, mechanical and chemical effects. Note: It is worth emphasizing that these effects are not independent, but occur simultaneously and interact with each other. |
US-Guided Thermal Effects
US energy is converted into heat energy and absorbed by the body, thus, raising the tissue temperature in the body to 40°C-45°C. Different tissues have different sensitivities to temperature, with 43°C typically as the cut-off. Temperatures below 43°C are considered mild hyperthermia, which can cause vasodilation, increase blood flow, and increase the permeability of vessel walls. Thermosensitive liposomes (TSLs) encapsulate hydrophilic drugs in a core surrounded by a lipid bilayer, and they respond to thermal signals and cause changes in the fluidity of the phospholipid bilayer. As the temperature increases, TSLs deform and eventually rupture, contributing to drug release at the site of the lesion.112 Some researchers used chemotherapy and thermotherapy in combination, where the loaded chemotherapeutic drug and the photothermal agent were released into the HCC tumor area in a controlled way, thus, enhancing the synergistic effect for the treatment.103–105,113 However, TSLs have some limitations in their practical application, such as causing damage to normal tissues and reduced drug release. To overcome such limitations, recent studies have focused on the structural design of liposomes. For example, TSLs were developed as a novel drug-controlled release system–ThermoDox®–with a relatively short half-life. To take advantage of the short half-life for cycling, thermotherapy is usually administered immediately before or after drug delivery, thus, overcoming the dependence of passive targeting of liposomes to solid tumor sites and ensuring free drug penetration through the tumor mesenchyme.114,115 Another thermal application of US—thermal ablation of tumors—is achieve by HIFU and occurs at temperatures above 43°C. Such high-temperature processes are classified as intense thermotherapy and lead to faster protein denaturation and necrosis.116 Thus, liposomes can enhance the therapeutic intensity. In a study by Feng et al, liposomes containing ammonium bicarbonate [Lip-ABC] produced bubbles after HIFU, which not only enhanced the quality of US imaging, but also further enhanced the ablation of tumors by vaporizing the liposomes into microbubbles through acoustic droplets.117 Liposomes can also increase the precision of treatment. Zhou et al developed adriamycin/indocyanine green (DOX/ICG)-loaded liposomes (DILPs) and combined them with radiofrequency ablation for the treatment of HCC. ICG is a contrast agent for multispectral photoacoustic tomography (MSOT) and facilitates the detection of lesions as small as 2.5 mm; a level of precision that is difficult to achieve by conventional imaging.118
Ultrasound-Guided Mechanical Effects
Mechanical effects mainly include the cavitation effect and the sonoporation effect.119 The sonoporation effect refers to the formation of pores in the cell membrane from US irradiation, which allow the transfer and accumulation of molecules and nanoparticles into the cell; thus, enhancing the delivery of liposomal drugs.120 The cavitation effect is classified into stable cavitation (SC) and inertial cavitation (IC) according to the way the US-induced bubbles burst.121 At low acoustic pressures, bubbles usually exhibit SC,122 while at high air pressure, bubbles collapse instantaneously, thus, leading to mechanical damage called IC.123 In addition, the cavitation effect produces ROS through the thermal dissociation of water.124 This is referred to as sonodynamic therapy in clinical applications; a combination of low-intensity US and chemotherapeutic agents, which has been explored as a promising alternative for cancer treatment.125
Zhao et al prepared multifunctional US molecular probes, hyaluronic acid-mediated cell-penetrating peptide-modified 10-hydroxycamptothecin-loaded phase-transformation lipid nanoparticles (HA/CPPs-10-HCPT-NPs), which actively target the CD44 receptor and accumulate in HCC cells. After low-intensity focused ultrasound (LIFU) irradiation of the lesion, the nanoparticles transform into gaseous microbubbles to enhance US imaging at the cellular level. In turn, US triggers targeted microbubble rupture (UTMD) for the synergistic physicochemical treatment of tumors.126 Similarly, Klibanov et al studied microbubble complexes coated with liposomes, where US was used to induce microbubble ruptures and trigger liposomes for drug delivery.127
US-Guided Chemical Effects
The process of US-guided chemical effects is closely linked to the cavitation effect, where vacuoles are formed after US waves pass through the blood-rich liver, and vacuole rupture generates large amounts of ROS that kill the diseased tissue.128
Echo-liposomes containing gas act as acoustic sensitizers to overcome the hypoxic tumor microenvironment. They are activated to produce ROS, including singlet oxygen (1O2), hydroxyl radicals (•OH), superoxide anions (O2•-), and hydrogen peroxide (H2O2).119,129 Lin et al described a 2, 2’-azobis [2-(2-imidazolin-2-yl) propane] dihydrochloride (AIPH)-loaded liposome (Lip-AIPH) that instantaneously generated bubbles and produced large amounts of ROS.130 It was also used as an ultrasound contrast agent because the air interface produced very high contrast between the circulation and the surrounding tissues.131
Near-Infrared (NIR)
