Back to Journals » Cancer Management and Research » Volume 15
Advances in Immunotherapy for the Treatment of Cutaneous T-Cell Lymphoma
Authors Pelcovits A, Ollila TA, Olszewski AJ
Received 28 February 2023
Accepted for publication 30 August 2023
Published 6 September 2023 Volume 2023:15 Pages 989—998
DOI https://doi.org/10.2147/CMAR.S330908
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Ari Pelcovits,1,2 Thomas A Ollila,1,2 Adam J Olszewski1,2
1Department of Medicine, Alpert Medical School of Brown University, Providence, RI, USA; 2Division of Hematology-Oncology, Rhode Island Hospital, Providence, RI, USA
Correspondence: Adam J Olszewski, Department of Medicine, Alpert Medical School of Brown University, Rhode Island Hospital, George Building, Ste 310, 593 Eddy St, Providence, RI, 02903, USA, Tel +1-401-444-2269, Fax +1-401-444-4184, Email [email protected]
Abstract: Cutaneous T-Cell Lymphoma (CTCL) is a heterogenous disease that consists of distinct clinicopathologic entities and presentations requiring a unique and expert approach to management. The most common subtype is mycosis fungoides, in which local disease has an excellent prognosis and is often managed with topical therapy alone. More extensive cutaneous involvement as well as involvement of lymph nodes and the peripheral blood (Sezary syndrome) require systemic therapies. Recent years have brought an expansion of therapeutic options, specifically with immune-based approaches that were developed using the knowledge gained regarding the biology and molecular pathology of CTCL. Previous systemic therapies such as retinoids, histone deacetylase inhibitors, and chemotherapeutic agents come with significant toxicity and only short-term response. Newer agents such as mogamulizumab and brentuximab vedotin use a targeted immune-based approach leading to longer periods of response with less systemic toxicity. While still in its infancy, the use of immune checkpoint inhibitors such as nivolumab and pembrolizumab appears promising, and while their current clinical application is limited, early data suggest possible future areas for research of immune manipulation to treat CTCL. Herein, we review these novel immune-based treatment strategies, their superiority over prior systemic options, and the ongoing need for further research and clinical trial enrollment.
Keywords: cutaneous T-cell lymphoma, Sézary syndrome, immunotherapy, brentuximab vedotin
Introduction
Cutaneous T-cell lymphoma (CTCL) is a malignancy of clonally expanded T-cells that infiltrate the skin.1 The International Consensus Classification (ICC) of Mature Lymphoid Neoplasms and the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours identify nine different clinicopathologic entities under the category of CTCL.2,3 The most common subtype is mycosis fungoides (MF), which has excellent survival rates in early stages and is usually treated with topical therapy. However, outcomes worsen with more extensive skin involvement, and when the lymphoma spreads beyond the skin, the 5-year survival rate drops to less than 20%.4 Sézary syndrome (SS) is a rare but aggressive form of CTCL characterized by erythroderma and blood involvement by malignant T-cells.1 In recent decades, there has been significant progress in understanding the biology and molecular pathology of CTCL, particularly the expression of molecules associated with cell trafficking, immune activation, and exhaustion.5,6 As current cytotoxic and cytostatic therapies for CTCL have limited efficacy and lack curative potential, there is growing interest in using immunotherapy for the treatment of advanced CTCL.7–9
CTCL (MF and SS) is staged using the TNMB (tumor, node, metastasis, blood) system, and responses to treatment can be described as global or specific to the T, N, or B compartments.10 The T stage is defined by the type of visible lesions (patches, plaques, or tumors) and the amount of body surface area involved, with T4 disease indicating erythema that covers at least 80% of the body surface area. The B (blood) classification distinguishes low blood burden designated as B0 (<250/µL circulating atypical [Sézary] cells with an aberrant phenotype, most commonly CD4+CD7− or CD4+CD26−), B2 (defined as ≥1000/µL circulating Sézary cells), and B1 (cases not falling into either category). Treatment of localized disease is focused initially on skin-directed therapy with the use of topical agents, phototherapy, or radiation therapy.11 Systemic treatment is reserved for more diffuse cutaneous spread, resistant disease, or extracutaneous involvement.11 Systemic treatment options include retinoids, interferon alpha, histone deacetylase inhibitors, and chemotherapeutic agents, which usually result in short-lived responses and are associated with significant toxicity.12 These agents, as well as the use of bone marrow transplantation (BMT), were extensively reviewed elsewhere.6,9,13
This paper reviews the immune-based treatments for CTCL (mainly monoclonal antibodies and antibody-drug conjugates) that are either currently available or under investigation. The immunopathogenesis of CTCL reflects the variety of relevant immune-targeting agents, and the differences in response to them in MF and SS (Figure 1). MF and SS likely evolve from distinct subsets of memory T-cells. SS is characterized by strong expression of CCR7 and L-selectin, which are both markers of central memory T-cells and play important roles in skin and lymph node homing.14 In contrast, MF cells typically lack these markers and display an immunophenotype more consistent with skin resident memory T-cells, although some plasticity is possible in different compartments.15 Both MF and SS T-cells show strong expression of CCR4, as well as the common T-cell antigens CD2, CD3, and CD4 (rarely CD8), with frequent aberrant loss of (one or more of) CD5, CD7, or CD26.2,6 Monoclonal antibodies can produce an anti-lymphoma effect through several mechanisms, which include antibody-dependent cell cytotoxicity (ADCC, primarily involving NK cells), antibody-dependent cell phagocytosis (ADCP, primarily involving macrophages), complement-dependent cytotoxicity (CDC). These effects may occur on the tumor cells as well as on other immune cells in the lymphoma microenvironment, and the relative contribution of different mechanisms may vary among the antibodies.
