Back to Journals » International Journal of Nanomedicine » Volume 18
Advances and Prospects of Prolamine Corn Protein Zein as Promising Multifunctional Drug Delivery System for Cancer Treatment
Authors Luo X , Wu S, Xiao M, Gu H, Zhang H, Chen J , Liu Y , Zhang C, Zhang J
Received 28 December 2022
Accepted for publication 6 May 2023
Published 15 May 2023 Volume 2023:18 Pages 2589—2621
DOI https://doi.org/10.2147/IJN.S402891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Xi Luo,1,* Sudan Wu,2,* Meng Xiao,1 Huan Gu,1 Huan Zhang,1 Jianping Chen,3 Yang Liu,4 Chen Zhang,1 Jinming Zhang1
1State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 2Blood Purification Center, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 3Lika Shing Faculty of Medicine, School of Chinese Medicine, the University of Hong KOng, Hong Kong, People’s Republic of China; 4Department of Vascular Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Liu, Hospital of Chengdu University of Traditional Chinese Medicine, No. 37, Shierqiao Road, Jinniu District, Chengdu, Sichuan, People’s Republic of China, Email [email protected] Jinming Zhang, State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, No.1166, Liutai Avenue, Wenjiang District, Chengdu, Sichuan, People’s Republic of China, Email [email protected]
Abstract: Zein is a type of prolamine protein that is derived from corn, and it has been recognized by the US FDA as one of the safest biological materials available. Zein possesses valuable characteristics that have made it a popular choice for the preparation of drug carriers, which can be administered through various routes to improve the therapeutic effect of antitumor drugs. Additionally, zein contains free hydroxyl and amino groups that offer numerous modification sites, enabling it to be hybridized with other materials to create functionalized drug delivery systems. However, despite its potential, the clinical translation of drug-loaded zein-based carriers remains challenging due to insufficient basic research and relatively strong hydrophobicity. In this paper, we aim to systematically introduce the main interactions between loaded drugs and zein, administration routes, and the functionalization of zein-based antitumor drug delivery systems, in order to demonstrate its development potential and promote their further application. We also provide perspectives and future directions for this promising area of research.
Keywords: zein, nanomedicine, drug delivery, antitumor
Graphical Abstract:
Introduction
Cancer is a growing concern globally, with an estimated 19.3 million new cases and 10 million cancer-related deaths reported in 2020 alone. Unfortunately, the situation may worsen in the future, with projections indicating a 47% rise in global cancer cases by 2040, to reach 28.4 million.1 Currently, various therapeutic approaches are employed to treat cancer, including chemotherapy, surgery, and radiotherapy.2 While chemotherapy remains the primary treatment for cancer, it comes with several drawbacks. Although it can improve the survival and quality of life for cancer patients, it also affects blood-forming cells in the bone marrow, hair follicles, and cells in the digestive tract and reproductive system.3,4
Nanoparticle technology is a promising new approach for cancer therapy, offering several advantages over traditional methods. For one, it can improve the bioavailability of insoluble drugs, control drug release, and reduce side effects.5,6 Additionally, nanoparticles can passively target cancer cells thanks to the enhanced permeability and retention (EPR) effect, which leads to increased accumulation of drug-loaded particles within tumors.7 Recent studies, however, have shown that nanoparticles primarily enter tumors through endothelial cells, rather than through gaps between them.8 Although nanoparticles are typically administered intravenously, there are now oral and pulmonary-inhalation methods available, which can improve patient compliance.9,10 To enhance the effectiveness of nanoparticle-based therapies, researchers have designed functionalized nanoparticles that can precisely deliver drugs to cancer cells based on the unique characteristics of the tumor microenvironment. These nanoparticles can be engineered to be pH-sensitive or GSH-sensitive, or to target highly expressed receptors on the tumor surface.11–13 Nanoparticles can combine phototherapy, chemotherapy, and immunotherapy to further inhibit drug resistance and tumor cell migration.14 Overall, nanoparticle technology offers a promising new approach to cancer therapy, with the potential to improve patient outcomes and reduce side effects.
