Back to Journals » International Journal of Nanomedicine » Volume 18
Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration
Authors Qin C, Qi Z, Pan S , Xia P, Kong W, Sun B, Du H , Zhang R , Zhu L, Zhou D, Yang X
Received 21 August 2023
Accepted for publication 8 November 2023
Published 6 December 2023 Volume 2023:18 Pages 7305—7333
DOI https://doi.org/10.2147/IJN.S436111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Cheng Qin, Zhiping Qi, Su Pan, Peng Xia, Weijian Kong, Bin Sun, Haorui Du, Renfeng Zhang, Longchuan Zhu, Dinghai Zhou, Xiaoyu Yang
Department of Orthopedic Surgery, the Second Hospital of Jilin University, Changchun, 130041, People’s Republic of China
Correspondence: Xiaoyu Yang, Department of Orthopedic Surgery, the Second Hospital of Jilin University, Changchun, TX, 130041, People’s Republic of China, Email [email protected]
Abstract: Spinal cord injury (SCI) treatment represents a major challenge in clinical practice. In recent years, the rapid development of neural tissue engineering technology has provided a new therapeutic approach for spinal cord injury repair. Implanting functionalized electroconductive hydrogels (ECH) in the injury area has been shown to promote axonal regeneration and facilitate the generation of neuronal circuits by reshaping the microenvironment of SCI. ECH not only facilitate intercellular electrical signaling but, when combined with electrical stimulation, enable the transmission of electrical signals to electroactive tissue and activate bioelectric signaling pathways, thereby promoting neural tissue repair. Therefore, the implantation of ECH into damaged tissues can effectively restore physiological functions related to electrical conduction. This article focuses on the dynamic pathophysiological changes in the SCI microenvironment and discusses the mechanisms of electrical stimulation/signal in the process of SCI repair. By examining electrical activity during nerve repair, we provide insights into the mechanisms behind electrical stimulation and signaling during SCI repair. We classify conductive biomaterials, and offer an overview of the current applications and research progress of conductive hydrogels in spinal cord repair and regeneration, aiming to provide a reference for future explorations and developments in spinal cord regeneration strategies.
Keywords: neural tissue engineering, electrical stimulation, electrical signal, conductive biomaterials, spinal cord injury microenvironment
Graphical Abstract:
Introduction
Spinal cord injury (SCI) is a debilitating trauma to the central nervous system (CNS) that results in permanent motor, sensory, and autonomic dysfunctions below the injury site, presenting a significant medical challenge.1 Globally, it is estimated that there are 250,000 to 500,000 new cases of SCI annually.2 The clinical management of SCI primarily involves surgical stabilization of the vertebrae and early spinal cord decompression, pharmacological intervention, and rehabilitative treatment.3 Despite considerable advancements in therapeutic techniques, a cure for SCI remains elusive.4 Treatment during the early stages primarily aims to stabilize the spinal cord and restore homeostasis immediately following the injury, while long-term management addresses symptoms caused by maladaptive plasticity and other secondary complications.5 Spinal cord injury can be classified into traumatic and non-traumatic causes. Traumatic spinal cord injuries occur when acute spinal cord injury is caused by an external physical shock, such as a motor vehicle collision, fall, sports-related injury, or direct trauma. In contrast, nontraumatic spinal cord injuries typically occur after an acute or chronic disease process, such as a tumor, an infection, or degenerative disc injury. In traumatic spinal cord injuries, the primary injury damages cells and triggers a complex cascade of secondary injuries involving neuronal and oligodendrocyte death, and leads to the deterioration of the environment surrounding the lesion site, resulting in progressive pathological changes like inflammation, oxidative damage, axonal demyelination, apoptosis, and the formation of irregular cystic cavities and glial fibrosis.6 It is now understood that glial fibrosis and fluid-filled cysts,7 along with endogenous remyelination and poor axonal regeneration, impede the electrical signaling and stimulus conduction in spinal cord tissue, thereby hindering neural regeneration.8 Therefore, the key to treating spinal cord injuries lies in reconnecting the disrupted neural pathways, stimulating neuronal differentiation and axonal growth, suppressing inflammation, and providing a regenerative environment and support for damaged neurons.9 However, current treatment approaches typically target only one aspect of these complex multifactorial causes and have limited efficacy.10 In recent years, biocompatible materials and tissue engineering approaches have emerged as promising new strategies for treating spinal cord injuries.11
Over the past few decades, hydrogels have been widely used as scaffolds in neural tissue engineering due to their exceptional biocompatibility, porosity, and mechanical flexibility.12–14 Scaffold designs based on hydrogels have been shown to possess similar biochemical and biophysical properties to the natural extracellular matrix of the brain/spinal cord, allowing effective filling of irregular lesions and mimicking the physiological environment of living tissues, thereby promoting the survival and differentiation of neurons.8,15 Moreover, they can enhance the microenvironment of the injury site and improve cell survival rates and axonal growth by incorporating cells, various neurotrophic factors, or drugs to release bioactive molecules.5
Although hydrogels have many desirable properties, they are typically electric insulators, impeding the transmission of intercellular electrical signals. Electrical signals are the foundation of neural system function, as the spinal cord communicates with other types of cells through neural networks and axonal bundles, conducting bioelectric signals.16 Neural networks not only participate in the differentiation and regeneration of neural stem cells,17 but also prevent scar tissue formation at injury sites and facilitate the transmission of biological signals to achieve specific biological functions.18,19 It has been established that the stimulation and transmission of electrical signals are crucial for neuronal function. Therefore, during the design and preparation of neural tissue regeneration scaffolds, it is imperative to mimic the native extracellular matrix (ECM) of the spinal cord by integrating conductive matrices into the cellular microenvironment to provide an artificial environment that promotes regeneration at injury sites.20 In neural repair applications, the advantage of conductive hydrogels lies in their ability to simultaneously provide physical and electrical characteristics, with the former being a unique property of hydrogels and the latter achieved through conductive materials.
This review article summarizes recent advances in conductive hydrogels for spinal cord injury repair, providing an overview of the characteristics of these hydrogels (Table 1) and their clinical applications, challenges, and prospects. Furthermore, we expound on the mechanisms underlying electrical stimulation and signaling in spinal cord injury repair.
 |
Table 1 Conductive Hydrogels Applied in Spinal Cord Injuries in Recent Years |
SCI Microenvironment
This section comprehensively explores the microenvironment following spinal cord injury. Spinal cord injury can be divided into primary and secondary injuries.33 Temporally, they can be categorized into acute (<48 hours), subacute (48 hours to 14 days), intermediate (14 days to 6 months), and chronic (>6 months) phases 9 (Figure 1). Primary injuries are often irreversible, including compression, stretching, tearing, transection, and bleeding,34 damaging neurons and oligodendrocytes (a type of glial cell in the central nervous system), disrupting the vascular system, and impairing the blood-spinal cord barrier, thus immediately initiating a cascade of secondary injury reactions. During the acute phase (within 0–48 hours after injury), damage to the microvessels supplying the spinal cord leads to increased cell permeability, infiltration of inflammatory cells, release of cytotoxic molecules, propagation of pro-apoptotic signaling, and ischemic damage.35 This secondary injury results in the necrosis and/or apoptosis of neurons and glial cells (such as oligodendrocytes), leading to demyelination and loss of neural circuits. During the subacute phase (2–4 days after injury), sustained edema, the formation of blood clots, and vasoconstriction further contribute to ischemia, causing glutamate release,36 lipid peroxidation,37 activation of calcium influx and calpain protease,38 edema, and excitotoxic cell death.39 The sustained infiltration of inflammatory cells contributes to further cell death,40,41 and the formation of cystic cavities due to the disruption of extracellular structures of cells and the spinal cord.42 In addition, astrocyte proliferation occurs and extracellular matrix molecules such as chondroitin sulfate proteoglycans (CSPGs), fibronectin, laminin, and collagen are deposited by astrocytes in the surrounding lesion area, limiting axonal growth.43,44 It has been shown that certain subtypes of astrocytes yield a neuroprotective effect during the injury process.45 During the intermediate and chronic phases (2 weeks to 6 months), axonal degeneration continues, and the astroglial scar matures, impeding axonal regeneration and extension.45,46 The fusion of cystic cavities further restricts axonal regeneration and cell migration.
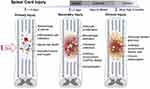 |
Figure 1 Pathophysiologic mechanisms of spinal cord injury. Notes: Reprinted from Acta Biomaterialia, 139, Kiyotake EA, Martin MD, Detamore MS. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. 43–64. Copyright 2020, with permission from Elsevier.47 |
In addition, inhibitory factors, including Nogo, myelin-associated glycoprotein (Mag), oligodendrocyte myelin glycoprotein (Omgp), Nogo receptor protein, and p75 neurotrophic receptor binding activation of RHOA and Rho-associated protein kinase (ROCK),48 strongly suppress axonal and myelin sheath regeneration which can result in the collapse of growth cones after SCI.49–53 The poor axonal growth capability and inhibitory factors hindering axonal regeneration lead to the failure of spinal cord regeneration and neural circuit reconstruction.54–56
The Mechanism of Electrical Stimulation/Electric Signal in the Repairing Process of Spinal Cord Injury
Spinal cord injury is a severe traumatic condition affecting the central nervous system, and it is characterized by the limited regenerative potential of neurons following damage. One significant obstacle in this process is the disruption of electrical signaling.57 In neural tissues, ion channels on the cell membrane of neural cells activate a cellular gap, leading to ion transport across the membrane, resulting in membrane polarization and the generation of an endogenous electric field. Bioelectric signals, stemming from changes in cell membrane potential, represent a primary mode of cellular signaling. These bioelectric signals play a vital role in processes such as neural cell proliferation, differentiation, migration, spreading, and apoptosis via various physiological mechanisms, including cytoskeletal rearrangement, cell membrane depolarization, conformational and positional changes of membrane proteins, and the regulation of transmembrane calcium influx.58 By facilitating signal transduction between cells and the extracellular matrix, the transmission and delivery of electrical signals regulate the morphology, quantity, location, migration, and differentiation of neural stem cells (NSCs), ultimately promoting the maturation of neuronal electrophysiological characteristics.59 Therefore, the transmission and delivery of electrical signals are essential for neuronal survival, regenerative differentiation, and the activation of neural circuits.