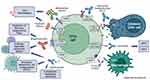 |
Figure 1 Cell surface targets and mechanisms of action of monoclonal antibodies in current use or under investigation in CTCL. Created with BioRender.com. |
Review
Denileukin Diftitox
Immune-targeted therapy has been used to treat advanced CTCL since the 1990s, starting with denileukin diftitox (DD), a recombinant protein that fuses the transmembrane domain protein (IL)-2 to the cytotoxic peptide of the diphtheria toxin.16 DD exerts its action through direct cytocidal effect after internalization of the agent and intracellular release of the toxin. Clinical trials of DD in CTCL, which has a high expression of the IL-2 receptor, showed an overall response rate (ORR) of up to 49% with a duration of response (DOR) approaching 1 year. However, manufacturing difficulties led to the discontinuation of the agent in 2014.17,18 An improved-purity form of DD (E7777) is currently being evaluated for potential reintroduction in CTCL and has shown an ORR of 36%, with a 9% complete response (CR) rate and a median DOR of 6 months.19,20 The primary toxicity of diphtheria toxin-based therapies is capillary leak syndrome, which can cause edema, hypotension, and hypoalbuminemia, typically during the first 2 courses of therapy.21
Alemtuzumab
Alemtuzumab, an anti-CD52 antibody, was initially developed to treat chronic lymphocytic leukemia, but it was later found to benefit patients with multiple sclerosis.22 CD52 is expressed on mature B- and T-cells, with particularly high levels on malignant T-cells.23 Alemtuzumab induces anti-lymphoma ADCC, but it unfortunately also effectively depletes effector T-cells. However, alemtuzumab may also exert direct cell-killing action via CDC.24,25 In a Phase 2 trial of 52 patients with mycosis fungoides (MF) or Sézary syndrome (SS) (stage ≥2) who had received ≥1 prior line of systemic therapy, alemtuzumab demonstrated an overall response rate (ORR) of 55%, with a complete response (CR) rate of 32% and median duration of response (DOR) of 12 months.23 Alemtuzumab cleared tumor cells from the blood in 6 out of 7 patients (86%), and the ORR in the skin was 56%. However, severe infections occurred in 50% of patients during treatment (2 of which were fatal), and 4 (18%) patients experienced reactivation of cytomegalovirus (CMV) infection. Furthermore, severe grade 4 neutropenia occurred in 18% of patients. Subsequent series of studies showed that the ORR in MF was less impressive (25%), although 15% of patients with SS maintained a CR for >2 years.26
Alternative administration schedules for alemtuzumab were investigated to mitigate the resulting immunosuppression. In a single-center study, 14 patients with SS (both previously treated and newly diagnosed) were treated with a reduced dose and slower dose escalation of subcutaneous alemtuzumab, along with strict hematologic parameters for holding therapy.27 The ORR was 86%, with a CR rate of 21% and median time to treatment failure of 12 months. Four patients experienced infectious complications (29%), with one death, all within the group receiving the maximum dose of alemtuzumab (15mg compared with 30mg in the prior studies).
No phase 3 randomized trials of alemtuzumab have been conducted in CTCL. Alemtuzumab is listed as an alternative treatment option in the National Comprehensive Center Network (NCCN) guidelines, which note the improved response in SS compared with MF and reduced infectious complications with the lower and subcutaneous dosing.11 However, alemtuzumab is currently only available for use in CTCL through the manufacturer’s compassionate use program. In our practice, we limit its use to patients with refractory SS, often as a bridge to allogeneic bone marrow transplantation. It is important to monitor patients frequently for cytopenias and CMV reactivation (using polymerase chain reaction [PCR]) and to provide early therapy for viremia to maintain safety.
Mogamulizumab
T-cell migration and homing to the skin, which is a critical pathophysiological mechanism in CTCL, is regulated by a range of chemokines and cell surface receptors. CC chemokine receptor 4 (CCR4) is a receptor that is frequently expressed on CTCL cells and plays a role in T-cell migration.14,28,29 Mogamulizumab, a defucosylated humanized IgG1κ anti-CCR4 monoclonal antibody, acts primarily through ADCC, but also leads to changes in the tumor microenvironment by selectively depleting tumor-residing Treg cells.30 It has been approved for the treatment of patients with relapsed or refractory CTCL.31 In a Phase 1/2 study of 41 patients with MF or SS, who had received at least one line of systemic therapy, the ORR to mogamulizumab (administered intravenously at 1.0 mg/kg weekly for 4 weeks and then every 2 weeks until disease progression) was 37%, with 47% ORR in SS.32 Three patients had a CR to treatment, and the median progression-free survival (PFS) was 11.4 months.
These promising results led to the phase 3 MAVORIC study of mogamulizumab in 372 patients with MF or SS who had progressed after at least one line of prior systemic therapy.33 Patients were randomized to receive either mogamulizumab or vorinostat (a histone deacetylase inhibitor), with a primary endpoint of PFS. CCR4 expression was not mandatory, and in an exploratory analysis, 97% of patients had CCR4 expression in the skin with no difference in response based on expression level.33 Mogamulizumab resulted in a significantly improved PFS of 7.7 months compared to 3.1 months in the vorinostat arm. In a prespecified subgroup analysis, treatment favored mogamulizumab in stage III/IV disease but not in stage IB/II. Mogamulizumab also showed a larger advantage in SS than in MF. ORR was 28% versus 5% with vorinostat, with a median DOR of 14.1 and 9.1 months, respectively. Responses to mogamulizumab were higher in the blood compartment (68%) than in the skin (42%) or nodes (17%). The most common adverse effects of mogamulizumab were largely grade 1 and included infusion-related reactions (32%), drug rash (20%), diarrhea (23%), and fatigue (22%). Subsequent studies showed that CTCL progression on mogamulizumab often occurs in the setting of loss of CCR4 expression by the tumor cells.34,35
Based on the observed durable responses and low toxicity, mogamulizumab is designated as the preferred NCCN option for patients with SS.11 However, several clinical aspects of mogamulizumab use warrant attention. The drug can cause infusion reactions as well as drug-related rash, which may rarely be severe. Hyperglycemia is noted in about 50% of patients with rare (4%) grade 3/4 glucose elevations. Furthermore, fatal graft versus host disease (GVHD) was observed in some patients with adult T-cell lymphoma/leukemia (ATLL) who underwent allogeneic BMT soon after mogamulizumab therapy, likely due to depletion of CCR4+ T-regs.36 Although only one out of eight patients with MF/SS undergoing allogeneic BMT developed severe GVHD after mogamulizumab in one case series, maintaining the recommended minimum 50-day interval between mogamulizumab and an allogeneic BMT is prudent.37 Finally, patients with large cell transformation of MF/SS were excluded from the pivotal trials, and the efficacy of mogamulizumab in this setting is unknown.