In the past few years, researchers have explored a variety of inorganic and organic materials as micro/nano delivery carriers for anticancer drugs. These materials include nonmetallic and metal inorganic materials, as well as natural polymers, liposomes, exosomes, and dendrimers.15 Currently, 16 anti-tumor nano drugs have been approved for marketing, not including polymer drug conjugates or antibody drug conjugates.16 Additionally, there are nearly 200 clinical trials involving a large number of nano drugs in various stages of development. In completed studies, the success rate of Phase I was about 94%, but dropped to 53% in Phase II and 18% in Phase III. Low effectiveness is the primary reason for clinical failure, and the toxicity and side effects of nano drugs are also major concerns.17,18 For example, liposomal encapsulation of doxorubicin with surface-bound methoxypolyethylene glycol (Caelyx®) is known to be less cardiotoxic and nephrotoxic than unbound doxorubicin, but it produces more dermal lesions primarily on the feet and legs.19 Side effects on the immune system were reported for three out of the four nanomedicinal liposome products, including liposomal amphotericin B, pegylated liposomal doxorubicin, and liposomal daunorubicin.19 To address these challenges, active-targeting nanomedicine drugs have become a research hotspot for drug delivery systems, offering a new way to clinically treat cancer.16 Therefore, it’s essential to find a new delivery carrier for anti-tumor drugs that is highly efficient, has low toxicity and side effects, and can be easily functionalized.
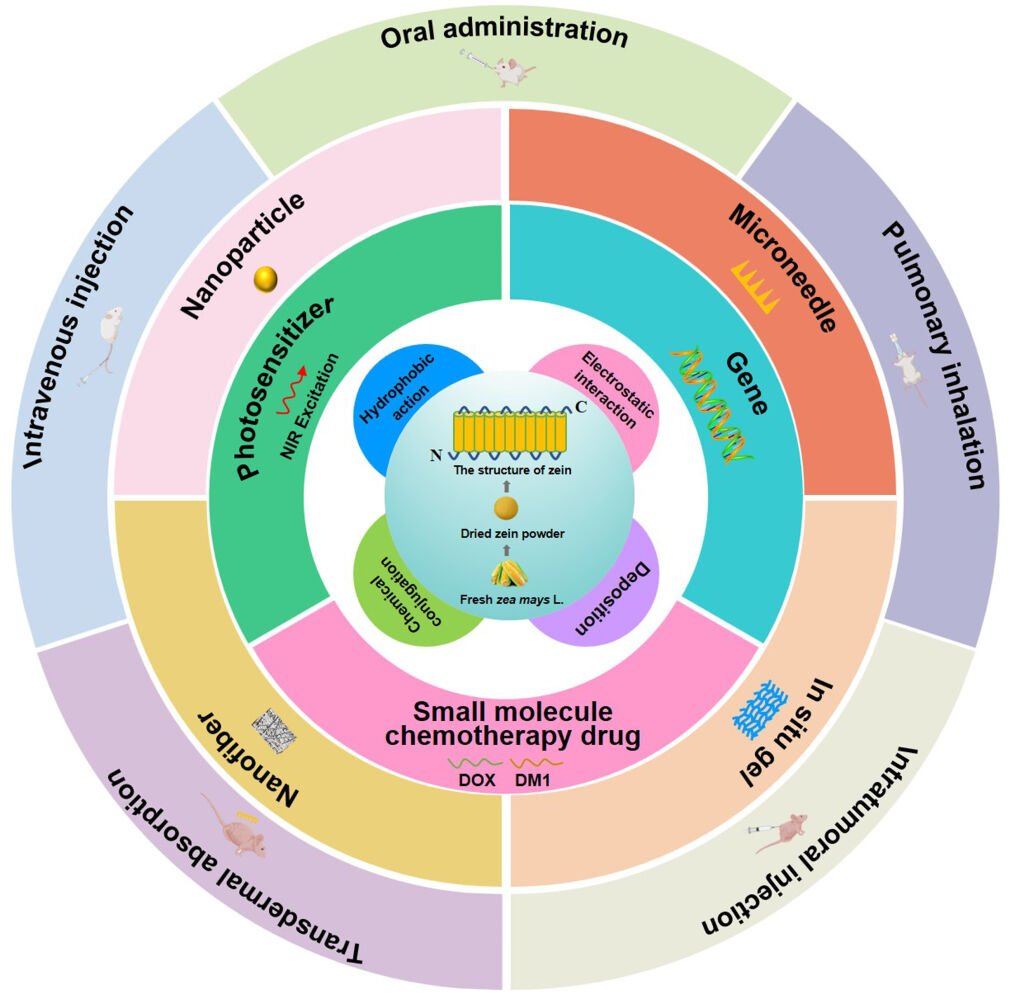
Zein is a type of prolamine protein derived from corn and has been recognized by the US FDA as one of the safest biological materials. It is also the most extensively researched plant protein due to its low immunogenicity, amphipathy, edibility, biodegradability, biocompatibility, and gastrointestinal resistance. Because of these advantages, zein is highly favored in the research of improving oral bioavailability and achieving sustained and targeted drug delivery.20–23 Its brick-like structure (Figure 1) allows for the interception and encapsulation of drugs, while the N-terminal region of γ-zein can interact with the cell membrane, making it a useful carrier for drugs that cross the cell membrane. Zein also contains free hydroxyl and amino groups that can serve as more modification sites, making it a versatile material for functionalized drug delivery.24–27 Currently, zein-based drug delivery systems include nanoparticle,28 nanofiber,29 microneedle30 and hydrogels31 prepared by hydrophobic