Neurons inherently possess the ability to transmit electrochemical signals throughout the nervous system and are significantly influenced by external electrical stimulation.60,61 Electrical stimulation has been shown to promote axon extension in vitro and nerve regeneration in vivo.62 Studies have shown that electrical stimulation accelerates axonal regeneration and promotes functional recovery when an electric field is established between nerve defects.63 Although the mechanisms by which electrical stimulation promotes neuronal growth are not fully understood, it is widely thought that multiple mechanisms exist64(Figure 2):
- Calcium ion influx and calcium signaling. ES can increase intracellular calcium levels by activating calcium channels (VGCC) to promote more calcium ions into cells or activating GPCRs to release Ca2+ from storage in the endoplasmic reticulum.65,66 Calcium signaling can negatively regulate the PTEN protein, a major intrinsic barrier to axon regeneration.67,68 After inhibiting PTEN expression, it promotes axon regeneration in corticospinal neurons by upregulating mTOR activity.69,70 As a second messenger, intracellular calcium ions are the key factors affecting functional activities such as migration, proliferation, and differentiation of neural stem cells.71,72 In addition, calcium influx induces phosphorylation of the transcription factor cAMP response element binding protein (CREB), which can regulate the initiation of transcriptional programs and promote the expression of neuronal genes (i.e.NeuroD1 and Neurogenin1), thus exerting important effects on neurogenesis.73
- Activation of intracellular signaling pathways, such as FAK and p38 ion channels and ERK, MAPK, PI3K/AKT, and ROCK, and reactive oxygen species (ROS) pathways.74–76 Phosphorylation of FAK (a junction that bridges integrins and the cytoskeleton) by electrical stimulation further stimulates actin remodeling and downstream mechanical signaling, such as the MAPK and ERK pathways closely related to neural cell proliferation and differentiation. The subsequent activation of JNK, which is required for neurogenesis, affects neuronal differentiation.77,78
- Gap junctions: Gap junctions enable neighboring cells to exchange small molecules, and create electrical and metabolic couplings between these cells.79,80 Electrical stimulation can alter cell gap junctions, affecting the exchange of signaling molecules such as calcium, potassium, cyclic nucleotides, and inositol phosphates, and promote the development and communication of electroactive cells.81,82
- Extracellular matrix composition: Electrical stimulation can alter ECM composition by affecting proteins, soluble ions, or charged groups, which cause electrophoretic redistribution of cell surface receptors and altered adhesion of adhesion proteins, thereby affecting neurite outgrowth.83,84
- Electric fields are introduced at the injury site to promote axonal growth and plasticity.85–87 The use of spinal cord electrical stimulation (ES) to activate or increase the excitability of neuronal networks below the injury directly promotes axon regeneration in the environment by mimicking the action potential of the central nervous system, maintaining the ability of the spinal cord to respond to peripheral stimuli,88–90 which increases the local synthesis of major proteins in myelin, thereby stimulating myelin induction.91–94 At the same time, electrical stimulation enhances the expression of neurotrophic factors, such as GDNF, ENO2, and BDNF.95,96
Research Progress of Conductive Hydrogel
Hydrogels are three-dimensional structures consisting of a porous polymer network. These materials mimic the extracellular matrix environment, allowing the exchange of nutrients with surrounding tissues, cell migration, and the diffusion of molecules. Hydrogels are characterized by their high water content and controlled release capabilities, making them ideal for implantation.97 They offer suitable pore sizes, porosity, biocompatibility, low toxicity, adjustable biodegradability, and mechanical properties. Injectable hydrogels, which can be filled into spinal cord injury sites through in-situ gelation, are advantageous for avoiding additional surgical damage.98,99 Therefore, hydrogels are considered the most suitable scaffold for spinal cord regeneration and neural tissue repair.100
Although hydrogels can generate ionic currents through ions dissolved in water, they typically act as electronic insulators, meaning they do not conduct electrons. Under physiological conditions, the electrical properties of hydrogels are generally similar to those of the surrounding tissue medium, and they are far inferior to conventional electronic conductors.101 One viable approach to enhance the electrical properties of hydrogels is to introduce electronic conductivity alongside their ionic conductivity achieved by incorporating conductive nanomaterials into the polymeric matrix of hydrogels. Consequently, resulting hydrogel composite materials possess both ionic and electronic conductivity while preserving the unique biomechanical advantages of hydrogels. Charge carriers migrate within conductive hydrogels through electronic/hole transport in the polymer network and ionic flow in the porous electrolyte. This movement of electronic/hole carriers induces changes in the polarization of the polymer network, which, in turn, triggers the orientation, migration, and rearrangement of ions in the pore electrolyte of hydrogels. This leads to alterations in the extracellular potential of cell membranes, activating ion channels and initiating the transmembrane flow of ions. Additionally, this process alters the polarization state of neuron cell membranes, initiating a cascade of intracellular signaling pathways.102 Additionally, hydrogel matrices play a regulatory role in the fate and behavior of neural cells within them, providing favorable conditions for neuroregeneration in treating spinal cord injuries.
To facilitate the repair and regeneration of SCI, researchers have extensively explored other properties of conductive hydrogels besides electrical conductivity. In this respect, Liu et al developed conductive hydrogels with a specific elastic modulus and mechanical strength, enabling them to prevent damage to surrounding residual tissues and maintain the local structure. Moreover, an appropriate elastic modulus (approximately 0.11–1 kPa) can enhance neural stem cell differentiation into neurons and reduce neurogliogenesis.99 Adequate porosity, permeability, and surface morphology have been found to facilitate material exchange and cell loading, ensuring good swelling properties and the overall stability of the hydrogel.103 Luo et al grafted dopamine (DA) molecules onto hyaluronic acid (HA) biomolecules to form HA-DA hydrogels, which resulted in HA-based hydrogels with good adhesion properties and stable adhesion to surrounding tissues.30 Zhang et al designed and prepared conductive hydrogels with good biocompatibility and degradation rates that align with axonal and tissue regeneration. These hydrogels could efficiently degrade in vivo as neural tissues regenerate, preventing long-term presence in the spinal cord tissues, which could impede neural tissue regeneration or cause chronic inflammation or toxicity.104 Zhao et al prepared PPM hydrogels with significant shear-thinning properties. By adjusting the angular frequency, these hydrogels could be easily injected with a syringe and spontaneously penetrated and filled irregularly shaped areas, owing to their shape memory properties. The reversible non-covalent crosslinking in PPM hydrogels provided remarkable self-healing properties, ensuring structural integrity and prolonging their lifespan.105 These properties facilitated the rapid injection of hydrogels into the target site, offering the possibility of minimally invasive treatment. Additionally, Chen et al developed a hydrogel based on polyvinyl alcohol (PVA), molybdenum sulfide, and graphene oxide, which exhibited excellent anti-oxidative properties and effectively attenuated ROS, inhibiting the destructive inflammatory response.106
The selection of appropriate manufacturing methods and materials plays a critical role in controlling and manipulating the characteristics of hydrogels. Depending on their origin, the most commonly used biopolymer-based hydrogels for SCI scaffolds in current research include natural polymers, synthetic polymers and self-assembled peptides. Natural polymers can be divided into two main categories: protein-based (collagen, gelatin, and matrix gel) and polysaccharide-based (chitosan, alginate, agarose, hyaluronic acid, and methylcellulose).107 Synthetic polymers are categorized into artificial materials such as methacrylic anhydride and polyethylene glycol.108 The synthesis of hydrogels can be achieved through physical and chemical crosslinking methods. Physical crosslinking methods encompass techniques like ion-pairing, complex coacervation, hydrogen bonding, phase transition (either thermal-responsive, involving heating or cooling a polymer solution or crystallization), and hydrophobic interactions. Chemical crosslinking methods involve processes like Michael addition reactions, Schiff base reactions, click chemistry, condensation reactions, crosslinking with aldehydes, thiol-disulfide exchange, radiation crosslinking, free-radical polymerization, photo-induced crosslinking, and enzyme-mediated crosslinking.109 In terms of design approaches, various hydrogel scaffold designs have been applied and studied in SCI tissue engineering, such as electrospun scaffolds,110 3D bioprinted scaffolds,111 patterned scaffolds, in-situ injectable scaffolds,98 microfluidic fabrication,112 and directed microstructures.113 For injectable conductive hydrogels, the rate of gelation of the material is critical . It is now understood that gelation should neither be too fast, allowing adequate time for injection and filling of the defect site, nor too slow, preventing the hydrogel from flowing out of the lesion. The gelation rate is typically adjusted through chemical or physical cross-linking methods, depending on the type of polymer, its relative molecular mass, concentration, and the type of cross-linking, such as ultraviolet light, pH, and temperature changes.
Currently, conductive hydrogels often employ electrical stimulation to promote neuroplasticity by providing a matrix that guides axonal growth in specific directions, aligning neural cells with fiber axons, and effectively promoting neurogenesis.114,115 Moreover, they can regenerate damaged tissues by delivering cells, drugs, and/or bioactive molecules, thus bridging these two distinct features.116 In the following sections, we will discuss recently used conductive biomaterials for constructing conductive hydrogels and their advantages and disadvantages in SCI treatment.
Conductive Hydrogel for Spinal Cord Injury Repair
Over the years, the application of conductive hydrogels for spinal cord injury treatment has gained significant momentum. The conductive nanomaterials used to construct these hydrogels include conductive polymers, carbon-based nanomaterials, phosphorus-based nanomaterials, MXene, and various combinations of these materials. Conductive polymers, such as polypyrrole (ppy), polyaniline, and poly 3,4-ethyl-dioxothiophene, are the most commonly used conductive polymers in the field of biomedical research. They offer good electrical properties and biocompatibility.117,118 Carbon-based materials, including graphene, reduced graphene oxide (rGO), and carbon nanotubes, are also utilized in the development of conductive biomaterials due to their high electrical conductivity (ranging from 103 to 104 S/cm) and large surface area, which allows for functionalizing biomolecules and modulating cellular responses.25 GeP, as an emerging material in the phosphorus-based family, is known for its excellent biocompatibility, degradability, and electrical conductivity, making it a multifunctional nanomaterial suitable for various applications.119,120
MXene is, a class of two-dimensional transition metal carbide and nitride materials, combines the electrical conductivity of a transition metal with the physicochemical characteristics of carbon- nitride. Its surface is rich in functional groups, making MXene hydrophilic and highly modifiable for various bioengineering applications. The functionalization of MXene materials has become a research hotspot in recent years.121,122
Table 2 provides a comprehensive list of the advantages and disadvantages associated with each of these materials.
 |
Table 2 Conductive Biomaterials for Use as Conductive Hydrogels |
Conductive Hydrogels Based on Conductive Polymers
Conductive polymers (CPs) are a class of organic materials that exhibit unique electrical and optical properties similar to inorganic semiconductors and metals. Typically, conductive polymers have conjugated chains with alternating single and double bonds. Due to the highly delocalized and polarizable nature of the π electrons, they can form pathways for charge carriers, allowing electrons to move along the polymer chains.
In recent years, conductive polymers have attracted significant interest as biomaterials in tissue engineering applications because of their physical and chemical properties, ability to transmit electrical signals to cells, and capacity to offer a conducive platform for specific cell responses like cell adhesion, growth, and proliferation. Among the conductive polymers frequently used in spinal cord injury repair are polypyrrole (PPy), polyaniline (PANi), polythiophene(PT), and its derivative, poly(3,4-ethylenedioxythiophene) (PEDOT). These conductive polymers have been widely employed in the development of conductive hydrogel. While these conductive polymers have relatively low intrinsic conductivity, their conductivity can be substantially enhanced through processes such as oxidation (p-doping) or reduction (n-doping), with dopant ions serving to maintain electrical neutrality. This enhancement enables them to reach the conductivity, range of semiconductors and metals.