NIR light imaging has been widely discussed in recent years. ICG is a fluorescent dye that absorbs and emits green light in NIR light and can identify and characterize tumors and metastatic lymph nodes. Thus, NIR fluorescence can be used to monitor cancer tissue resection during surgery.132 NIR light causes the least damage to the human body because its wavelength is in the biologically harmless range (650–950 nm).133
Experts have proposed photodynamic therapy (PDT) and photothermal therapy (PTT) as new options for tumor ablation. PDT can directly kill tumor cells, because during the therapy, ROS cause the apoptosis of tumor cells, while the immune response can also kill tumor cells.134 Kaneko et al evaluated the effectiveness of PDT using ICG and NIR, and found that the dye was directly absorbed by the HuH-7 tumor cell line.135 Liposomes can convert light energy into thermal energy,136 and with the aid of NIR-induced photothermal therapy, tumor ablation was achieved by heat stress and the thermal-induced release of therapeutic molecules.137 Peng et al prepared liposomes responsive to NIR to co-deliver DOX and the molecular targeting agent, sorafenib (SF). The disintegration of liposome structures under NIR light resulted in rapid drug release at the tumor site, which greatly enhanced the synergistic chemotherapeutic effect.138 Mu et al designed liposomes targeting Glypican-3 (GPC3) containing SF and IR780 iodide (IR780) (GSI-Lip) to perform NIR fluorescence imaging, and found that this system responded to photothermal therapy to improve the accuracy of HCC diagnoses.139
MRI
In recent years, liposomes have become an integrated platform for MRI imaging and therapy due to their excellent carrier properties.140 Platform can be combined with metallic elements to achieve therapeutic implications using their unique properties. For example, Fe3O4 is commonly used as a T2 contrast agent for MRI due to its unique superparamagnetic properties.141 Recent studies have shown that Fe3O4 exhibits photothermal conversion under NIR irradiation.142 In Shen’s study, multifunctional magnetic nanoparticle-loaded thermosensitive liposomes (Fe3O4-TSL) were developed for NIR-triggered release and combined with the photothermal-chemical treatment of tumors. After intravenous injection, Fe3O4-TSL was enriched in tumors over time and exhibited significant MRI and photothermal effects.
Gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) is also a widely used contrast agent for MRI.143 Xiao et al prepared SF and Gd co-loaded liposomes (SF/Gd-liposomes) to improve the aqueous solubility of SF and the accurate monitoring of its distribution.144 It was shown that SF/Gd-liposomes facilitated the MRI-guided visualization of liposome delivery and in vivo biodistribution with longer visualization times and higher signal enhancements in tumor tissues.145
Opportunities and Challenges for Future Applications of Liposomes
Liposomes are ideal tools for the application of HCC therapy. They are easy to prepare, highly biocompatible, adjustable in size, and capable of encapsulating highly toxic chemotherapeutic drugs. They can also respond to stimuli such as high temperatures, pH value, and US to release drugs into the diseased tissue in a targeted manner.146,147 Given these advantages, why are liposomes still limited to basic research and have not been widely used for clinical applications? Liposomes are prone to degradation through hydrolysis and oxidation, which is due to their inherent instability.12 Therefore, adjusting the structure of liposomes, controlling the drug loading and drug release rate, overcoming the rapid clearance of liposomes, and increasing the residence time of liposomes in tissues will accelerate their clinical applicability.148 To this end, this paper makes the following recommendations for the future use of liposomal drugs.
(1) Active targeting of ligands to control the release of drugs. There are many studies on highly expressed ligands for HCC cell membranes, however, few studies have targeted HCC vascular endothelium, or the organelle. Integrin-modified liposomes have been reported to modulate the production of breast cancer.149 Through reading much documents, we found studies in HCC are still lacking but potential.
(2) Triggered release. There are two main trigger types, including remote triggers (eg, heat, US, and light), and local triggers (eg, enzymes and pH changes) at the disease site or in organelles.150 For remote trigger release, we must further consider the issue of metastatic foci, and a whole-body Positron Emission Tomography-Computed Tomography (PET/CT) scan is required to determine the location of the lesion and improve the efficiency of treatment before operating.151
(3) Combination therapies. Combination therapies with different mechanisms can be used for the development of liposomes. In addition to traditional drug therapies, immunotherapies and gene therapies also have broad applicability, but how to combine them will be a primary area of focus in the field of nanomedicine in the future.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. The authors are grateful to the Jilin Health Technology Innovation (2020SCZT066); Science and Technology Development Plan of Jilin (20190201214JC) and Bethune Project of Jilin University (2020B06) for the financial support of this study. Graphial abstract is reprinted with permission from John Wiley and Sons, Inc. Ishizawa T, Bandai Y, Harada N, et al. Indocyanine green-fluorescent imaging of hepatocellular carcinoma during laparoscopic hepatectomy: An initial experience. Asian Journal of Endoscopic Surgery. 2010. © 2010 Asia Endosurgery Task Force and Blackwell Publishing Asia Pty Ltd.