Brentuximab Vedotin
Brentuximab vedotin (BV) is an antibody–drug conjugate that targets CD30 and is linked to a microtubule toxin called monomethyl auristatin E. BV has been approved for the treatment of several malignancies that are characterized by CD30 expression, including Hodgkin lymphoma, anaplastic large cell lymphoma, and CD30+ peripheral T-cell lymphoma.38–41 Additionally, a significant subset of CTCL cells express CD30, which is a hallmark of primary cutaneous anaplastic large cell lymphoma (pcALCL).2,3,42
CD30 targeted therapy’s effectiveness in CTCL was first demonstrated in a phase 2 study of a naked anti-CD30 antibody SGN-30, with an ORR of 70%.43 BV was subsequently tested in two phase 2 trials for relapsed/refractory CD30+ cutaneous lymphomas.44,45 The first trial enrolled 48 patients with CD30+ CTCL after ≥1 prior systemic therapy, showing an ORR of 73% and CR rate of 35%.44 Responses were assessed specifically in patients with MF according to the CD30 expression level, with no significant differences seen. ORR for patients with MF was 54% compared with 100% for typical CD30+ cutaneous lymphoproliferative disorders (lymphomatoid papulosis and pcALCL). The second multicenter trial was conducted strictly in patients with CD30+ MF/SS, also progressing after ≥1 prior line of systemic therapy.45 The ORR was similar to the prior experience (70%), but a global CR was observed in only 1 subject. Patients were similarly grouped into categories of CD30 expression, and a statistically significant inferior response was noted among 6 patients with low (<10%) expression.
The ALCANZA trial was a randomized phase 3 trial of patients with CD30+ (defined as ≥10% expression) MF or pcALCL after ≥1 prior line of systemic therapy, comparing BV against physicians’ choice of standard of care (oral methotrexate or bexarotene).46 BV was administered intravenously at 1.8 mg/kg for up to 16 three-week courses; similarly, methotrexate or bexarotene were continued for up to 48 weeks. The primary endpoint was an objective response lasting at least 4 months (ORR4), which was attained by 56% of patients in the BV arm compared with 12% in the control arm. CR rates were 16% and 2%, respectively, median PFS was 16.7 and 3.5 months, respectively, and median DOR was 15.1 and 18.3 months, respectively. The toxicity of BV was similar to prior experience. Peripheral neuropathy was the most common adverse event occurring in 67% of patients, but only 9% experienced grade 3 neuropathy, and 82% reported improvement or resolution of symptoms. Nausea (36%), diarrhea (29%), and fatigue (29%) were also common, but low-grade in most cases. Serious adverse events occurred in 29% of patients treated with BV, and 24% of patients discontinued the drug due to toxicity.
Observational data confirm high responses to BV in both MF and SS. Among 67 CTCL patients from the Spanish Primary Cutaneous Lymphoma Registry, ORR was 63% in MF, 71% in SS, and 84% in other CD30+ lymphoproliferative disorders.47 Similar ORR rates (69% and 62%, respectively) were reported in a retrospective analysis from nine European Organization for Research and Treatment of Cancer (EORTC) centers. The median duration of response (DOR) was 9 months, with higher ORR in the skin (72%) than in the lymph nodes (47%) or blood (40%).48 However, a small single-institution series of 13 patients with relapsed/refractory SS reported lower response rates: 38% global, 38% in the skin, 63% in the blood, and 50% in the lymph nodes, with a median DOR of only 5.5 months.49 Based on the summary of the evidence, BV is an excellent immunotherapy option for patients with either MF or SS expressing the CD30.