PPY
PPy is one of the most commonly used conductive polymers in the treatment of spinal cord injuries. Doped PPy exhibits high conductivity, excellent environmental stability, ease of preparation, and easy modification. Its electrical conductivity can reach up to approximately 103 S cm−1, and PPy can be easily synthesized in large quantities using various common organic solvents and water at room temperature. Furthermore, its good biocompatibility can significantly promote the adhesion, proliferation, and differentiation of different types of cells in vitro.123 At the same time, it can be integrated into materials with large surface areas and different porosity, and can be easily modified by incorporating bioactive molecules, making it suitable for biomedical applications.
Despite the above-mentioned advantages of PPy, it still has some limitations. Previous studies have shown that PPy can be challenging to further process due to its crystalline, mechanically rigid, brittle, and insoluble, nature, which makes it unsuitable for soft natural spinal cord tissue. It can readily trigger stress and induce inflammatory reactions, significantly limiting its application in the field of central nervous system regeneration. To address these issues, researchers have found that PPy’s limitations can be mitigated by carefully selecting dopants and optimizing synthesis methods and conditions. A common strategy involves blending PPy with other degradable synthetic or natural polymers, such as PLA, PLGA, PCL, chitosan, and silk fibroin, to create conductive hydrogels. For instance, Xu et al synthesized a shape-recoverable, self-healing conductive hydrogel by utilizing Schiff base reactions, incorporating N-carboxyethyl chitosan (CEC), chitosan-modified polypyrrole (DCP) nanoparticles, and a unique aldehyde-terminated difunctional polyurethane (DFPU) crosslinker. This hydrogel was found to promote the attachment, proliferation, and differentiation of neural stem cells, resulting in remarkable recovery of motor function in brain-injured zebrafish.124 Huang et al developed a conductive hydrogel by blending collagen with PPy, achieving the most suitable Young’s modulus and electrical conductivity similar to spinal cord tissue. Combined with electrical stimulation, this hydrogel led to increased cell proliferation and differentiation into neurons, inhibiting differentiation into astrocytes.125 It has been established that the electrical signal transmission level in the natural spinal cord is 4.2×10−4 S cm−1, and the elastic modulus is 202.2 Pa. Dai et al harnessed PPy’s conductivity, gelatin’s tissue affinity, and tannic acid’s (TA) supramolecular interaction to create a gelatin-based conductive hydrogel (GCH) with mechanical properties and electrical conductivity comparable to the natural spinal cord by controlling the concentrations of gelatin and PPy (Figure 3). By utilizing glutathione (GSH) conjugation, this hydrogel allowed the on-demand release of bFGF in response to the SCI microenvironment. In vivo results showed significant attenuation of matrix metalloproteinases (MMPs) expression, creating a conductive microenvironment that promoted axon regeneration at the injury site, increasing vessel density.126
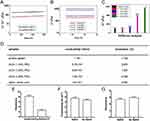 |
Figure 3 Optimizing GCHs with spinal cord-like mechanical property and conductivity. (A) Time sweep of the native spinal cord (frequency = 1 Hz, temperature = 37°C). (B) Time-dependent G’ of hydrogels with different gelatin concentrations. (C) G’ comparison of the natural spinal cord and different hydrogels. (D) Electrical conductivity and impedance of pure gelatin, three GCHs, and the native spinal cord. (E) Resistance of conductive and non-conductive hydrogels. (F) Conductivity of GCHs with or without 50 mM NaIO4. (G) Resistance of GCHs with or without 50 mM NaIO4.. Notes: Reprinted from Biomaterials, 288, Fan CX, Yang W, Zhang LL, et al Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Copyright 2022, with permission from Elsevier.126 |
One major challenge in spinal cord injury repair is the secondary injury cascade reactions, including elevated ROS levels, lipid peroxidation, and other oxidative pathological microenvironments.40 Wu et al developed a conductive hydrogel composed of PPy and methacrylic anhydride (MA)-modified hyaluronic acid and collagen. The incorporated PPy within the matrix demonstrated antioxidant properties, helping to regulate the oxidative pathological microenvironment, protect exogenous cells from oxidative damage, and maintain electrical signals. The hydrogel also facilitated the delivery of exogenous stem cells to the lesion site, restoring the transmission of endogenous electrical signals.127
Importantly, the spinal cord transmits information in the form of electrical impulses along highly conductive nerve fibers. Endogenous bioelectrical signals are essential for maintaining neuronal function and axonal growth. Therefore, the design and fabrication of scaffolds for spinal cord regeneration should mimic the highly conductive properties of the spinal cord. Zhou et al used tannic acid (TA) as a dopant and crosslinking agent to prepare a conductive hydrogel with polypyrrole as the backbone. Compared with the traditional conductive hydrogel, this hydrogel synthesized a polymer without insulation due to PPy’s natural crosslinking with tannic acid through intermolecular interactions, resulting in good conductivity. Implanted highly conductive hydrogel bridges in a severe mouse spinal cord injury model promoted neuronal differentiation, inhibited astrogliogenesis and promoted endogenous neurogenesis, leading to functional recovery by restoring disrupted spinal cord circuits.128
The pathological microenvironment after spinal cord injury can impair the electrical signal transmission of living nerve cells in the lesion, disrupt the electrical connection of the injured spinal cord, and ultimately destroy the neural circuit. To address this issue, Yang et al developed an agarose/gelatin/poly pyrrole (Aga/Gel/PPy, AGP3) hydrogel with similar electrical conductivity and modulus to those of the spinal cord. The physical crosslinking method employed makes it suitable for injection. In vivo studies have shown that AGP3 hydrogels could provide a suitable microenvironment for the migration and differentiation of neural stem cells, which could significantly promote endogenous neurogenesis and reduce the formation of glial fibrosis. SCI mice exhibited significant functional recovery based on functional assessments of Basso-Beattie-Bresnahan (BBB) scores, footprint tests, and inclined plane test (IPT) scores. Further analysis using RNA-sequencing revealed that the AGP3 hydrogel significantly notably regulated the expression of genes related to neurogenesis through an intracellular Ca2+ signaling cascade. Moreover, the incorporation of electroactive materials amplifies the weak local electric field generated by the cell membrane, thus creating a transmembrane voltage gradient that influences the inflow of ions across the cell membrane. Consequently, intracellular signaling significantly impacts the proliferation and differentiation of neural stem cells (Figure 4).21
 |
Figure 4 Investigations of RNA-seq and assessments of intracellular calcium involved in neurogenesis. (A) Heat map of neurogenesis-related gene expression. (B) The gene enrichment KEGG pathway analysis. (C and D) Immunofluorescence images of L-VGCC, p-CREB and BDNF in each group on day 7. (E) WB analysis of L-VGCC, p-CREB and BDNF. (F) Quantitative analysis of L-VGCC, p-CREB and BDNF. (G) Pseudo-color pictures of representative calcium images before and after glutamate stimulation. (H) Image-derived fluo-4 intensity measurements over time in NSCs. Notes: Reprinted from Acta Biomaterialia, 15, Yang B, Liang C, Chen D, et al A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. 103–119. Copyright 2022, with permission from Elsevier.21 |
PANI
Polyaniline is another commonly used conducting polymer, which can be synthesized chemically or electrochemically from the monomeric aniline. Similar to PPy, PANI boasts high environmental stability, cost-effectiveness in synthesis, and the ability to transition between conductive and resistive states, as well as various structural forms. However, the drawbacks of polyaniline and its derivatives in the field of biological tissue engineering primarily pertain to their limited cytocompatibility, processability, flexibility, and non-biodegradability, which can result in chronic inflammation and pain.129 Furthermore, as hydrogels swell under physiological conditions, ionic or physically loaded PANI may leach out, resulting in decreased conductivity and toxicity. Liu et al addressed these issues by creating an electroactive hydrogel based on poly amino acid and electroresponsive polyaniline. It has been established that polyamino acid possesses excellent characteristics such as biocompatibility, safety after degradation, non-toxicity, and ease of modification, which mitigates the challenges associated with using PANI in neural tissue engineering. Implanting this hydrogel into a rat model of spinal cord injury and applying electrical stimulation, led to the inhibition of astrocyte proliferation, prompting damaged neural stem cells in the spinal cord to differentiate into neurons and stimulating endogenous neurogenesis. Evaluations through footprint tests and the BBB score demonstrated that the electroactive hydrogel, when combined with electrical stimulation, significantly enhanced the recovery of motor function in spinal cord injury rats. Histological and ultrastructural observations confirmed that the damaged area predominantly comprised newly generated neuronal cells, regenerated myelin, and axons.130
PEDOT
A third common conjugated polymer is poly(3,4-ethylenedioxythiophene) (PEDOT), a polythiophene (PTh) derivative. Compared to PPy, PEDOT offers superior electrical conductivity and chemical stability. However, its low solubility makes it challenging to uniformly disperse in water. Typically, polystyrene sulfonate (PSS) is used as a dopant and templating agent to enhance the water solubility and stability of PEDOT. Nonetheless, the disadvantages of PSS, such as its insulating properties, hydrophilicity, and strong acidity, limit its applicability in tissue engineering. To overcome these limitations, researchers synthesized PEDOT nanoparticles (NPs) through chemical oxidative polymerization within microemulsions. These NPs can be uniformly integrated into hydrogel-based scaffolds, substantially increasing the electrical conductivity of the scaffolds to 8.3×10−4 ± 8.1×10−5 S/cm without biocompatibility issues.131 Gao et al introduced sulfonated lignin (LS) as a dopant, which not only improved the dispersion and conductivity of PEDOT but also demonstrated the excellent biocompatibility of the synthesized PEDOT: LS. The conductive hydrogel prepared by 3D bioprinting simulated the composition of spinal cord ECM with gelatin and hyaluronic acid, promoted the growth and adhesion of nerve cells, and its conductivity was comparable to that of spinal cord white matter (0.60 S m−1). At the same time, it simulated the structure of the parallel arrangement of the white matter of the spinal cord, facilitating the orderly parallel growth of new neurofilaments along the bioprinted scaffold, thus enabling efficient spinal cord injury repair.104 Moreover, Song et al advanced the bioinks for 3D bioprinted conductive hydrogel scaffolds. By integrating PECT (PEDOT: CSMA, TA) with a photocrosslinked gelatin/polyethylene glycol physical gel (GP) matrix, followed by a cooling process, they achieved optimal shear-thinning behavior and suitable rheological properties. This design bolstered cell-cell and cell-matrix interactions within a conductive microenvironment, enhancing the therapeutic effect of neural stem cells and promoting nerve regeneration in spinal cord injury repair.132
Hydrogels formed with conductive polymers have attracted significant interest in recent years. Through the modification and amalgamation of various substances, they offer an appropriate microenvironment conducive to the healing and rejuvenation of spinal cord injuries. Importantly, as biomaterials degrade over time, ensuring enduring electrical stability poses a crucial challenge that demands further exploration in the future.