Author Contributions
Yitong Li, Ruihang Zhang, Zhen Xu and Zhicheng Wang designed the research, Yitong Li, Ruihang Zhang and Zhen Xu wrote the manuscript, Zhicheng Wang completed the revising. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
2. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastro Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
4. Makary MS, Ramsell S, Miller E, Beal EW, Dowell JD. Hepatocellular carcinoma locoregional therapies: outcomes and future horizons. World J Gastroenterol. 2021;27(43):7462–7479. doi:10.3748/wjg.v27.i43.7462
5. Patel K, Lamm R, Altshuler P, Dang H, Shah AP. Hepatocellular carcinoma-the influence of immunoanatomy and the role of immunotherapy. Int J Mol Sci. 2020;21(18):6757. doi:10.3390/ijms21186757
6. Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis after resection of barcelona clinic liver cancer (BCLC) Stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26(11):3693–3700. doi:10.1245/s10434-019-07580-9
7. Liang L, Xing H, Zhang H, et al. Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20(2):110–119. doi:10.1016/j.hpb.2017.10.004
8. Gao Y, Lyu L, Feng Y, Li F, Hu Y. A review of cutting-edge therapies for hepatocellular carcinoma (HCC): perspectives from patents. Int J Med Sci. 2021;18(14):3066–3081. doi:10.7150/ijms.59930
9. Rommasi F, Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res Lett. 2021;16(1):95. doi:10.1186/s11671-021-03553-8
10. Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315.
11. Wang J, Gong J, Wei Z. Strategies for liposome drug delivery systems to improve tumor treatment efficacy. AAPS PharmSciTech. 2021;23(1):27. doi:10.1208/s12249-021-02179-4
12. Nakhaei P, Margiana R, Bokov DO, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886. doi:10.3389/fbioe.2021.705886
13. Yan W, Leung SSY, To KKW. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine. 2020;15(3):303–318. doi:10.2217/nnm-2019-0308
14. Koirala N, Das D, Fayazzadeh E, et al. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J Biomed Mater Res A. 2019;107(11):2522–2535. doi:10.1002/jbm.a.36758
15. Pittala S, Krelin Y, Shoshan-Barmatz V. Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1-based peptide. Neoplasia. 2018;20(6):594–609. doi:10.1016/j.neo.2018.02.012
16. D’Souza AA, Devarajan PV. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J Control Release. 2015;203:126–139. doi:10.1016/j.jconrel.2015.02.022
17. Arranja AG, Pathak V, Lammers T, Shi Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol Res. 2017;115:87–95. doi:10.1016/j.phrs.2016.11.014
18. Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi:10.1016/j.msec.2019.01.066
19. Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5). doi:10.1002/wnan.1450
20. Mokdad AA, Singal AG, Yopp AC. Jama Patient Page. Treatment of liver cancer. JAMA. 2016;315(1):100. doi:10.1001/jama.2015.15431
21. Bray F, Ren J-S, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. doi:10.1002/ijc.27711
22. Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. 2018;24(16):1679–1707. doi:10.3748/wjg.v24.i16.1679
23. Ho SY, Hsu CY, Liu PH, et al. Survival of patients with hepatocellular carcinoma in renal insufficiency: prognostic role of albumin-bilirubin grade. Cancers (Basel). 2020;12(5):1130. doi:10.3390/cancers12051130
24. Lee EC, Kim SH, Park H, Lee SD, Lee SA, Park SJ. Survival analysis after liver resection for hepatocellular carcinoma: a consecutive cohort of 1002 patients. J Gastroenterol Hepatol. 2017;32(5):1055–1063. doi:10.1111/jgh.13632
25. Cao Y, Xu L, Kuang Y, Xiong D, Pei R. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. J Mater Chem B. 2017;5(19):3431–3461. doi:10.1039/c7tb00382j
26. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
27. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–700. e3. doi:10.1053/j.gastro.2014.02.032
28. Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011650. doi:10.1002/14651858.CD011650.pub2
29. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi:10.1097/01.sla.0000197706.21803.a1
30. Vivarelli M, Cucchetti A, La Barba G, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248(5):857–862. doi:10.1097/SLA.0b013e3181896278
31. Ko KL, Mak LY, Cheung KS, Yuen MF. Hepatocellular carcinoma: recent advances and emerging medical therapies. F1000Res. 2020;9:F1000 Faculty Rev–620. doi:10.12688/f1000research.24543.1
32. Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10(5):425–434. doi:10.3348/kjr.2009.10.5.425
33. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308
34. Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
35. Wang T, Suita Y, Miriyala S, Dean J, Tapinos N, Shen J. Advances in lipid-based nanoparticles for cancer chemoimmunotherapy. Pharmaceutics. 2021;13(4):520. doi:10.3390/pharmaceutics13040520
36. Chan C, Du S, Dong YZ, Cheng XL. Computational and experimental approaches to investigate lipid nanoparticles as drug and gene delivery systems. Curr Top Med Chem. 2021;21(2):92–114. doi:10.2174/1568026620666201126162945
37. Duan L, Yang L, Jin J, et al. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics. 2020;10(2):462–483. doi:10.7150/thno.37593
38. Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi:10.1016/j.addr.2020.06.005
39. Kiaie SH, Mojarad-Jabali S, Khaleseh F, et al. Axial pharmaceutical properties of liposome in cancer therapy: recent advances and perspectives. Int J Pharm. 2020;581:119269. doi:10.1016/j.ijpharm.2020.119269
40. Li RR, Kowalski PS, Morselt HWM, et al. Endothelium-targeted delivery of dexamethasone by anti-VCAM-1 SAINT-O-Somes in mouse endotoxemia. PLoS One. 2018;13(5):e0196976. doi:10.1371/journal.pone.0196976
41. Li XC, Diao WB, Xue HT, et al. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett. 2020;489:163–173. doi:10.1016/j.canlet.2020.06.017
42. Chen JD, Jiang H, Wu Y, Li YD, Gao Y. A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver-targeting and in vitro/vivo evaluation. Drug Des Dev Ther. 2015;9:2265–2275. doi:10.2147/Dddt.S81722
43. Lian B, Wei H, Pan RY, et al. Galactose modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. Int J Nanomed. 2021;16:457–467. doi:10.2147/Ijn.S283793
44. Chen XY, Hu XX, Hu JJ, Qiu ZP, Yuan M, Zheng GH. Celastrol-loaded galactosylated liposomes effectively inhibit AKT/c-met-triggered rapid hepatocarcinogenesis in mice. Mol Pharm. 2020;17(3):738–747. doi:10.1021/acs.molpharmaceut.9b00428
45. Farinha D, de Lima MCP, Faneca H. Specific and efficient gene delivery mediated by an asialofetuin-associated nanosystem. Int J Pharm. 2014;473(1–2):366–374. doi:10.1016/j.ijpharm.2014.07.019
46. Bansal D, Yadav K, Pandey V, Ganeshpurkar A, Agnihotri A, Dubey N. Lactobionic acid coupled liposomes: an innovative strategy for targeting hepatocellular carcinoma. Drug Deliv. 2016;23(1):140–146. doi:10.3109/10717544.2014.907373
47. Oh HR, Jo HY, Park JS, et al. Galactosylated liposomes for targeted co-delivery of doxorubicin/vimentin siRNA to hepatocellular carcinoma. Nanomaterials (Basel). 2016;6(8):141. doi:10.3390/nano6080141
48. Pireddu R, Pibiri M, Valenti D, et al. A novel lactoferrin-modified stealth liposome for hepatoma-delivery of triiodothyronine. Int J Pharm. 2018;537(1–2):257–267. doi:10.1016/j.ijpharm.2017.12.048
49. Sun D, Tan SY, Xiong YL, et al. MicroRNA biogenesis is enhanced by liposome-encapsulated Pin1 inhibitor in hepatocellular carcinoma. Theranostics. 2019;9(16):4704–4716. doi:10.7150/thno.34588
50. Liu MC, Liu L, Wang XR, et al. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int J Nanomed. 2016;11:1395–1412. doi:10.2147/Ijn.S96862
51. Zhang WD, Peng FQ, Zhou TT, et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomed. 2015;10:4825–4836. doi:10.2147/Ijn.S79598
52. Wei YH, Gu XL, Cheng L, Meng FH, Storm G, Zhong ZY. Low-toxicity transferrin-guided polymersomal doxorubicin for potent chemotherapy of orthotopic hepatocellular carcinoma in vivo. Acta Biomater. 2019;92:196–204. doi:10.1016/j.actbio.2019.05.034
53. Alanazi SA, Harisa GI, Badran MM, et al. Crosstalk of low density lipoprotein and liposome as a paradigm for targeting of 5-fluorouracil into hepatic cells: cytotoxicity and liver deposition. Bioengineered. 2021;12(1):914–926. doi:10.1080/21655979.2021.1896202
54. Fang Y, Yang WJ, Cheng L, Meng FH, Zhang J, Zhong ZY. EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017;64:323–333. doi:10.1016/j.actbio.2017.10.013
55. Gao J, Chen HW, Yu YS, et al. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of Adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34(38):10084–10098. doi:10.1016/j.biomaterials.2013.08.088
56. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin. 2004;130(7):417–422. doi:10.1007/s00432-004-0552-0
57. Ren Y, He S, Huttad L, et al. An NIR-II/MR dual modal nanoprobe for liver cancer imaging. Nanoscale. 2020;12(21):11510–11517. doi:10.1039/d0nr00075b
58. Wallnofer EA, Thurner GC, Kremser C, et al. Albumin-based nanoparticles as contrast medium for MRI: vascular imaging, tissue and cell interactions, and pharmacokinetics of second-generation nanoparticles. Histochem Cell Biol. 2021;155(1):19–73. doi:10.1007/s00418-020-01919-0
59. Šimečková P, Hubatka F, Kotouček J, et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: in vitro safety study in liver cells and macrophages. Sci Rep. 2020;10(1):4780. doi:10.1038/s41598-020-60284-z
60. Fouillet X, Tournier H, Khan H, et al. Enhancement of computed tomography liver contrast using iomeprol-containing liposomes and detection of small liver tumors in rats. Acad Radiol. 1995;2(7):576–583. doi:10.1016/s1076-6332(05)80118-7
61. Lee SY, Jeon SI, Jung S, Chung IJ, Ahn CH. Targeted multimodal imaging modalities. Adv Drug Deliv Rev. 2014;76:60–78. doi:10.1016/j.addr.2014.07.009
62. Lamichhane N, Dewkar GK, Sundaresan G, Mahon RN, Zweit J. [(18)F]-Fluorinated Carboplatin and [(111)In]-liposome for image-guided drug delivery. Int J Mol Sci. 2017;18(5):1079. doi:10.3390/ijms18051079
63. Guan T, Shang W, Li H, et al. From detection to resection: photoacoustic tomography and surgery guidance with indocyanine green loaded gold Nanorod@liposome core-shell nanoparticles in liver cancer. Bioconjug Chem. 2017;28(4):1221–1228. doi:10.1021/acs.bioconjchem.7b00065
64. Wang Y, Chen C, Luo Y, et al. Experimental study of tumor therapy mediated by multimodal imaging based on a biological targeting synergistic agent. Int J Nanomedicine. 2020;15:1871–1888. doi:10.2147/ijn.S238398
65. Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213. doi:10.1016/j.tcb.2014.11.006
66. Li YL, Zhu XM, Liang H, Orvig C, Chen ZF. Recent advances in asialoglycoprotein receptor and glycyrrhetinic acid receptor-mediated and/or pH-responsive hepatocellular carcinoma-targeted drug delivery. Curr Med Chem. 2021;28(8):1508–1534. doi:10.2174/0929867327666200505085756
67. Cai Y, Xu Y, Chan HF, Fang X, He C, Chen M. Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy. Mol Pharm. 2016;13(3):699–709. doi:10.1021/acs.molpharmaceut.5b00677
68. Kindrat I, Tryndyak V, de Conti A, et al. MicroRNA-152-mediated dysregulation of hepatic transferrin receptor 1 in liver carcinogenesis. Oncotarget. 2016;7(2):1276–1287. doi:10.18632/oncotarget.6004
69. Joo I, Kim H, Lee JM. Cancer stem cells in primary liver cancers: pathological concepts and imaging findings. Korean J Radiol. 2015;16(1):50–68. doi:10.3348/kjr.2015.16.1.50
70. Dou L, Shi X, He X, Gao Y. Macrophage phenotype and function in liver disorder. Front Immunol. 2019;10:3112. doi:10.3389/fimmu.2019.03112
71. You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48(3):978–990. doi:10.1002/hep.22395
72. Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381–389. doi:10.1016/j.humpath.2008.08.011
73. Li XG, Yao WB, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. doi:10.1136/gutjnl-2015-310514
74. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002
75. Li T, Yu P, Chen Y, et al. N-acetylgalactosamine-decorated nanoliposomes for targeted delivery of paclitaxel to hepatocellular carcinoma. Eur J Med Chem. 2021;222:113605. doi:10.1016/j.ejmech.2021.113605
76. Zhang J, Hu XX, Zheng GH, Yao H, Liang HL. In vitro and in vivo antitumor effects of lupeol-loaded galactosylated liposomes. Drug Deliv. 2021;28(1):709–718. doi:10.1080/10717544.2021.1905749
77. Wang S, Xu H, Xu J, et al. Sustained liver targeting and improved antiproliferative effect of doxorubicin liposomes modified with galactosylated lipid and PEG-lipid. AAPS PharmSciTech. 2010;11(2):870–877. doi:10.1208/s12249-010-9450-8
78. Meier A, Mehrle S, Weiss TS, Mier W, Urban S. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology. 2013;58(1):31–42. doi:10.1002/hep.26181
79. Yan H, Zhong GC, Xu GW, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi:10.7554/eLife.00049
80. Blank A, Markert C, Hohmann N, et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65(3):483–489. doi:10.1016/j.jhep.2016.04.013