PD-1/PD-L1 Blockade
Immune checkpoint inhibitors, specifically PD-1-directed monoclonal antibodies like nivolumab and pembrolizumab, have been effective in treating various solid malignancies and hematologic cancers, including Hodgkin lymphoma and primary mediastinal B-cell lymphoma.50,51 Their success is attributed to their ability to disrupt the inhibitory PD-1/PD-L1 interaction, which unleashes the activity of cytotoxic tumor-infiltrating CD8+ T-cells.52,53 However, using PD-1 inhibitors in T-cell lymphomas, such as CTCL, is challenging due to the fact that T-cell lymphoma cells may themselves express PD-1, which maintains an antiproliferative effect.54,55 In some cases, checkpoint inhibition can trigger a massive and potentially fatal “hyperprogression” of a T-cell lymphoma, as observed in ATLL.56 In CTCL, PD-1 expression is common in SS (89%) but less so in MF (13%).57,58 PDCD1, the gene encoding PD-1, is deleted in 1/3 of SS cases and is mutated in cases with accelerated proliferation, suggesting a potential mechanism of escape from the negative PD-1-driven regulation as the disease progresses.54,59 Despite increased proliferation of SS cells upon PD-1 blockade in pre-clinical models,60 only one case of hyperprogression has been reported in the clinical setting, with worsening skin lesions, lymphocytosis, and upregulation of the activation marker CD69 on malignant T-cells.61
Clinical data on immune checkpoint inhibitors in CTCL are promising, leading to the inclusion of off-label pembrolizumab in the NCCN guidelines.11 The initial evidence of efficacy came from a phase 1 trial of patients with various hematologic malignancies treated with nivolumab.62 Out of 15 patients with CTCL included in this study, two (both MF) showed responses. A phase 2 trial of pembrolizumab enrolled 24 patients with CTCL who had received a median of four prior lines of systemic therapy.63 The observed ORR was 38%, with 2 patients achieving a CR. Responses occurred in 56% of patients with MF and 27% of those with SS, and median DOR was not reached after median 58 weeks of follow-up. Thirty-eight percent of patients experienced an immune-related adverse event, but grade 4 or 5 toxicities were absent. Responders showed an expansion of CD8+ cytotoxic T-cells, suggesting that the efficacy of pembrolizumab is mediated by activation of anti-tumor immunity.64 The increased proliferation of malignant T-cells was not observed in patients treated with pembrolizumab, although some may experience transient flares of erythroderma.64
Other PD-1 and PD-L1 inhibitors are under investigation for CTCL. Tislelizumab, a humanized anti-PD-1 monoclonal antibody, showed a promising ORR of 45% (with 9% CR rate) in 11 patients with CTCL enrolled in a phase 2 trial.65 Other anti-PD-1 antibodies under investigation include durvalumab and sintilimab. PD-L1 is not overexpressed on CTCL cells compared to healthy controls66 but becomes overexpressed in cases with large-cell transformation that acquire structural variants in CD274 (the gene encoding PD-L1).67 Initial data from the phase 2 trial of atezolizumab in relapsed/refractory MF/SS suggested a disappointing ORR of 15% with a median PFS of 3 months and 15% risk of sepsis (fatal in 7%).68
Novel Targets: KIR3DL2 and CD47
KIR3DL2 (CD158k) is a surface antigen expressed on CTCL cells (85% of SS) and is a member of the killer cell immunoglobulin-like receptor (KIR) class.69,70 Its binding to MHC class I molecules inhibits cytotoxicity for NK cells and some CD8+ T-cells, but it is expressed on few normal immune cells.71 Lacutamab (IP4102) is a humanized anti-KIR3DL2 monoclonal antibody currently in clinical development for CTCL therapy.71,72 Lacutamab acts primarily through ADCC and ADCP, but it generally does not bind complement.72 In a phase 1 trial, the agent showed no dose-limiting toxicities up to the recommended phase 2 dose of 750 mg. The most common adverse events were related to grade 1/2 peripheral edema (27%), fatigue (20%), fever, diarrhea, and arthralgia (16% each).73 Preliminary results from the phase 2 trial indicate global confirmed ORR of 22%, including skin in 35% and blood in 38% in a relapsed/refractory SS after at least 2 lines of therapy including mogamulizumab.74 The combination of lacutamab with PD-1 inhibitors may be worth investigating because decreased KIR3DL2 expression is associated with a higher chance of response to pembrolizumab.64
TTI-621 and TTI-622 (PF-07901801) are fusion proteins containing the CD47-binding domain of SIRPα and an Fc portion of the human IgG1 or IgG4 (respectively). CD47 is highly expressed on Sézary cells both in the blood and cutaneous compartments, where it can serve as a receptor for thrombospondin-1 (which stimulates Sézary cell migration and growth),75 as well as a surface checkpoint inhibitor molecule that binds SIRPα on macrophages.76 TTI-621 and TTI-622 act by blocking the immune checkpoint interaction between the CD47 on MF/SS tumor cells with SIRPα on macrophages, enabling the phagocytosis of the tumor cells.77,78 In a phase 1 setting, responses to TTI-621 were recorded in 1 of 4 patients with SS and 5 of 19 with MF, for an ORR in CTCL of 21% (including one CR).77 TTI-622/PF-07901801 has produced a response in 3 of 6 CTCL patients in a phase 1 trial, including 1 CR.79 Toxicities of both agents were low-grade, reversible, and primarily hematologic (most frequently, thrombocytopenia). In contrast to other CD47-directed agents, TTI-621 and 622 were not associated with high rates of severe anemia. Preliminary data suggest that TTI-621 may also enhance the response to PD-1 inhibition in CTCL cell lines.80
Ongoing Investigations
Several ongoing trials (referred herein by their identifiers in the ClinicalTrials.gov database) are examining combinations of the immunotherapeutic agents reviewed above, as well as alternative targets. NCT05414500 is a phase 1 dose-escalation study evaluating the combination of BV and mogamulizumab in patients with previously treated CTCL, including those who were previously treated with BV. Although lenalidomide, an immunomodulatory agent, has limited activity as a single agent against CTCL, it is currently being evaluated in at least two trials as an augmentation to immunotherapy.81 NCT03409432 is a phase 2 trial combining BV with lenalidomide in relapsed/refractory T-cell lymphomas, including CTCL. NCT03011814 is a phase 1/2 trial of lenalidomide and durvalumab in relapsed/refractory CTCL. The preliminary report of the phase 1 portion indicated promising activity with 9 out of 12 enrolled subjects responding.82 Another phase 1 trial (NCT04652960) is investigating the combination of nivolumab with the phosphoinositide 3-kinase inhibitor duvelisib. Other potential targets for immunotherapy in CTCL under consideration include ICOS,83 TIGIT,35,84 CD70 (the ligand of CD27),85 and CD38, which is also overexpressed on CTCL cells.86 Zanolimumab, an anti-CD4 antibody, was investigated with preliminary success in a phase 2 trial, and a phase 3 trial was initiated, but the development of the drug was subsequently halted for business reasons.87
CAR T-cell therapy is an immunotherapy approach that has revolutionized the management of relapsed/refractory B-cell lymphomas, leukemias, and myeloma.88 However, developing suitable CAR T-cell products against CTCL and other T-cell lymphomas is hampered by several issues, including the antigen overlap between malignant and infused T-cells, the potential for inadvertent transduction of harvested Sézary T-cells, fratricide, and complications of prolonged T-cell aplasia.89 Allogeneic products or CAR NK-cells may help overcome some of these limitations (ability to generate autologous product from immunosuppressed donors, reinfusion of SS tumor cells, fratricide), but pose other challenges related to their persistence and the theoretical risk of GVHD.90,91 Nevertheless, early clinical investigations are underway for CAR T-cells targeting CD4, CD7, CD37, CD70, CCR4 and other antigens (with suitable knockouts to prevent fratricide and GVHD) in various T-cell lymphomas.92–95 For a more detailed information on this emerging field of research, including a comprehensive listing of targets and ongoing clinical trials, we refer the reader to recent specialized reviews.89,96
Conclusions: Our Current Approach and Future Directions
The currently available immunotherapy agents for CTCL include mogamulizumab, BV, and pembrolizumab (Table 1). While effective and tolerable, they are not approved for first-line treatment and require long-term intravenous administration with frequent visits. Standard first-line therapy options include bexarotene, oral methotrexate, or oral histone deacetylase inhibitors, which may be less burdensome to patients, but their efficacy becomes very limited in more advanced MF or SS. Cytotoxic chemotherapy is rarely used in advanced CTCL, except as bridging disease control before consolidative BMT in cases with aggressive, large cell transformation.