Conductive Hydrogels Based on Carbon-Based Materials
Numerous studies have demonstrated the excellent physicochemical and mechanical properties, as well as substantial electrical conductivity (ranging from 103 to 104 S/cm), of carbon-based nanomaterials. These properties enable these materials to facilitate axon regeneration and encourage stem cell differentiation via neural electrical signals within spinal cord tissue. Their large surface area is advantageous for functionalizing biomolecules and modulating cellular responses. However, the presence of peripheral hydrophobic groups limits their interaction with hydrophilic polymers. To address these limitations, researchers have either modified the surface of nanomaterials with various polar functional groups, such as -OH, -NH2, and carboxyl (-COOH), or enhanced their dispersion by grafting different polymer chains. Conjugated and modified carbon-based nanomaterials, including graphene and its derivatives and carbon nanotubes (CNTs), have shown significant potential in the context of nerve regeneration.
Graphene
Graphene is a two-dimensional material composed of a single layer of carbon atoms arranged in a hexagonal honeycomb lattice. Its distinct structure and geometry account for its exceptional physicochemical properties, including high fracture strength, a high Young’s modulus, excellent thermal and electrical conductivity, a large specific surface area, and biocompatibility.133 While graphene exhibits biocompatibility as an immobilizing surface for neuronal growth and can induce human neural stem cells (hNSCs) to differentiate more towards neurons while preventing their differentiation into glial cells,134–136 its smoothness, chemical inertness, and lack of biological cues hinder its use as a conductive material for synthesizing conductive hydrogels. To address these limitations, researchers have modified graphene and its derivatives to form nanocomposites. For example, Agarwal et al developed collagen-based cryogels utilizing amino-functionalized graphene nanocrosslinkers. The addition of amino-functionalized graphene improved the order and crosslinking degree of collagen molecules in the cryogel, resulting in sufficient porosity and interconnectivity that facilitated cell penetration and unimpeded nutrient transfer, necessary for accelerated axon regeneration.137 The implantation of 0.5% w/v graphene collagen cryogel into the injured spinal cord promoted cell proliferation from proximal to distal, enhanced the secretion of immune regulators, established neural connections, and facilitated spinal cord regeneration and repair of injuries.103
Graphene oxide (GO), the main derivative of graphene,138 contains carboxyl groups (-OOH) on the edges of its structure, with epoxy groups (-O) and hydroxyl groups (-OH) on the basal plane. The negatively charged carboxylate groups increase colloidal stability and hydrophilicity, rendering them more conducive to nerve cell surface attachment, proliferation, and differentiation. Over the years, graphene oxide GO has been extensively used in neural engineering due to its outstanding electrical properties and affinity for neural cells.139 Studies have shown that GO can promote the differentiation of neural stem cells, sustain the survival of neurons, accelerate the growth of neurons, and enhance neuroelectric properties.140 Zhang et al developed a supramolecular conductive hydrogel by crosslinking graphene oxide and diacerein-functionalized four-arm polyethylene glycol. The presence of GO imparted high electrical conductivity to the hydrogel, which facilitated neuron growth and axon myelination. The incorporation of the anti-inflammatory drug diacerene provided a sustainable anti-inflammatory environment for spinal cord injury repair. In vivo experiments in rat models confirmed evident spinal cord regeneration.141 However, the electrical conductivity and mechanical strength of graphene oxide were reduced due to high vacancy defects and hole defects caused by the presence of functional groups. In response, Chen et al developed a composite hydrogel using polyvinyl alcohol (PVA) and molybdenum sulfide/graphene oxide (MoS2/GO) nanomaterials (Figure 5). The defects in GO were leveraged to enhance fast electron transfer and high electrical conductivity after reacting with MoS2. This combination effectively promoted the differentiation of neural stem cells into neural cells. Additionally, the mechanical properties of PVA were improved by the addition of MoS2 and GO conductive nanoparticles, resulting in a lower elastic modulus that reduced growth resistance after spinal cord injury and significantly increased the proliferation rate of neural stem cells. The results demonstrated that implantation of the composite hydrogel promoted the repair of spinal cord tissue and enhanced the motor function of injured mice.
 |
Figure 5 The schematic diagram shows the preparation steps of MoS2/GO/PVA nanocomposite hydrogel and the treatment process of mouse SCI. Notes: Reprinted from Journal of Nanobiotechnology, 20, Chen LL, Wang WS, Lin ZF, et al Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. Copyright 2022, with permission from Spring Nature. Creative Commons.106 |
rGO is a structural intermediate between graphene and GO that can be synthesized in large quantities from GO through various reduction conditions. Compared with GO, the reduction of oxygen-containing functional groups and the restoration of π-π bonds in rGO result in increased conductivity. The remaining oxygenated groups can also be further modified or functionalized, enhancing rGO’s performance in both organic and aqueous environments. In the realm of neural tissue engineering, rGO boasts several advantageous attributes, including electrical conductivity, biocompatibility, biodegradability, antibacterial properties,142 and pro-angiogenic characteristics.143 Ankor et al demonstrated that rGO is biocompatible with neurons and glial cells in vivo. Reduced graphene oxide could adhere to cells and induce cell migration based on the π-π interaction between reduced graphene oxide and cells.144 Serrano et al explored microfiber-shaped rGO materials (rGO-MF), revealing their potential in promoting nerve cell regeneration and the possibility of aiding axon and blood vessel regeneration in injured spinal cords.145 Xue et al prepared a conductive, porous, soft gel scaffold for spinal cord injury repair using xanthan gum and rGO. The gel scaffold exhibited an elastic modulus and high electrical conductivity similar to spinal cord tissue, as well as a 3D porous structure mimicking extracellular matrix, providing growth space and nutrient transport channels for spinal cord tissue regeneration. The endogenous bioelectrical signals generated by this scaffold, along with the required electric field for nerve cell growth, enhance nerve differentiation into neurons. Additionally, the π-π interaction between rGO and amino acids on cell membranes facilitates nerve cell adhesion within the scaffold. Both in vitro and in vivo experiments confirm the ability of the conductive hydrogel to inhibit astrocyte proliferation around the spinal cord injury area and promote motor function recovery in rats with spinal cord injuries.146
Carbon Nanotubes
Carbon nanotubes (CNTs) are cylindrical nanostructures arranged in single-walled or multi-walled nanotubes composed of a lattice of carbon atoms. Carbon nanotubes exhibit unique properties such as flexibility, electrical conductivity, mechanical strength, and ease of functionalization, making them well-suited for interaction with electrically active tissues. Current studies suggest that carbon nanotube substrates can support neuron survival and promote the growth of neuronal processes,147–149 and the high conductivity of carbon nanotubes improves the transmission efficiency of neuronal electrical signals. For example, hippocampal neurons grown on CNT substrates showed increased spontaneous synaptic activity and firing frequency.150 CNTs can also facilitate synaptogenesis and, thus, neural connectivity of neural circuits.151,152 However, a significant drawback of carbon nanotubes is their insolubility in water, leading to aggregation and cytotoxicity in the central nervous system. This aggregation can trigger inflammation, excessive production of ROS, mitochondrial dysfunction, and impaired synaptic plasticity. To address this issue, Sang and colleagues used a multifunctional crosslinking agent to polymerize single-walled carbon nanotubes, forming a thermosensitive hydrogel. The inclusion of dodecylamine, an amphiphilic coupling agent, and long hydrophobic alkyl chains stabilizes carbon nanotubes and promotes SH-SY5Y cell and neurite outgrowth when coupled with in vitro electrical stimulation. This SWNT-PNIPAAM hydrogel enhances neural tissue regeneration and reduces scar formation in a spinal cord injury model.153 In addition, Koppes and colleagues investigated the synergistic effects of electrical stimulation when combined with conductive 3D hydrogels. They found that SWCNTs loaded at various concentrations promoted neurite outgrowth. Interestingly, 20 μg/mL displayed the most significant advantages, particularly regarding total neurite outgrowth and the longest neurite length. Lower concentrations of more dispersed SWNTs increased electrical conductivity and did not significantly impact the elastic modulus, highlighting the dose-independent advantages (Figure 6). Combining these nanomaterial-loaded hydrogels with electrical stimulation further enhanced neurite length.154
 |
Figure 6 Electrical stimulation enhances neurite outgrowth in SWCNT composite hydrogels. (A) DRG cultured in nanomaterial-free hydrogel and without electrical stimulation. (B) DRG cultured in nanomaterial-free hydrogel but using electrical stimulation (50 mV/mm, 8h, 1mA). (C) DRG cultured in hydrogels with 20 µg/mL SWCT without electrical stimulation. (D) DRG cultured in hydrogels containing 20 µg/mL SWCNTs and using electrical stimulation (50 mV/mm, 8h, 1mA). (E) DRG using electrical stimulation (50 mV/mm, 8h, 1mA) and cultured in hydrogels containing 20 µg/mL SWCNT had the largest total neurite outgrowth compared with the other three control groups. (F) DRG using electrical stimulation (50 mV/mm, 8h, 1mA) and cultured in hydrogels containing 20 µg/mL SWCNT had the longest neurite length compared with the other three control groups. Green = β-III-Tubulin neurites, Red = Phalloidin actin, Blue = DAPI nuclei, Bar = 500 µm, 20x. * = p < 0.05 compared to all conditions, # = p < 0.05 compared to all conditions, n = 3, standard error shown. Notes: Reprinted with permission from Acta biomaterialia, 39, Koppes AN, Keating KW, McGregor AL, et al Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. 34–43. Copyright 2016, with permission from Elsevier.154 |
The low solubility of carbon nanotubes makes it challenging for non-functionalized carbon nanotubes with high conductivity to disperse in aqueous polymer solutions. Existing studies have attempted to modify carbon nanotubes with various functional groups, including hydroxyl, carboxyl, and amino groups. However, traditional carbon nanotube functionalization processes often resulted in the destruction of the sample, causing defects and reducing electrical conductivity. Inspired by mussel foot proteins.155 Inspired by mussel podiacin, Shin et al combined and efficiently dispersed single-walled carbon nanotubes and PPy into catechol-functionalized HA hydrogels via oxidative catechol chemistry. This approach created a biocompatible, dynamic, and conductive 3D extracellular matrix for neuronal regeneration. The formation of HA-CA hydrogels overcame the difficulties in uniformly dispersing non-functionalized conductive materials, such as oxidized PPy and/or CNTs, within 3D HA hydrogels.156,157 Experimental results indicated that the conductive 3D environment provided by the CNT/PPy-containing HA-CA hydrogel promoted neurogenesis in human stem cells by increasing the expression of functional calcium channels (Cav1.2) and intracellular calcium levels.158
While conductive hydrogels based on carbon-based materials hold promise for spinal cord injury repair and regeneration, more extensive safety assessments are essential for their future clinical application. These assessments should explore long-term toxicity, genotoxicity, biodegradation, distribution, metabolism, and organ accumulation.