81. Zhang X, Zhang Q, Peng Q, et al. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014;35(23):6130–6141. doi:10.1016/j.biomaterials.2014.04.037
82. Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15(17):1193–1199. doi:10.1038/gt.2008.60
83. Witzigmann D, Uhl P, Sieber S, et al. Optimization-by-design of hepatotropic lipid nanoparticles targeting the sodium-taurocholate cotransporting polypeptide. Elife. 2019;8:e42276. doi:10.7554/eLife.42276
84. An Y, Yang R, Wang X, et al. Facile assembly of thermosensitive liposomes for active targeting imaging and synergetic chemo-/magnetic hyperthermia therapy. Front Bioeng Biotechnol. 2021;9:691091. doi:10.3389/fbioe.2021.691091
85. Salzano G, Marra M, Porru M, et al. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int J Pharm. 2011;403(1–2):292–297. doi:10.1016/j.ijpharm.2010.10.046
86. Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222–229. doi:10.1016/j.molimm.2017.02.018
87. Böttcher JP, Knolle PA, Stabenow D. Mechanisms balancing tolerance and immunity in the liver. Dig Dis. 2011;29(4):384–390. doi:10.1159/000329801
88. Zhao QH, Zhang XS, Wu K, et al. Preparation of Zoledronate liposome and its impact on apoptosis of Kupffer cells in rat liver. Acta Cir Bras. 2018;33(12):1052–1060. doi:10.1590/s0102-865020180120000002
89. Jin Z, Yi X, Yang J, Zhou M, Wu P, Yan G. Liposome-coated arsenic-manganese complex for magnetic resonance imaging-guided synergistic therapy against carcinoma. Int J Nanomedicine. 2021;16:3775–3788. doi:10.2147/ijn.S313962
90. Zhao Z, Wang X, Zhang Z, et al. Real-time monitoring of arsenic trioxide release and delivery by activatable T(1) imaging. ACS Nano. 2015;9(3):2749–2759. doi:10.1021/nn506640h
91. Zhang K, Lin H, Mao J, et al. An extracellular pH-driven targeted multifunctional manganese arsenite delivery system for tumor imaging and therapy. Biomater Sci. 2019;7(6):2480–2490. doi:10.1039/c9bm00216b
92. Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, Van Der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–363. doi:10.1016/j.addr.2020.06.026
93. Jiménez Calvente C, Sehgal A, Popov Y, et al. Specific hepatic delivery of procollagen α1(I) small interfering RNA in lipid-like nanoparticles resolves liver fibrosis. Hepatology. 2015;62(4):1285–1297. doi:10.1002/hep.27936
94. Entzian K, Aigner A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics. 2021;13(8):1135. doi:10.3390/pharmaceutics13081135
95. Ho W, Gao M, Li F, Li Z, Zhang XQ, Xu X. Next-generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv Healthc Mater. 2021;10(8):e2001812. doi:10.1002/adhm.202001812
96. Kulkarni JA, Witzigmann D, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc Chem Res. 2019;52(9):2435–2444. doi:10.1021/acs.accounts.9b00368
97. Watanabe T, Umehara T, Yasui F, et al. Liver target delivery of small interfering RNA to the HCV gene by lactosylated cationic liposome. J Hepatol. 2007;47(6):744–750. doi:10.1016/j.jhep.2007.06.015
98. Kullberg M, McCarthy R, Anchordoquy TJ. Systemic tumor-specific gene delivery. J Control Release. 2013;172(3):730–736. doi:10.1016/j.jconrel.2013.08.300
99. Wang K, Shang F, Chen D, et al. Protein liposomes-mediated targeted acetylcholinesterase gene delivery for effective liver cancer therapy. J Nanobiotechnology. 2021;19(1):31. doi:10.1186/s12951-021-00777-9
100. Yanez Arteta M, Kjellman T, Bartesaghi S, et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci U S A. 2018;115(15):E3351–E3360. doi:10.1073/pnas.1720542115
101. Miao L, Lin J, Huang Y, et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun. 2020;11(1):2424. doi:10.1038/s41467-020-16248-y
102. Fu LH, Hu YR, Qi C, et al. Biodegradable manganese-doped calcium phosphate nanotheranostics for traceable cascade reaction-enhanced anti-tumor therapy. ACS Nano. 2019;13(12):13985–13994. doi:10.1021/acsnano.9b05836
103. Yoshiji H, Kuriyama S, Yoshii J, et al. Vascular endothelial growth factor tightly regulates in vivo development of murine hepatocellular carcinoma cells. Hepatology. 1998;28(6):1489–1496. doi:10.1002/hep.510280607
104. Yoshiji H, Kuriyama S, Hicklin DJ, et al. KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells. Hepatology. 1999;30(5):1179–1186. doi:10.1002/hep.510300509
105. Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60. doi:10.1186/s13046-017-0533-1