 |
Table 1 Currently Available Immunotherapy Agents for CTCL |
Therefore, we usually institute immunotherapy as a second-line therapy, as the risk/benefit ratio becomes favorable. If CD30 expression (>5%) is detected and the patient does not have underlying neuropathy, BV is our preferred agent, although there is uncertainty about the minimal necessary cutoff of CD30 expression. For other patients, mogamulizumab is typically the most reasonable alternative, providing durable responses even in advanced SS (without large cell transformation). Pembrolizumab is an acceptable alternative, but vigilance is necessary regarding the risk of erythrodermic flare or hyperprogression. Alemtuzumab is limited by toxicity and availability, but it can be helpful for refractory SS patients with close surveillance for opportunistic infections. Allogeneic BMT, a form of radical immunotherapy, retains curative potential for select patients and should be considered during or after the first line of immunotherapy, given the high chance of further progression.
As none of the available agents provide a cure, we prioritize enrollment in clinical trials of novel approaches whenever possible. Combinations of immunotherapeutic antibodies have demonstrated safety in other malignancies, such as BV and PD-1 antibodies in Hodgkin lymphoma or mogamulizumab and PD-1 antibodies in solid tumors, and should be further investigated in CTCL.97,98 Agents targeting KIR3DL2 and CD47 may potentiate checkpoint inhibitors, but further establishment as single agents is necessary. In the near future, CAR T-cell therapy may offer a potentially curative, immune effector cell-engaging approach.
Disclosure
AJO is a Clinical Research Scholar of the Leukemia & Lymphoma Society, and reports consultancy role with Genmab; Blue Cross/Blue Shield of Rhode Island; TG Therapeutics; Schrodinger; ADC Therapeutics; BeiGene, and research funding from Genetech/Roche; Adaptive Biotechnologies; Precision Biosciences; Genmab; Kymera Therapeutics. TAO reports research funding from ONO Pharmaceuticals. AP reports no relevant conflicts of interest in this work.
References
1. Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–1714. doi:10.1182/blood-2018-11-881268
2. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
3. Campo E, Jaffe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140(11):1229–1253.
4. Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised international society for cutaneous lymphomas/European organisation for research and treatment of cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739. doi:10.1200/JCO.2009.27.7665
5. Tensen CP, Quint KD, Vermeer MH. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood. 2022;139(1):15–33. doi:10.1182/blood.2019004256
6. Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
7. Whittaker S, Hoppe R, Prince HM. How I treat mycosis fungoides and Sezary syndrome. Blood. 2016;127(25):3142–3153. doi:10.1182/blood-2015-12-611830
8. Duvic M. Choosing a systemic treatment for advanced stage cutaneous T-cell lymphoma: mycosis fungoides and Sezary syndrome. Hematology Am Soc Hematol Educ Program. 2015;2015:529–544. doi:10.1182/asheducation-2015.1.529
9. Khodadoust MS, Mou E, Kim YH. Integrating novel agents into the treatment of advanced mycosis fungoides and Sezary syndrome. Blood. 2023;141(7):695–703. doi:10.1182/blood.2020008241
10. Olsen EA, Whittaker S, Willemze R, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140(5):419–437. doi:10.1182/blood.2021012057
11. Mehta-Shah N, Horwitz SM, Ansell S, et al. NCCN guidelines insights: primary cutaneous lymphomas, version 2.2020. J Natl Compr Canc Netw. 2020;18(5):522–536. doi:10.6004/jnccn.2020.0022
12. Hughes CF, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sezary syndrome: a comparative study of systemic therapy. Blood. 2015;125(1):71–81. doi:10.1182/blood-2014-07-588236
13. Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193–209. doi:10.1002/ajh.26760
14. Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767–771. doi:10.1182/blood-2009-11-251926
15. Roelens M, Delord M, Ram-Wolff C, et al. Circulating and skin-derived Sezary cells: clonal but with phenotypic plasticity. Blood. 2017;130(12):1468–1471. doi:10.1182/blood-2017-03-772996
16. Saleh MN, Kuzel TM, Foss F, et al. Antitumor activity of DAB389IL-2 fusion toxin in mycosis fungoides. J Am Acad Dermatol. 1998;39(1):63–73. doi:10.1016/S0190-9622(98)70403-7
17. Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(11):1870–1877. doi:10.1200/JCO.2009.26.2386
18. Duvic M, Geskin L, Prince HM. Duration of response in cutaneous T-cell lymphoma patients treated with denileukin diftitox: results from 3 phase III studies. Clin Lymphoma Myeloma Leuk. 2013;13(4):377–384. doi:10.1016/j.clml.2013.02.020
19. Kawai H, Ando K, Maruyama D, et al. Phase II study of E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T-cell lymphoma. Cancer Sci. 2021;112(6):2426–2435. doi:10.1111/cas.14906