Conductive Hydrogels Based on Phosphorus-Based Materials
Conductive hydrogels based on phosphorus-based materials offer significant advantages in the context of spinal cord injury repair. Unlike conventional conductive materials such as conductive polymers and carbon nanomaterials, phosphorus-based conductive hydrogels provide the high conductivity required for nerve tissue regeneration while gradually degrading alongside the regeneration of new nerve tissue, leaving no foreign body residues.159 In recent years, phosphorus-based 2D nanomaterials, such as black phosphorus(BP), silicon phosphide, and germanium phosphide (GeP), have attracted significant attention for applications in nerve repair and regeneration.160,161 Black phosphorus, which has a similar structure and properties to graphene, boasts a high conductivity of up to 300S/M and offers both conductivity and degradation characteristics.162 Moreover, being an allotrope of phosphorus, a basic element of the human body, it can naturally degrade into non-toxic phosphorous acid, phosphate, and other PxOy substances under physiological conditions, ensuring good biocompatibility.163 Silicon Phosphorus (Si P) introduces biologically active silicon elements; in addition to providing conductivity and degradability, it promotes angiogenesis. Germanium phosphorus has a narrower band gap, larger charge carrier mobility, and higher conductivity.164 The inclusion of silicon and germanium effectively slows down the oxidation process. To enhance the biostability and biocompatibility of GeP nanosheets, Xu and colleagues doped polydopamine (PDA)-modified GeP nanosheets into a hyaluronic acid hydrogel matrix. In this system, PDA improved the stability of GeP nanosheets, while dopamine molecules grafted onto HA biomolecules endowed the hydrogel with excellent tissue adhesion properties. This injectable hydrogel, crosslinked using horseradish peroxidase (HRP)/H2O2 as an initiator system, enhanced neuron-directed differentiation of neural stem cells in vitro. In a rat model of complete spinal cord injury, the implantation of this hydrogel promoted immune regulation, reduced local inflammatory responses, facilitated endogenous angiogenesis and NSC neurogenesis, and significantly improved motor function recovery.30 The unique advantages of phosphorus-based materials, such as their biocompatibility, conductivity, and degradability, position them as promising candidates in the field of spinal cord injury repair. Given that phosphorus-based conductive materials are natural constituents of the human body, they offer inherent degradation properties. However, long-term studies are warranted to assess whether excessive phosphate ions in the body could lead to phosphate poisoning and the loss of other essential metal ions, such as calcium and magnesium.
MXene-Based Conductive Hydrogels
MXenes represent a new class of 2D materials consisting of nitrides, carbides, and carbonitrides of transition metals,165 with the general formula Mn+1Xn (n=1–3), where M is an early transition metal (eg Sc, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo) and X is carbon or nitrogen.166 These materials offer rich physical and chemical properties and functionalities, owing to the various types and quantities of transition elements and the diverse functionalizations they undergo. MXenes combine the metallic conductivity of transition metal carbides/nitrides with the hydrophilicity of their hydroxyl/oxygen/fluorine-terminated surfaces in the presence of intact metal atomic layers and abundant surface functional groups.167 This endows MXenes with fascinating properties such as good electrical conductivity,168 antibacterial properties,169 hydrophilicity,170 degradability171 and biocompatibility.172 Notably, MXene’s increased hydrophilic groups make it more amenable to surface modifications,173,174 rendering it well-suited for diverse applications in spinal cord injury repair. Yu et al successfully developed hydrogels using phytic acid (PA), polyvinylpyrrolidone (PVP), and MXenes, offering properties like injectability, conductivity, adhesion, and self-healing capabilities. The electrically stimulating microenvironment enabled by MXenes triggers the MEK/ERK signaling pathway, leading to increased secretion of nerve regeneration-related cytokines and eventual promotion of spinal nerve regeneration (Figure 7). In vivo experiments have further verified that hydrogels containing MXene groups create an optimal microenvironment that accelerates nerve regeneration, angiogenesis, remyelination, axon regeneration, and calcium channel activation.105 To achieve targeted neuronal growth, Cai et al designed a composite hydrogel of gelatin methacrylate (GelMA) and MXene with a microgroove structure, which effectively promoted the adhesion, proliferation, and targeted differentiation of NSCs, attributed to MXene’s excellent electrical conductivity.175 In addition, MXenes and their derivatives can be effectively integrated or hybridized with various materials, including metals, graphene and its derivatives, carbon dots, MOFs, and CNTs, to enhance their overall performance and functionality.176 Kong et al combined gold nanoparticles (AuNPs) and MXene, harnessing the good electrical conductivity and biocompatibility of metal nanoparticles (AuNPs), as well as the characteristics of promoting the differentiation of NSCs and the growth of neurons. Coupled with the anti-oxidative ability of MXene to reduce the inflammatory response in the spinal cord injury area, an MAu-GelMA hydrogel loaded with NSCs combined with ES was constructed (Figure 8). The experimental results showed that MAu-GelMA hydrogel could reduce the accumulation of inflammatory cells by scavenging ROS, creating a more suitable microenvironment for NSC growth in the injured area compared to the MXene-GelMA control group. Combined with electrical stimulation, it further promoted the differentiation of NSCs into neural cells and reduced the generation of glial scars, thereby improving the establishment of intercellular synaptic connections and promoting the functional recovery of SCI.177
 |
Figure 7 Possible molecular mechanisms of PPM hydrogel promoting spinal cord regeneration. (a) Western blot analysis of regenerated nerves in SCI, PP and PPM groups; (b) p-MEK/MEK relative protein expression level analysis; (c) MEK/GAPDH relative protein expression level analysis; (d) p-MEK/MEK relative protein expression level analysis; (e) ERK/GAPDH relative protein expression level analysis; (f) Possible molecular mechanisms of PPM hydrogel promoting spinal cord regeneration; *: P < 0.05 compared with SCI group; #: P < 0.05 compared with PP group; n = 5. Notes: Reprinted with permission from Chemical Engineering Journal. Yu QN, Jin SC, Wang SC, Xiao HN, Zhao YT. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Copyright 2022, with permission from Elsevier.105 |
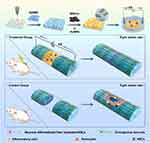 |
Figure 8 A novel multifunctional hydrogel containing MXene-Au composite and NSCs combined with electrical stimulation therapy was constructed to promote motor function recovery after SCI in rats. The combination of MAu-GelMA with NSCs and ES could promote the proliferation and differentiation of NSCs and stimulate the growth of nerve cell synapses. Notes: Reprinted from Ceramics International, 49, Kong WJ, Zhao YL, Yang XY, et al Combined treatment using novel multifunctional MAu-GelMA hydrogel loaded with neural stem cells and electrical stimulation promotes functional recovery from spinal cord injury. 20623–20,636. Copyright 2023, with permission from Elsevier.177 |
Benefiting from its excellent conductivity and diverse surface functional groups, MXene holds the potential to serve multiple roles within conductive hydrogels. The development of improved design and preparation methods for MXene composite hydrogels, addressing drawbacks such as poor dispersion and rapid oxidation,178 is expected to pave the way for their application in the repair and regeneration of spinal cord injuries.
Conclusions and Prospects
The process of repairing and regenerating spinal cord injury is a multifaceted one influenced by numerous factors. Despite extensive research in this area, no single therapeutic approach has yet emerged to achieve full motor and sensory recovery after a spinal cord injury. A promising avenue involves integrating regenerative medicine and rehabilitation approaches to stimulate the regeneration of neural pathways and facilitate functional rehabilitation post-SCI. This comprehensive approach encompasses regenerative medicine179 (eg, cells, drugs and bioactive molecules, biomaterials) and rehabilitation therapies (eg, motor training, electrical stimulation). While rehabilitation therapies have demonstrated positive results in previous clinical studies, their effectiveness diminishes in cases where the injury is severe and significant neural tissue loss has occurred. On the other hand, regenerative medicine approaches, relying on cells, drugs, and bioactive molecules, face their own set of limitations, such as constraints on cell sources, challenges in cell culturing, and difficulties ensuring cell survival following implantation.120 While capable of reducing secondary damage at the injury site and promoting regeneration, drugs and bioactive molecules require multiple administrations over extended periods to maintain therapeutic efficacy, rendering clinical treatment challenging. In light of advancements in biomaterials science and research pertaining to the microenvironment following spinal cord injuries, conductive biomaterials have emerged as a promising component of regenerative medicine. These biomaterials offer the potential to promote SCI repair while being compatible with electrical stimulation to enhance neuroplasticity. For instance, preformed catheters and fibrous scaffolds created through electrostatic spinning, multilayer stacking of electrically conductive materials, or nanofibers can guide the directional growth of axons and serve as carriers for cells, drugs, and bioactive molecules.180,181 However, these scaffolds often lack the mechanical strength required to match spinal cord tissue, presenting challenges in bridging irregular defects caused by contusion or compression-type injuries and potentially leading to secondary damage.
Biomaterials, particularly conductive hydrogels, have proven effective in addressing the limitations of the aforementioned treatment approaches. Currently, these conductive hydrogel scaffolds are designed to closely mimic native spinal cord tissues in terms of properties such as swelling rate, porosity, mechanical strength, electrical conductivity, structural features, and biochemical characteristics. Injectable hydrogels can fill irregular injury gaps and improve the microenvironment at the injury site by providing damaged spinal cord tissue with a matrix for delivering cells, drugs, and bioactive molecules. Leveraging their electroactivity, these conductive hydrogels can reestablish interrupted neuroelectrical signal transmission, deliver electrical stimuli to enhance axonal regeneration and neurogenesis and accelerate the formation of synaptic connections and neurogenesis, ultimately promoting injury repair. Simultaneously, the incorporation of electrical stimulation within the spinal cord injury repair process can lead to more effective functional recovery. While there is limited research on the application of conductive hydrogels in spinal cord injury, the reported results thus far are highly promising and underscore the potential of conductive hydrogels within current tissue engineering and regenerative medicine strategies for enhancing spinal cord injury repair.