106. Torimura T, Ueno T, Sata M. Liposome-mediated gene transfer of K1-5 suppresses tumor development and improves the prognosis of hepatocellular carcinoma in mice. Med Mol Morphol. 2006;39(2):72–78. doi:10.1007/s00795-006-0319-6
107. Dou L, Ono Y, Chen YF, Thomson AW, Chen XP. Hepatic dendritic cells, the tolerogenic liver environment, and liver disease. Semin Liver Dis. 2018;38(2):170–180. doi:10.1055/s-0038-1646949
108. Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18(2):291–297. doi:10.1016/j.aohep.2019.04.003
109. Li G, Liu D, Kimchi ET, et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology. 2018;154(4):1024–1036.e9. doi:10.1053/j.gastro.2017.10.050
110. Iwama T, Uchida T, Sawada Y, et al. Vaccination with liposome-coupled glypican-3-derived epitope peptide stimulates cytotoxic T lymphocytes and inhibits GPC3-expressing tumor growth in mice. Biochem Biophys Res Commun. 2016;469(1):138–143. doi:10.1016/j.bbrc.2015.11.084
111. Tehrani Fateh S, Moradi L, Kohan E, Hamblin MR, Shiralizadeh Dezfuli A. Comprehensive review on ultrasound-responsive theranostic nanomaterials: mechanisms, structures and medical applications. Beilstein J Nanotechnol. 2021;12:808–862. doi:10.3762/bjnano.12.64
112. Zhu L, Altman MB, Laszlo A, et al. Ultrasound hyperthermia technology for radiosensitization. Ultrasound Med Biol. 2019;45(5):1025–1043. doi:10.1016/j.ultrasmedbio.2018.12.007
113. Sun Y, Zhai W, Liu X, et al. Homotypic cell membrane-cloaked biomimetic nanocarrier for the accurate photothermal-chemotherapy treatment of recurrent hepatocellular carcinoma. J Nanobiotechnology. 2020;18(1):60. doi:10.1186/s12951-020-00617-2
114. Dou Y, Hynynen K, Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J Control Release. 2017;249:63–73. doi:10.1016/j.jconrel.2017.01.025
115. Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–1201.
116. van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–258. doi:10.1007/s00262-016-1891-9
117. Feng G, Hao L, Xu C, et al. High-intensity focused ultrasound-triggered nanoscale bubble-generating liposomes for efficient and safe tumor ablation under photoacoustic imaging monitoring. Int J Nanomedicine. 2017;12:4647–4659. doi:10.2147/ijn.S135391
118. Zhou Q, Wang K, Dou J, et al. Theranostic liposomes as nanodelivered chemotherapeutics enhanced the microwave ablation of hepatocellular carcinoma. Nanomedicine (Lond). 2019;14(16):2151–2167. doi:10.2217/nnm-2018-0424
119. Zhao P, Deng Y, Xiang G, Liu Y. Nanoparticle-assisted sonosensitizers and their biomedical applications. Int J Nanomedicine. 2021;16:4615–4630. doi:10.2147/ijn.S307885
120. Feng Y, Tian Z, Wan M. Bioeffects of low-intensity ultrasound in vitro: apoptosis, protein profile alteration, and potential molecular mechanism. J Ultrasound Med. 2010;29(6):963–974. doi:10.7863/jum.2010.29.6.963
121. Pellicori P, Platz E, Dauw J, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail. 2021;23(5):703–712. doi:10.1002/ejhf.2032
122. Tharkar P, Varanasi R, Wong WSF, Jin CT, Chrzanowski W. Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front Bioeng Biotechnol. 2019;7:324. doi:10.3389/fbioe.2019.00324
123. Pang X, Xiao Q, Cheng Y, et al. Bacteria-responsive nanoliposomes as smart sonotheranostics for multidrug resistant bacterial infections. ACS Nano. 2019;13(2):2427–2438. doi:10.1021/acsnano.8b09336
124. Lin X, Song J, Chen X, Yang H. Ultrasound-activated sensitizers and applications. Angew Chem Int Ed Engl. 2020;59(34):14212–14233. doi:10.1002/anie.201906823
125. Son S, Kim JH, Wang X, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–3261. doi:10.1039/c9cs00648f
126. Zhao H, Wu M, Zhu L, et al. Cell-penetrating peptide-modified targeted drug-loaded phase-transformation lipid nanoparticles combined with low-intensity focused ultrasound for precision theranostics against hepatocellular carcinoma. Theranostics. 2018;8(7):1892–1910. doi:10.7150/thno.22386
127. Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: a tool for targeted drug delivery. J Control Release. 2010;148(1):13–17. doi:10.1016/j.jconrel.2010.07.115
128. Costley D, Mc Ewan C, Fowley C, et al. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31(2):107–117. doi:10.3109/02656736.2014.992484