20. Foss FM, Kim YH, Prince HMM, et al. Efficacy and Safety of E7777 (improved purity Denileukin diftitox [ONTAK]) in Patients with relapsed or refractory cutaneous T-cell lymphoma: results from pivotal study 302. Blood. 2022;140(Supplement 1):1491–1492. doi:10.1182/blood-2022-166916
21. Prince HMM, Geskin LJ, Akilov OE, et al. Safety and Tolerability of E7777 (improved purity Denileukin diftitox [ONTAK]) in patients with relapsed or refractory cutaneous T-cell lymphoma: results from pivotal study 302. Blood. 2022;140(Supplement 1):6577–6578. doi:10.1182/blood-2022-167564
22. Keating MJ, Jain V, Binet JL, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–35561. doi:10.1182/blood.V99.10.3554
23. Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267–4272. doi:10.1182/blood-2002-09-2802
24. Gribben JG, Hallek M. Rediscovering alemtuzumab: current and emerging therapeutic roles. Br J Haematol. 2009;144(6):818–831. doi:10.1111/j.1365-2141.2008.07557.x
25. Heit W, Bunjes D, Wiesneth M, et al. Ex vivo T-cell depletion with the monoclonal antibody Campath-1 plus human complement effectively prevents acute graft-versus-host disease in allogeneic bone marrow transplantation. Br J Haematol. 1986;64(3):479–486. doi:10.1111/j.1365-2141.1986.tb02203.x
26. de Masson A, Guitera P, Brice P, et al. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014;170(3):720–724. doi:10.1111/bjd.12690
27. Bernengo MG, Comessatti A, Ortoncelli M, Novelli M, Lisa F, Fierro MT. Low-dose intermittent alemtuzumab in the treatment of Sézary syndrome: clinical and immunologic findings in 14 patients. Haematologica. 2007;92(6):784–794. doi:10.3324/haematol.11127
28. Jones D, O’Hara C, Kraus MD, et al. Expression pattern of T-cell–associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96(2):685–690. doi:10.1182/blood.V96.2.685
29. Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. 2003;121(5):1045–1052. doi:10.1046/j.1523-1747.2003.12555.x
30. Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531. doi:10.1158/1078-0432.CCR-09-2697
31. Ollila TA, Sahin I, Olszewski AJ. Mogamulizumab: a new tool for management of cutaneous T-cell lymphoma. Onco Targets Ther. 2019;12:1085–1094. doi:10.2147/OTT.S165615
32. Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883–1889. doi:10.1182/blood-2014-09-600924
33. Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. doi:10.1016/S1470-2045(18)30379-6
34. Beygi S, Duran GE, Fernandez-Pol S, Rook AH, Kim YH, Khodadoust MS. Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma. Blood. 2022;139(26):3732–3736. doi:10.1182/blood.2021014468
35. Roelens M, de Masson A, Andrillon A, et al. Mogamulizumab induces long-term immune restoration and reshapes tumour heterogeneity in Sezary syndrome. Br J Dermatol. 2022;186(6):1010–1025. doi:10.1111/bjd.21018
36. Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation Anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426–3433. doi:10.1200/JCO.2016.67.8250
37. Dai J, Almazan TH, Hong EK, et al. Potential association of anti-CCR4 antibody mogamulizumab and graft-vs-host disease in patients with mycosis fungoides and sezary syndrome. JAMA Dermatol. 2018;154(6):728–730. doi:10.1001/jamadermatol.2018.0884
38. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi:10.1200/JCO.2011.38.0410
39. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi:10.1200/JCO.2011.38.0402
40. Connors JM, Jurczak W, Straus DJ, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med. 2018;378(4):331–344. doi:10.1056/NEJMoa1708984
41. Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–240. doi:10.1016/S0140-6736(18)32984-2
42. Liu HL, Hoppe RT, Kohler S, Harvell JD, Reddy S, Kim YH. Cd30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49(6):1049–1058. doi:10.1016/S0190-9622(03)02484-8
43. Duvic M, Reddy SA, Pinter-Brown L, et al. A phase II study of SGN-30 in cutaneous anaplastic large cell lymphoma and related lymphoproliferative disorders. Clin Cancer Res. 2009;15(19):6217–6224. doi:10.1158/1078-0432.CCR-09-0162
44. Duvic M, Tetzlaff MT, Gangar P, Clos AL, Sui D, Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015;33(32):3759–3765. doi:10.1200/JCO.2014.60.3787
45. Kim YH, Tavallaee M, Sundram U, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and sezary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015;33(32):3750–3758. doi:10.1200/JCO.2014.60.3969
46. Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555–566. doi:10.1016/S0140-6736(17)31266-7
47. Muniesa C, Gallardo F, Garcia-Doval I, et al. Brentuximab vedotin in the treatment of cutaneous T-cell lymphomas: data from the Spanish primary cutaneous lymphoma registry. J Eur Acad Dermatol Venereol. 2023;37(1):57–64. doi:10.1111/jdv.18563
48. Papadavid E, Kapniari E, Pappa V, et al. Multicentric EORTC retrospective study shows efficacy of brentuximab vedotin in patients who have mycosis fungoides and Sezary syndrome with variable CD30 positivity. Br J Dermatol. 2021;185(5):1035–1044. doi:10.1111/bjd.20588