Despite the excellent physicochemical properties and applications of conductive hydrogels in spinal cord repair, there is room for improvement when it comes to translating them into clinical practice. The challenge lies in fabricating conductive hydrogels with various combinations of functionalities. Future conductive hydrogels should not only provide spatial and directional support for spinal cord regeneration but should also mimic the electrophysiological microenvironment of neural tissues, transmit endogenous bioelectrical signals, and regulate the microenvironment and endogenous neural stem cells. Further refinement is necessary to ensure these hydrogels possess more appropriate and effective properties, such as maintaining long-term electrical stability, aligning the rate of scaffold degradation with the rate of tissue regeneration, and autonomously responding to changes in the microenvironment to meet the specific repair requirements during different stages of spinal cord injury. Moreover, additional preclinical assessments are required, encompassing long-term toxicity studies, evaluations of cellular uptake, and assessments of effects on metabolic pathways to ensure safe clinical application. Subsequent in vivo studies should include multiple long-term assays examining scaffold components in various body sites, organs, blood, and cells. Furthermore, future research should focus on determining the type and location of electrical stimulation and the parameters of electrical stimulation in synergy with conductive biomaterials. In summary, several challenges exist regarding the development of biomaterials and their clinical application, necessitating further research and development to unlock the full potential of conductive hydrogels as a new direction in spinal cord injury treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137(3):654–667. doi:10.1093/brain/awt262
2. Anjum A, Yazid MD, Daud MF, et al Spinal Cord Injury: pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21(20):7533. doi:10.3390/ijms21207533
3. Karsy M, Hawryluk G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci. 2019;19(9):65. doi:10.1007/s11910-019-0984-1
4. Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi:10.1016/j.pneurobio.2013.11.002
5. Lv ZS, Dong C, Zhang TJ, Zhang SK. Hydrogels in Spinal Cord Injury Repair: a Review. Front Bioeng Biotech. 2022;10:931800. doi:10.3389/fbioe.2022.931800
6. Llorens-Bobadilla E, Chell JM, Le Merre P, et al A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020;370:6512. doi:10.1126/science.abb8795
7. Tran AP, Warren PM, Silver J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev. 2018;98(2):881–917. doi:10.1152/physrev.00017.2017
8. Hong LTA, Kim YM, Park HH, et al An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8(1):533. doi:10.1038/s41467-017-00583-8
9. Orr MB, Gensel JC. Spinal Cord Injury Scarring and Inflammation: therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics. 2018;15(3):541–553. doi:10.1007/s13311-018-0631-6
10. Mohammed R, Opara K, Lall R, Ojha U, Xiang JP. Evaluating the effectiveness of anti-Nogo treatment in spinal cord injuries. Neural Dev. 2020;15(1):1. doi:10.1186/s13064-020-0138-9
11. Wang HY, Xia YL, Li BQ, Li YH, Fu CF. Reverse Adverse Immune Microenvironments by Biomaterials Enhance the Repair of Spinal Cord Injury. Front Bioeng Biotech. 2022;10:812340. doi:10.3389/fbioe.2022.812340
12. Zou JL, Liu S, Sun JH, et al Peripheral Nerve-Derived Matrix Hydrogel Promotes Remyelination and Inhibits Synapse Formation. Adv Funct Mater. 2018;28(13):1705739. doi:10.1002/adfm.201705739
13. Zou Y, Ma D, Shen H, et al Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomaterials sci. 2020;8(18):5145–5156. doi:10.1039/d0bm00431f
14. Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34(15):3775–3783. doi:10.1016/j.biomaterials.2013.02.002
15. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. doi:10.1126/science.aaf3627
16. Hahn G, Ponce-Alvarez A, Deco G, Aertsen A, Kumar A. Portraits of communication in neuronal networks. Nat Rev Neurosci. 2019;20(2):117–127. doi:10.1038/s41583-018-0094-0
17. Bonizzato M, Pidpruzhnykova G, DiGiovanna J, et al Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun. 2018;9(1):3015. doi:10.1038/s41467-018-05282-6
18. Alves-Sampaio A, García-Rama C, Collazos-Castro JE. Biofunctionalized PEDOT-coated microfibers for the treatment of spinal cord injury. Biomaterials. 2016;89:98–113. doi:10.1016/j.biomaterials.2016.02.037
19. Xu C, Xu Y, Yang M, et al Black-Phosphorus-Incorporated Hydrogel as a Conductive and Biodegradable Platform for Enhancement of the Neural Differentiation of Mesenchymal Stem Cells. Adv Funct Mater. 2020;30(39):2000177. doi:10.1002/adfm.202000177
20. Ashammakhi N, Kim HJ, Ehsanipour A, et al Regenerative Therapies for Spinal Cord Injury. Tissue Eng Part B-Reviews. 2019;25(6):471–491. doi:10.1089/ten.teb.2019.0182
21. Yang B, Liang C, Chen D, et al A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact Mater. 2022;15:103–119. doi:10.1016/j.bioactmat.2021.11.032
22. Tandon B, Magaz A, Balint R, Blaker JJ, Cartmell SH. Electroactive biomaterials: vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv Drug Deliv Rev. 2018;129:148–168. doi:10.1016/j.addr.2017.12.012
23. Zare EN, Makvandi P, Ashtari B, Rossi F, Motahari A, Perale G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: a Review. J Med Chem. 2020;63(1):1–22. doi:10.1021/acs.jmedchem.9b00803
24. Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015;5(47):37553–37567. doi:10.1039/c5ra01851j
25. Aleemardani M, Zare P, Seifalian A, Bagher Z, Seifalian AM. Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury. Biomedicines. 2022;10(1):73. doi:10.3390/biomedicines10010073
26. Hu Y, Chen Z, Wang H, et al Conductive Nerve Guidance Conduits Based on Morpho Butterfly Wings for Peripheral Nerve Repair. ACS Nano. 2022;16(2):1868–1879. doi:10.1021/acsnano.1c11627
27. Liu XF, Miller AL, Park S, et al Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl Mater Interfaces. 2017;9(17):14677–14690. doi:10.1021/acsami.7b02072
28. Yang BW, Yin JH, Chen Y, et al 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv Mater. 2018;30(10):1705611. doi:10.1002/adma.201705611
29. Xie DM, Sun CW, Tu QQ, et al Modified black phosphorus quantum dots promotes spinal cord injury repair by targeting the AKT signaling pathway. J Tissue Eng. 2023:1420417314231180033. doi:10.1177/20417314231180033
30. Xu C, Chang YK, Wu P, et al Two-Dimensional-Germanium Phosphide-Reinforced Conductive and Biodegradable Hydrogel Scaffolds Enhance Spinal Cord Injury Repair. Adv Funct Mater. 2021;31(41):2104440. doi:10.1002/adfm.202104440
31. Maleki A, Ghomi M, Nikfarjam N, et al Biomedical Applications of MXene-Integrated Composites: regenerative Medicine, Infection Therapy, Cancer Treatment, and Biosensing. Adv Funct Mater. 2022;32(34):2203430. doi:10.1002/adfm.202203430
32. Zhang YZ, El-Demellawi JK, Jiang Q, et al MXene hydrogels: fundamentals and applications. Chem Soc Rev. 2020;49(20):7229–7251. doi:10.1039/d0cs00022a
33. Quadri SA, Farooqui M, Ikram A, et al Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43(2):425–441. doi:10.1007/s10143-018-1008-3
34. Sterner RC, Sterner RM. Immune response following traumatic spinal cord injury: pathophysiology and therapies. Front Immunol. 2023;13:1084101. doi:10.3389/fimmu.2022.1084101
35. Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurgery Spine. 2007;6(3):255–266. doi:10.3171/spi.2007.6.3.255
36. Li S, Mealing GA, Morley P. injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19(14):RC16.
37. Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32(11):1102–1115. doi:10.1016/s0891-5849(02)00826-2
38. Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206(4419):700–702. doi:10.1126/science.386513
39. Li SX, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20(3):1190–1198. doi:10.1523/jneurosci.20-03-01190.2000
40. Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflamm. 2021;18(1):284. doi:10.1186/s12974-021-02337-2
41. Shi ZJ, Yuan SY, Shi LL, et al Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Proliferat. 2021;54(3):e12992. doi:10.1111/cpr.12992
42. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathology. 1995;5(4):407–413. doi:10.1111/j.1750-3639.1995.tb00619.x
43. Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11(11):3398–3411.
44. Yang R, Zhang Y, Kang J, Zhang C, Ning B. Chondroitin Sulfate Proteoglycans Revisited: its Mechanism of Generation and Action for Spinal Cord Injury. Aging Dis. 2023. doi:10.14336/AD.2023.0512
45. Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res. 2018;126:39–43. doi:10.1016/j.neures.2017.10.004
46. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi:10.1038/nrn1326
47. Kiyotake EA, Martin MD, Detamore MS. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta biomaterialia. 2022;139:43–64. doi:10.1016/j.actbio.2020.12.021
48. Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi:10.3389/fnins.2014.00338
49. Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–713. doi:10.1038/nrn1195
50. Forgione N, Fehlings MG. Rho-ROCK Inhibition in the Treatment of Spinal Cord Injury. World Neurosurg. 2014;82(3–4):E535–E539. doi:10.1016/j.wneu.2013.01.009
51. Baldwin KT, Giger RJ. Insights into the physiological role of CNS regeneration inhibitors. Front Mol Neurosci. 2015;8:23. doi:10.3389/fnmol.2015.00023
52. Boghdadi AG, Teo L, Bourne JA. The Involvement of the Myelin-Associated Inhibitors and Their Receptors in CNS Plasticity and Injury. Mol Neurobiol. 2018;55(3):1831–1846. doi:10.1007/s12035-017-0433-6
53. Geoffroy CG, Zheng BH. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol. 2014;27:31–38. doi:10.1016/j.conb.2014.02.012
54. Fawcett J. Repair of spinal cord injuries: where are we, where are we going?. Spinal Cord. 2002;40(12):615–623. doi:10.1038/sj.sc.3101328
55. Nash M, Pribiag H, Fournier AE, Jacobson C. Central Nervous System Regeneration Inhibitors and their Intracellular Substrates. Mol Neurobiol. 2009;40(3):224–235. doi:10.1007/s12035-009-8083-y
56. Fan BY, Wei ZJ, Yao X, et al Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853–866. doi:10.1177/0963689718755778
57. Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20(5):543–556. doi:10.1016/j.semcdb.2009.04.013
58. Bertucci C, Koppes R, Dumont C, Koppes A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res Bull. 2019;152:265–284. doi:10.1016/j.brainresbull.2019.07.016
59. Arocena M, Zhao M, Collinson JM, Song B. A Time-Lapse and Quantitative Modelling Analysis of Neural Stem Cell Motion in the Absence of Directional Cues and in Electric Fields. J Neurosci Res. 2010;88(15):3267–3274. doi:10.1002/jnr.22502