129. Nguyen AT, Wrenn SP. Acoustically active liposome-nanobubble complexes for enhanced ultrasonic imaging and ultrasound-triggered drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(3):316–325. doi:10.1002/wnan.1255
130. Lin XH, Qiu Y, Song L, et al. Ultrasound activation of liposomes for enhanced ultrasound imaging and synergistic gas and sonodynamic cancer therapy. Nanoscale Horiz. 2019;4(3):747–756. doi:10.1039/c8nh00340h
131. Huang SL. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60(10):1167–1176. doi:10.1016/j.addr.2008.03.003
132. Egloff-Juras C, Bezdetnaya L, Dolivet G, Lassalle HP. NIR fluorescence-guided tumor surgery: new strategies for the use of indocyanine green. Int J Nanomedicine. 2019;14:7823–7838. doi:10.2147/ijn.S207486
133. Highley CB, Kim M, Lee D, Burdick JA. Near-infrared light triggered release of molecules from supramolecular hydrogel-nanorod composites. Nanomedicine (Lond). 2016;11(12):1579–1590. doi:10.2217/nnm-2016-0070
134. Hou YJ, Yang XX, Liu RQ, et al. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomedicine. 2020;15:6827–6838. doi:10.2147/ijn.S269321
135. Kaneko J, Inagaki Y, Ishizawa T, et al. Photodynamic therapy for human hepatoma-cell-line tumors utilizing biliary excretion properties of indocyanine green. J Gastroenterol. 2014;49(1):110–116. doi:10.1007/s00535-013-0775-4
136. Reismann M, Bretschneider JC, von Plessen G, Simon U. Reversible photothermal melting of DNA in DNA-gold-nanoparticle networks. Small. 2008;4(5):607–610. doi:10.1002/smll.200701317
137. Li Z, Ye E, Lakshminarayanan R, Loh XJ. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small. 2016;12(35):4782–4806. doi:10.1002/smll.201601129
138. Peng Y, Su Z, Wang X, et al. Near-infrared light laser-triggered release of doxorubicin and sorafenib from temperaturesensitive liposomes for synergistic therapy of hepatocellular carcinoma. J Biomed Nanotechnol. 2020;16(9):1381–1393. doi:10.1166/jbn.2020.2975
139. Mu W, Jiang D, Mu S, Liang S, Liu Y, Zhang N. Promoting early diagnosis and precise therapy of hepatocellular carcinoma by glypican-3-targeted synergistic chemo-photothermal theranostics. ACS Appl Mater Interfaces. 2019;11(26):23591–23604. doi:10.1021/acsami.9b05526
140. Reeßing F, Stuart MCA, Samplonius DF, et al. A light-responsive liposomal agent for MRI contrast enhancement and monitoring of cargo delivery. Chem Commun (Camb). 2019;55(72):10784–10787. doi:10.1039/c9cc05516a
141. Amiri M, Salavati-Niasari M, Akbari A. Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv Colloid Interface Sci. 2019;265:29–44. doi:10.1016/j.cis.2019.01.003
142. Zhou P, Pan L, Deng G, et al. Fe@Fe3Ge2 nanoparticles for MR imaging-guided NIR-driven photodynamic therapy in vivo. J Mater Chem B. 2019;7(37):5661–5668. doi:10.1039/c9tb01173k
143. Lux F, Sancey L, Bianchi A, Crémillieux Y, Roux S, Tillement O. Gadolinium-based nanoparticles for theranostic MRI-radiosensitization. Nanomedicine (Lond). 2015;10(11):1801–1815. doi:10.2217/nnm.15.30
144. Xiao Y, Liu Y, Yang S, et al. Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids Surf B Biointerfaces. 2016;141:83–92. doi:10.1016/j.colsurfb.2016.01.016
145. Chen J, Sheu AY, Li W, et al. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–17. doi:10.1016/j.jconrel.2014.04.008
146. Kumar S, Dutta J, Dutta PK, Koh J. A systematic study on chitosan-liposome based systems for biomedical applications. Int J Biol Macromol. 2020;160:470–481. doi:10.1016/j.ijbiomac.2020.05.192
147. Wang GW, Wu BH, Li QY, et al. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16(44):e2004172. doi:10.1002/smll.202004172
148. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
149. Zhou B, Li M, Xu X, et al. Integrin alpha2beta1 targeting DGEA-modified liposomal doxorubicin enhances antitumor efficacy against breast cancer. Mol Pharm. 2021;18(7):2634–2646. doi:10.1021/acs.molpharmaceut.1c00132
150. Bibi S, Lattmann E, Mohammed AR, Perrie Y. Trigger release liposome systems: local and remote controlled delivery? J Microencapsul. 2012;29(3):262–276. doi:10.3109/02652048.2011.646330
151. Lu RC, She B, Gao WT, et al. Positron-emission tomography for hepatocellular carcinoma: current status and future prospects. World J Gastroenterol. 2019;25(32):4682–4695. doi:10.3748/wjg.v25.i32.4682
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.