49. Lewis DJ, Haun PL, Samimi SS, et al. Brentuximab vedotin for relapsed or refractory sezary syndrome. JAMA Dermatol. 2021;157(3):317–321. doi:10.1001/jamadermatol.2020.4901
50. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi:10.1056/NEJMoa1411087
51. Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291–3299. doi:10.1200/JCO.19.01389
52. Ansell SM. Where do programmed death-1 inhibitors fit in the management of malignant lymphoma? J Oncol Pract. 2016;12(2):101–106. doi:10.1200/JOP.2015.009191
53. Kline J, Godfrey J, Ansell SM. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood. 2020;135(8):523–533. doi:10.1182/blood.2019000847
54. Park J, Daniels J, Wartewig T, et al. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood. 2021;138(14):1225–1236. doi:10.1182/blood.2020009655
55. Neuwelt A, Al-Juhaishi T, Davila E, Haverkos B. Enhancing antitumor immunity through checkpoint blockade as a therapeutic strategy in T-cell lymphomas. Blood Adv. 2020;4(17):4256–4266. doi:10.1182/bloodadvances.2020001966
56. Rauch DA, Conlon KC, Janakiram M, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood. 2019;134(17):1406–1414. doi:10.1182/blood.2019002038
57. Cetinozman F, Jansen PM, Vermeer MH, Willemze R. Differential expression of programmed death-1 (PD-1) in Sezary syndrome and mycosis fungoides. Arch Dermatol. 2012;148(12):1379–1385. doi:10.1001/archdermatol.2012.2089
58. Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Arch Dermatol. 2010;146(12):1382–1388. doi:10.1001/archdermatol.2010.200
59. Wang L, Ni X, Covington KR, et al. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47(12):1426–1434. doi:10.1038/ng.3444
60. Saulite I, Ignatova D, Chang YT, et al. Blockade of programmed cell death protein 1 (PD-1) in Sezary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. Oncoimmunology. 2020;9(1):1738797. doi:10.1080/2162402X.2020.1738797
61. Gao Y, Hu S, Li R, et al. Hyperprogression of cutaneous T cell lymphoma after anti-PD-1 treatment. JCI Insight. 2023;8(4). doi:10.1172/jci.insight.164793
62. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase ib study. J Clin Oncol. 2016;34(23):2698–2704. doi:10.1200/JCO.2015.65.9789
63. Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and sezary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20–28. doi:10.1200/JCO.19.01056
64. Su T, Duran GE, Kwang AC, et al. Single-cell RNA-sequencing reveals predictive features of response to pembrolizumab in Sezary syndrome. Oncoimmunology. 2022;11(1):2115197. doi:10.1080/2162402X.2022.2115197
65. Bachy E, Savage KJ, Huang H, et al. Tislelizumab, a PD-1 inhibitor for relapsed/refractory mature T/NK-cell neoplasms: results from a phase 2 study. J Clin Oncol. 2022;40(16_suppl):7552. doi:10.1200/JCO.2022.40.16_suppl.7552
66. Querfeld C, Leung S, Myskowski PL, et al. Primary T cells from cutaneous T-cell lymphoma skin explants display an exhausted immune checkpoint profile. Cancer Immunol Res. 2018;6(8):900–909. doi:10.1158/2326-6066.CIR-17-0270
67. Beygi S, Fernandez-Pol S, Duran G, et al. Pembrolizumab in mycosis fungoides with PD-L1 structural variants. Blood Adv. 2021;5(3):771–774. doi:10.1182/bloodadvances.2020002371
68. Stadler R, Romero PO, Bagot M, et al. Phase II trial of atezolizumab (anti-PD-L1) in the treatment of stage IIb–IVB mycosis fungoides/Sézary syndrome patients relapsed/refractory after a previous systemic treatment (PARCT). Eur J Cancer. 2021;156:S22–S23. doi:10.1016/S0959-8049(21)00668-7
69. Vergnolle I, Douat-Beyries C, Boulinguez S, et al. CD158k and PD-1 expressions define heterogeneous subtypes of Sezary syndrome. Blood Adv. 2022;6(6):1813–1825. doi:10.1182/bloodadvances.2021005147
70. Moins-Teisserenc H, Daubord M, Clave E, et al. CD158k is a reliable marker for diagnosis of Sezary syndrome and reveals an unprecedented heterogeneity of circulating malignant cells. J Invest Dermatol. 2015;135(1):247–257. doi:10.1038/jid.2014.356
71. Van Der Weyden C, Bagot M, Neeson P, Darcy PK, Prince HM. IPH4102, a monoclonal antibody directed against the immune receptor molecule KIR3DL2, for the treatment of cutaneous T-cell lymphoma. Expert Opin Investig Drugs. 2018;27(8):691–697. doi:10.1080/13543784.2018.1498081
72. Marie-Cardine A, Viaud N, Thonnart N, et al. IPH4102, a humanized KIR3DL2 antibody with potent activity against cutaneous T-cell lymphoma. Cancer Res. 2014;74(21):6060–6070. doi:10.1158/0008-5472.CAN-14-1456
73. Bagot M, Porcu P, Marie-Cardine A, et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: an international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019;20(8):1160–1170. doi:10.1016/S1470-2045(19)30320-1
74. Bagot M, Kim YH, Ortiz-Romero PL, et al. Lacutamab in patients with advanced sezary syndrome: results from an interim analysis of the tellomak phase 2 trial. Blood. 2022;140(Supplement 1):3760–3761. doi:10.1182/blood-2022-160239