60. Ye H, Steiger A. Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J Neuroeng Rehabil. 2015;12:65. doi:10.1186/s12984-015-0061-1
61. Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17(6):261–270. doi:10.1016/j.tcb.2007.04.007
62. Goganau I, Sandner B, Weidner N, Fouad K, Blesch A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp Neurol. 2018;300:247–258. doi:10.1016/j.expneurol.2017.11.011
63. Ming GL, Henley J, Tessier-Lavigne M, Song HJ, Poo MM. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29(2):441–452. doi:10.1016/S0896-6273(01)00217-3
64. Liu ZR, Wan XY, Wang ZL, Li LL. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: design and Applications. Adv Mater. 2021;33(32):2007429. doi:10.1002/adma.202007429
65. Song YQ, Li D, Farrelly O, et al The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron. 2019;102(2):373. doi:10.1016/j.neuron.2019.01.050
66. Murillo G, Blanquer A, Vargas-Estevez C, et al Electromechanical Nanogenerator-Cell Interaction Modulates Cell Activity. Adv Mater. 2017;29(24):1605048. doi:10.1002/adma.201605048
67. Zeng Q, Zhou Z, Qin S, et al Rapamycin inhibits B-cell activating factor (BAFF)-stimulated cell proliferation and survival by suppressing Ca(2+)-CaMKII-dependent PTEN/Akt-Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells. Cell Calcium. 2020;87:102171. doi:10.1016/j.ceca.2020.102171
68. Ohtake Y, Hayat U, Li SX. PTEN inhibition and axon regeneration and neural repair. Neural Regeneration Res. 2015;10(9):1363–1368. doi:10.4103/1673-5374.165496
69. Fan L, Liu C, Chen X, et al Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci. 2022:e2105586. doi:10.1002/advs.202105586
70. Park KK, Liu K, Hu Y, et al Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–6. doi:10.1126/science.1161566
71. Zhu R, Sun ZQ, Li CP, Ramakrishna S, Chiu K, He LM. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 2019;319:112963. doi:10.1016/j.expneurol.2019.112963
72. Feng JF, Liu J, Zhang L, et al Electrical Guidance of Human Stem Cells in the Rat Brain. Stem Cell Rep. 2017;9(1):177–189. doi:10.1016/j.stemcr.2017.05.035
73. Batty NJ, Fenrich KK, Fouad K. The role of cAMP and its downstream targets in neurite growth in the adult nervous system. Neurosci Lett. 2017;652:56–63. doi:10.1016/j.neulet.2016.12.033
74. He XG, Li Y, Deng B, et al The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Proliferat. 2022;55(9):e13275. doi:10.1111/cpr.13275
75. Dong ZY, Pei Z, Wang YL, Li Z, Khan A, Meng XT. Ascl1 Regulates Electric Field-Induced Neuronal Differentiation Through PI3K/Akt Pathway. Neuroscience. 2019;404:141–152. doi:10.1016/j.neuroscience.2019.02.004
76. Huang HT, Liu HW, Yan RZ, Hu M. PI3K/Akt and ERK/MAPK Signaling Promote Different Aspects of Neuron Survival and Axonal Regrowth Following Rat Facial Nerve Axotomy. Neurochem Res. 2017;42(12):3515–3524. doi:10.1007/s11064-017-2399-1
77. Joo MC, Jang CH, Park JT, et al Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats. Neural Regen Res. 2018;13(2):340–346. doi:10.4103/1673-5374.226404
78. Chang LF, Jones Y, Ellisman MH, Goldstein LSB, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4(4):521–533. doi:10.1016/S1534-5807(03)00094-7
79. Coulon P, Landisman CE. The Potential Role of Gap Junctional Plasticity in the Regulation of State. Neuron. 2017;93(6):1275–1295. doi:10.1016/j.neuron.2017.02.041
80. Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci. 2014;15(4):250–263. doi:10.1038/nrn3708
81. Curti S, O’Brien J. Characteristics and plasticity of electrical synaptic transmission. Bmc Cell Biol. 2016;17:13. doi:10.1186/s12860-016-0091-y
82. Gordon T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics. 2016;13(2):295–310. doi:10.1007/s13311-015-0415-1
83. Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22(10):1055–1064. doi:10.1016/S0142-9612(00)00344-6
84. Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: a tissue engineering perspective. Biomaterials. 2018;150:60–86. doi:10.1016/j.biomaterials.2017.10.003
85. Karamian BA, Siegel N, Nourie B, et al The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J Orthop Traumatol. 2022;23(1):2. doi:10.1186/s10195-021-00623-6
86. McLaughlin KA, Levin M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev Biol. 2018;433(2):177–189. doi:10.1016/j.ydbio.2017.08.032
87. Hillen BK, Abbas JJ, Jung R. Accelerating locomotor recovery after incomplete spinal injury. Ann Ny Acad Sci. 2013;1279:164–174. doi:10.1111/nyas.12061
88. Eisdorfer JT, Smit RD, Keefe KM, Lemay MA, Smith GM, Spence AJ. Epidural Electrical Stimulation: a Review of Plasticity Mechanisms That Are Hypothesized to Underlie Enhanced Recovery From Spinal Cord Injury With Stimulation. Front Mol Neurosci. 2020;13:163. doi:10.3389/fnmol.2020.00163
89. Kapadia N, Moineau B, Popovic MR. Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping After Spinal Cord Injury and Stroke. Front Neurosci. 2020;14:718. doi:10.3389/fnins.2020.00718
90. Gill ML, Grahn P, Calvert JS, et al Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677. doi:10.1038/s41591-018-0175-7
91. Luo SY, Xu HN, Zuo Y, Liu XG, All AH. A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury. Neuromol Med. 2020;22(4):447–463. doi:10.1007/s12017-019-08589-9
92. Wagner FB, Mignardot JB, Le goff-mignardot CG, et al Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65. doi:10.1038/s41586-018-0649-2
93. Titushkin I, Cho M. Regulation of Cell Cytoskeleton and Membrane Mechanics by Electric Field: role of Linker Proteins. Biophys J. 2009;96(2):717–728. doi:10.1016/j.bpj.2008.09.035
94. Jack AS, Hurd C, Martin J, Fouad K. Electrical Stimulation as a Tool to Promote Plasticity of the Injured Spinal Cord. J Neurotrauma. 2020;37(18):1933–1953. doi:10.1089/neu.2020.7033
95. Song S, Amores D, Chen C, et al Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci Rep. 2019;9:19565. doi:10.1038/s41598-019-56021-w
96. Wenjin W, Wenchao L, Hao Z, et al Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca(2+)- and Erk-dependent signaling pathways. Cell Mol Neurobiol. 2011;31(3):459–467. doi:10.1007/s10571-010-9639-0
97. Wang Y, Lv HQ, Chao X, et al Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Military Med Res. 2022;9(1):16. doi:10.1186/s40779-022-00376-1
98. Cornelison RC, Gonzalez-Rothi EJ, Porvasnik SL, et al Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed Mater. 2018;13(3):034110. doi:10.1088/1748-605X/aaab82
99. Zhu TX, Ni YM, Biesold GM, et al Recent advances in conductive hydrogels: classifications, properties, and applications. Chem Soc Rev. 2023;52(2):473–509. doi:10.1039/d2cs00173j
100. Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials. 2016;111:40–54. doi:10.1016/j.biomaterials.2016.09.020
101. Yuk H, Lu BY, Zhao XH. Hydrogel bioelectronics. Chem Soc Rev. 2019;48(6):1642–1667. doi:10.1039/c8cs00595h
102. Guo YH, Bae J, Fang ZW, Li PP, Zhao F, Yu GH. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem Rev. 2020;120(15):7642–7707. doi:10.1021/acs.chemrev.0c00345
103. Agarwal G, Kumar N, Srivastava A. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Materials Sci Eng C. 2021;118111518. doi:10.1016/j.msec.2020.111518
104. Gao C, Li YX, Liu XY, Huang J, Zhang ZJ. 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem Eng J. 2023;451:138788. doi:10.1016/j.cej.2022.138788
105. Yu QN, Jin SC, Wang SC, Xiao HN, Zhao YT. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chem Eng J. 2023;452:139252. doi:10.1016/j.cej.2022.139252
106. Chen LL, Wang WS, Lin ZF, et al Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J Nanobiotechnology. 2022;20(1):210. doi:10.1186/s12951-022-01396-8
107. Chedly J, Soares S, Montembault A, et al Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91–107. doi:10.1016/j.biomaterials.2017.05.024
108. Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regeneration Res. 2019;14(8):1352–1363. doi:Pmid 30964053. doi:10.4103/1673-5374.253512
109. Ghane N, Beigi MH, Labbaf S, Nasr-Esfahani MH, Kiani A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. Review. J Materials Chem B. 2020;8(47):10712–10738. doi:10.1039/d0tb01842b
110. Mozhdehbakhsh Mofrad Y, Shamloo A. The effect of conductive aligned fibers in an injectable hydrogel on nerve tissue regeneration. Int J Pharm. 2023;645:123419. doi:10.1016/j.ijpharm.2023.123419
111. Serafin A, Culebras M, Oliveira JM, Koffler J, Collins MN. 3D printable electroconductive gelatin-hyaluronic acid materials containing polypyrrole nanoparticles for electroactive tissue engineering. Article. Adv Compos Hybrid Mater. 2023;6(3):
112. Wu C, Liu A, Chen S, et al Cell-Laden Electroconductive Hydrogel Simulating Nerve Matrix To Deliver Electrical Cues and Promote Neurogenesis. ACS Appl Mater Interfaces. 2019;11(25):22152–22163. doi:10.1021/acsami.9b05520
113. Yucel D, Kose GT, Hasirci V. Tissue Engineered, Guided Nerve Tube Consisting of Aligned Neural Stem Cells and Astrocytes. Biomacromolecules. 2010;11(12):3584–3591. doi:10.1021/bm1010323
114. Yang Y, Sun J, Liu X, et al Wet-spinning fabrication of shear-patterned alginate hydrogel microfibers and the guidance of cell alignment. Regen Biomater. 2017;4(5):299–307. doi:10.1093/rb/rbx017
115. Yan L, Zhao B, Liu X, et al Aligned Nanofibers from Polypyrrole/Graphene as Electrodes for Regeneration of Optic Nerve via Electrical Stimulation. ACS Appl Mater Interfaces. 2016;8(11):6834–6840. doi:10.1021/acsami.5b12843
116. Jeong HJ, Yun Y, Lee SJ, Ha Y, Gwak SJ. Biomaterials and strategies for repairing spinal cord lesions. Neurochem Int. 2021;144:104973. doi:10.1016/j.neuint.2021.104973
117. Uz M, Mallapragada SK. Conductive Polymers and Hydrogels for Neural Tissue Engineering. J Indian Inst Sci. 2019;99(3):489–510. doi:10.1007/s41745-019-00126-8
118. Peressotti S, Koehl GE, Goding JA, Green RA. Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomat Sci Eng. 2021;7(9):4136–4163. doi:10.1021/acsbiomaterials.1c00030
119. Shao JD, Ruan CS, Xie HH, et al Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv Sci. 2018;5(5):1700848. doi:10.1002/advs.201700848
120. Xu Y, Xu C, Yang K, et al Copper Ion-Modified Germanium Phosphorus Nanosheets Integrated with an Electroactive and Biodegradable Hydrogel for Neuro-Vascularized Bone Regeneration. Adv Healthcare Mater. 2023. doi:10.1002/adhm.202301151
121. Wang QH, Pan XF, Lin CM, et al Modified Ti3C2TX (MXene) nanosheet-catalyzed self-assembled, anti-aggregated, ultra-stretchable, conductive hydrogels for wearable bioelectronics. Ar Chem Eng J. 2020;401:
122. Mahmud ST, Hasan MM, Bain S, et al Multilayer MXene Heterostructures and Nanohybrids for Multifunctional Applications: a Review. Acs Materials Lett. 2022;4(6):1174–1206. doi:10.1021/acsmaterialslett.2c00175
123. Zhang L, Stauffer WR, Jane EP, Sammak PJ, Cui XT. Enhanced differentiation of embryonic and neural stem cells to neuronal fates on laminin peptides doped polypyrrole. Macromol Biosci. 2010;10(12):1456–1464. doi:10.1002/mabi.201000176
124. Xu JP, Wong CW, Hsu SH. An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing. Chem Materials. 2020;32(24):10407–10422. doi:10.1021/acs.chemmater.0c02906
125. Xu XZ, Wang L, Jing JH, et al Conductive Collagen-Based Hydrogel Combined With Electrical Stimulation to Promote Neural Stem Cell Proliferation and Differentiation. Front Bioeng Biotech. 2022;10:912497. doi:10.3389/fbioe.2022.912497
126. Fan CX, Yang W, Zhang LL, et al Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Biomaterials. 2022;288:121689. doi:10.1016/j.biomaterials.2022.121689
127. Wu CH, Chen SP, Zhou T, et al Antioxidative and Conductive Nanoparticles-Embedded Cell Niche for Neural Differentiation and Spinal Cord Injury Repair. ACS Appl Mater Interfaces. 2021;13(44):52346–52361. doi:10.1021/acsami.1c14679
128. Zhou L, Fan L, Yi X, et al Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. Acs Nano. 2018;12(11):10957–10967. doi:10.1021/acsnano.8b04609
129. Rai R, Roether JA, Boccaccini AR. Polyaniline based polymers in tissue engineering applications: a review. Prog Biomed Eng. 2022;4(4):042004. doi:10.1088/2516-1091/ac93d3
130. Liu W, Luo Y, Ning C, et al Thermo-sensitive electroactive hydrogel combined with electrical stimulation for repair of spinal cord injury. J Nanobiotechnology. 2021;19(1). doi:10.1186/s12951-021-01031-y
131. Serafin A, Rubio MC, Carsi M, et al Electroconductive PEDOT nanoparticle integrated scaffolds for spinal cord tissue repair. Biomater Res. 2022;26(1):63. doi:10.1186/s40824-022-00310-5
132. Song S, Li Y, Huang J, Cheng S, Zhang Z. Inhibited astrocytic differentiation in neural stem cell-laden 3D bioprinted conductive composite hydrogel scaffolds for repair of spinal cord injury. Biomater Adv. 2023;148:213385. doi:10.1016/j.bioadv.2023.213385
133. Geim AK. Graphene: status and prospects. Science. 2009;324(5934):1530–1534. doi:10.1126/science.1158877
134. Silva GA, Czeisler C, Niece KL, et al Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi:10.1126/science.1093783
135. Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10(9):682–692. doi:10.1038/nrn2685
136. Tang ML, Song Q, Li N, Jiang ZY, Huang R, Cheng GS. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials. 2013;34(27):6402–6411. doi:10.1016/j.biomaterials.2013.05.024
137. Bozkurt A, Lassner F, O’Dey D, et al The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012;33(5):1363–1375. doi:10.1016/j.biomaterials.2011.10.069
138. Bitounis D, Ali-Boucetta H, Hong BH, Min DH, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv Mater. 2013;25(16):2258–2268. doi:10.1002/adma.201203700
139. Thompson BC, Murray E, Wallace GG. Graphite Oxide to Graphene. Biomaterials to Bionics. Adv Mater. 2015;27(46):7563–7582. doi:10.1002/adma.201500411
140. Ding X, Liu H, Fan Y. Graphene-Based Materials in Regenerative Medicine. Adv Healthcare Mater. 2015;4(10):1451–1468. doi:10.1002/adhm.201500203
141. Zhang K, Li J, Jin J, et al Injectable, anti-in fl ammatory and conductive hydrogels based on graphene oxide and diacerein-terminated four-armed polyethylene glycol for spinal cord injury repair. Mater Des. 2020:196109092. doi:10.1016/j.matdes.2020.109092
142. Liu SB, Zeng TH, Hofmann M, et al Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: membrane and Oxidative Stress. Acs Nano. 2011;5(9):6971–6980. doi:10.1021/nn202451x
143. Yao XY, Yan ZW, Wang X, Jiang HQ, Qian Y, Fan CY. The influence of reduced graphene oxide on stem cells: a perspective in peripheral nerve regeneration. Regenerative Biomaterials. 2021;8(4):rbab032. doi:10.1093/rb/rbab032
144. Gonzalez-Mayorga A, Lopez-Dolado E, Gutierrez MC, et al Favorable Biological Responses of Neural Cells and Tissue Interacting with Graphene Oxide Microfibers. Acs Omega. 2017;2(11):8253–8263. doi:10.1021/acsomega.7b01354
145. Lopez-Dolado E, Gonzalez-Mayorga A, Gutierrez MC, Serrano MC. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials. 2016;99:72–81. doi:10.1016/j.biomaterials.2016.05.012
146. Xue F, Liu TY, Liu X, Chen KX, Duan LJ, Gao GH. Electroconductive and porous graphene-xanthan gum gel scaffold for spinal cord regeneration. Eur Polym J. 2022;173:111225. doi:10.1016/j.eurpolymj.2022.111225
147. Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14(3):175–182. doi:10.1385/jmn:14:3:
148. Hu H, Ni YC, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4(3):507–511. doi:10.1021/nl035193d
149. Fabbro A, Toma FM, Cellot G, Prato M, Ballerini L. Carbon nanotubes and neuronal performance. Nanomed Nervous System. 2012:183–206.
150. Lovat V, Pantarotto D, Lagostena L, et al Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5(6):1107–1110. doi:10.1021/nl050637m
151. Cellot G, Toma FM, Varley ZK, et al Carbon Nanotube Scaffolds Tune Synaptic Strength in Cultured Neural Circuits: novel Frontiers in Nanomaterial-Tissue Interactions. J Neurosci. 2011;31(36):12945–12953. doi:10.1523/jneurosci.1332-11.2011
152. Fabbro A, Villari A, Laishram J, et al Spinal Cord Explants Use Carbon Nanotube Interfaces To Enhance Neurite Outgrowth and To Fortify Synaptic Inputs. Acs Nano. 2012;6(3):2041–2055. doi:10.1021/nn203519r
153. Sang LL, Liu YQ, Hua WX, et al Thermally sensitive conductive hydrogel using amphiphilic crosslinker self-assembled carbon nanotube to enhance neurite outgrowth and promote spinal cord regeneration. RSC Adv. 2016;6(31):26341–26351. doi:10.1039/c5ra20780k
154. Koppes AN, Keating KW, McGregor AL, et al Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta biomaterialia. 2016;39:34–43. doi:10.1016/j.actbio.2016.05.014
155. Fujigaya T, Nakashima N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci Tech Adv Materials. 2015;16(2):024802. doi:10.1088/1468-6996/16/2/024802
156. Hong S, Yang K, Kang B, et al Hyaluronic Acid Catechol: a Biopolymer Exhibiting a pH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv Funct Mater. 2013;23(14):1774–1780. doi:10.1002/adfm.201202365
157. Shin J, Lee JS, Lee C, et al Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv Funct Mater. 2015;25(25):3814–3824. doi:10.1002/adfm.201500006
158. Shin J, Choi EJ, Cho JH, et al Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules. 2017;18(10):3060–3072. doi:10.1021/acs.biomac.7b00568
159. Tang ZM, Kong N, Ouyang J, et al Phosphorus Science-Oriented Design and Synthesis of Multifunctional Nanomaterials for Biomedical Applications. Matter-Us. 2020;2(2):297–322. doi:10.1016/j.matt.2019.12.007
160. Guo J, Huang DZ, Zhang Y, et al 2D GeP as a Novel Broadband Nonlinear Optical Material for Ultrafast Photonics. Laser Photonics Rev. 2019;13(9):1900123. doi:10.1002/lpor.201900123
161. Shao JD, Xie HH, Huang H, et al Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat Commun. 2021;12(1):3923. doi:10.1038/s41467-021-23210-z
162. Zeng GD, Chen YP. Surface modification of black phosphorus-based nanomaterials in biomedical applications: strategies and recent advances. Acta biomaterialia. 2020;118:1–17. doi:10.1016/j.actbio.2020.10.004
163. Liu XF, Miller AL, Park S, et al Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl Mater Interfaces. 2019;11(26):23558–23572. doi:10.1021/acsami.9b04121
164. Yu TT, Nie HK, Wang SP, et al Two-Dimensional GeP-Based Broad-Band Optical Switches and Photodetectors. Adv Opt Mater. 2020;8(2):1901490. doi:10.1002/adom.201901490
165. Naguib M, Mochalin VN, Barsoum MW, Gogotsi Y. Anniversary Article: mXenes: a New Family of Two-Dimensional Materials. Adv Mater. 2014;26(7):992–1005. doi:10.1002/adma.201304138
166. Lin H, Chen Y, Shi JL. Insights into 2D MXenes for Versatile Biomedical Applications: current Advances and Challenges Ahead. Adv Sci. 2018;5(10):1800518. doi:10.1002/advs.201800518
167. Huang K, Li ZJ, Lin J, Han G, Huang P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem Soc Rev. 2018;47(14):5109–5124. doi:10.1039/c7cs00838d
168. Guo JH, Yu YR, Zhang DG, Zhang H, Zhao YJ. Morphological Hydrogel Microfibers with MXene Encapsulation for Electronic Skin. Research-China. 2021;2021:7065907. doi:10.34133/2021/7065907
169. Wang LF, Li Y, Zhao L, et al Recent advances in ultrathin two-dimensional materials and biomedical applications for reactive oxygen species generation and scavenging. Nanoscale. 2020;12(38):19516–19535. doi:10.1039/d0nr05746k
170. Wychowaniec JK, Litowczenko J, Tadyszak K, et al Unique cellular network formation guided by heterostructures based on reduced graphene oxide - Ti3C2Tx MXene hydrogels. Acta biomaterialia. 2020;115:104–115. doi:10.1016/j.actbio.2020.08.010
171. Li XB, He LZ, Li YF, et al Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. Acs Nano. 2021;15(4):7765–7773. doi:10.1021/acsnano.1c01751
172. Zheng H, Wang SQ, Cheng F, et al Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy. Chem Eng J. 2021;424:130148. doi:10.1016/j.cej.2021.130148
173. Karahan HE, Goh K, Zhang CF, et al MXene Materials for Designing Advanced Separation Membranes. Adv Mater. 2020;32(29):1906697. doi:10.1002/adma.201906697
174. Sun LY, Fan L, Bian FK, Chen GP, Wang YT, Zhao YJ. MXene-Integrated Microneedle Patches with Innate Molecule Encapsulation for Wound Healing. Research-China. 2021;2021:9838490. doi:10.34133/2021/9838490
175. Cai JY, Zhang H, Hu YN, et al GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair. J Nanobiotechnology. 2022;20(1):460. doi:10.1186/s12951-022-01669-2
176. Zarepour A, Ahmadi S, Rabiee N, Zarrabi A, Iravani S. Self-Healing MXene- and Graphene-Based Composites: properties and Applications. Nanomicro Lett. 2023;15(1):100. doi:10.1007/s40820-023-01074-w
177. Kong WJ, Zhao YL, Yang XY, et al Combined treatment using novel multifunctional MAu-GelMA hydrogel loaded with neural stem cells and electrical stimulation promotes functional recovery from spinal cord injury. Ceram Int. 2023;49(12):20623–20636. doi:10.1016/j.ceramint.2023.03.193
178. Zhou C, Zhao XH, Xiong YS, et al A review of etching methods of MXene and applications of MXene conductive hydrogels. Eur Polym J. 2022;167:111063. doi:10.1016/j.eurpolymj.2022.111063
179. Hashimoto S, Nagoshi N, Nakamura M, Okano H. Regenerative medicine strategies for chronic complete spinal cord injury. Neural Regen Res. 2024;19(4):818–824. doi:10.4103/1673-5374.382230
180. Haeri N, Hashamdar S, Hamblin MR, Ranezani F. Effects of electrospun nanofibers on motor function recovery after spinal cord injury; a systematic review and meta-analysis. World Neurosurg. 2023. doi:10.1016/j.wneu.2023.10.065
181. Li X, Liu D, Xiao Z, et al Scaffold-facilitated locomotor improvement post complete spinal cord injury: motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20–31. doi:10.1016/j.biomaterials.2019.01.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