75. Kamijo H, Miyagaki T, Takahashi-Shishido N, et al. Thrombospondin-1 promotes tumor progression in cutaneous T-cell lymphoma via CD47. Leukemia. 2020;34(3):845–856. doi:10.1038/s41375-019-0622-6
76. Johnson LDS, Banerjee S, Kruglov O, et al. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019;3(7):1145–1153. doi:10.1182/bloodadvances.2018030577
77. Ansell SM, Maris MB, Lesokhin AM, et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2021;27(8):2190–2199. doi:10.1158/1078-0432.CCR-20-3706
78. Patel K, Orlowski RZ, Doucette K, et al. TTI-622-01: a phase 1a/1b dose-escalation and expansion trial of TTI-622 in patients with advanced hematologic malignancies, including multiple myeloma. J Clin Oncol. 2022;40(16_suppl):TPS8071–TPS8071. doi:10.1200/JCO.2022.40.16_suppl.TPS8071
79. Patel K, Zonder JA, Sano D, et al. CD47-blocker TTI-622 shows single-agent activity in patients with advanced relapsed or refractory lymphoma: update from the ongoing first-in-human dose escalation study. Blood. 2021;138(Supplement 1):3560. doi:10.1182/blood-2021-153683
80. Han Z, Wu X, Yuan Y-C, et al. Blockade of the immune checkpoint CD47 By TTI-621 potentiates the response to anti-PD-L1 in cutaneous T cell lymphoma. Blood. 2022;140(Supplement 1):6376–6377. doi:10.1182/blood-2022-167670
81. Querfeld C, Guitart J, Duvic M, Kim YH, Dusza SW, Kuzel TM. Results of an open-label multicenter phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sézary syndrome. Blood. 2014;123(8):1159–1166. doi:10.1182/blood-2013-09-525915
82. Querfeld C, Tsai N-C, Palmer J, et al. Phase 1 results of anti-PD-Ligand 1 (Durvalumab) & lenalidomide in patients with cutaneous T cell lymphoma and correlation with programmed death ligand 1 expression and gene expression profile. Blood. 2020;136(Supplement 1):20. doi:10.1182/blood-2020-143354
83. Amatore F, Ortonne N, Lopez M, et al. ICOS is widely expressed in cutaneous T-cell lymphoma, and its targeting promotes potent killing of malignant cells. Blood Adv. 2020;4(20):5203–5214. doi:10.1182/bloodadvances.2020002395
84. Anzengruber F, Ignatova D, Schlaepfer T, et al. Divergent LAG-3 versus BTLA, TIGIT, and FCRL3 expression in Sézary syndrome. Leuk Lymphoma. 2019;60(8):1899–1907. doi:10.1080/10428194.2018.1564827
85. Leupin N, Zinzani PL, Morschhauser F, et al. Cusatuzumab for treatment of CD70-positive relapsed or refractory cutaneous T-cell lymphoma. Cancer. 2022;128(5):1004–1014. doi:10.1002/cncr.34005
86. Isabelle C, McConnell K, Boles AE, et al. Therapeutic potential and role of CD38 in cutaneous T-cell lymphoma pathogenesis. Blood. 2022;140(Supplement 1):9216–9218. doi:10.1182/blood-2022-170550
87. Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109(11):4655–4662. doi:10.1182/blood-2006-12-062877
88. Mohty M, Gautier J, Malard F, et al. CD19 chimeric antigen receptor-T cells in B-cell leukemia and lymphoma: current status and perspectives. Leukemia. 2019;33(12):2767–2778. doi:10.1038/s41375-019-0615-5
89. To V, Evtimov VJ, Jenkin G, Pupovac A, Trounson AO, Boyd RL. CAR-T cell development for cutaneous T cell lymphoma: current limitations and potential treatment strategies. Front Immunol. 2022;13:968395. doi:10.3389/fimmu.2022.968395
90. Berrien-Elliott MM, Jacobs MT, Fehniger TA. Allogeneic natural killer cell therapy. Blood. 2023;141(8):856–868. doi:10.1182/blood.2022016200
91. Qasim W. Genome-edited allogeneic donor “universal” chimeric antigen receptor T cells. Blood. 2023;141(8):835–845. doi:10.1182/blood.2022016204
92. Zhang Y, Li C, Jiang H, et al. Allogeneic and autologous anti-CD7 CAR-T cell therapies in relapsed or refractory T cell malignancies. Blood. 2022;140(Supplement 1):4592–4594. doi:10.1182/blood-2022-170819
93. Iyer SP, Sica RA, Ho PJ, et al. S262: the Cobalt-Lym study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered car T cells in patients with relapsed/refractory (r/r) T-cell malignancies. HemaSphere. 2022;6:163–164. doi:10.1097/01.HS9.0000843940.96598.e2
94. Frigault MJ, Chen Y-B, Gallagher KME, et al. Phase 1 Study of CD37-Directed CAR T cells in patients with relapsed or refractory CD37+ Hematologic malignancies. Blood. 2021;138(Supplement 1):653. doi:10.1182/blood-2021-146236
95. Watanabe K, Aznar MA, Kuramitsu S, et al. Identifying highly active anti-CCR4-CAR T cells for the treatment of T-cell lymphoma. Blood Adv. 2023;7:3416–3430. doi:10.1182/bloodadvances.2022008327
96. Atilla PA, Atilla E. Are we there yet? Cellular therapies for cutaneous T cell lymphoma. Curr Res Transl Med. 2023;71(2):103390. doi:10.1016/j.retram.2023.103390
97. Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–1194. doi:10.1182/blood-2017-10-811224
98. Hong DS, Rixe O, Chiu VK, et al. Mogamulizumab in Combination with Nivolumab in a Phase I/II study of patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2022;28(3):479–488. doi:10.1158/1078-0432.CCR-21-2781
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