interaction, chemical conjugation, and electrostatic interaction with drugs. These systems have successfully delivered small-molecule chemotherapeutic drugs, anti-tumor genes, and photosensitizers through various administration routes such as intravenous injection, oral administration, pulmonary inhalation, transdermal absorption, and intratumoral injection. Additionally, due to its hydrophobicity, zein gel can also be used to form 4D-printing drug delivery systems that can change shape, property, and function over time when stimulated.32
 |
Figure 1 The origin and structure of Zein, the drug-loading zein-based carriers and appropriate routes of administration, and the functionalization of zein-based nanoparticle. |
This paper aims to systematically introduce the advantages and disadvantages of current antitumor drugs and how zein-based drug delivery systems can improve their effectiveness (Figure 1). It also discusses the administration routes and functionalization of zein-based nanoparticles, in order to promote further development and application of zein as an antitumor drug carrier.
Drug Loading
Zein has shown promising results in delivering various types of antitumor drugs, including small-molecule chemotherapeutic drugs, genes, and photosensitizers. Due to its amphiphilic nature, zein can interact with drugs through hydrophobic interactions, as well as chemical conjugation and electrostatic interaction, thanks to its free hydroxyl and amino groups that exhibit different charges under different pH conditions. Additionally, metal nanoparticles or phenolic acid/metal ion membranes can be deposited on zein-based nanoparticles to enhance drug delivery efficiency. In the following sections, we will discuss the different types of antitumor drugs that can be loaded onto zein carriers and the primary mechanisms of interaction between zein and these drugs.
Small Molecule Chemotherapy Drug
Currently, chemotherapy is one of the main methods used to treat tumors,33 Commonly used drugs include doxorubicin,34 paclitaxel,35 and camptothecin,36 etc. Although these drugs have strong antitumor activity, their clinical application is limited due to drawbacks such as cellular drug resistance, systemic toxicity, poor water solubility and short half-life period.37–39 To address these limitations, zein, with its amphipathy, can form nanoparticles by self-assembly. The hydrophobic inner core can effectively encapsulate lipid-soluble drugs through hydrophobic interaction and other forces to improve the solubility of the drugs. Additionally, based on the enhanced permeability and retention (EPR) effect, zein-based nanoparticles can reduce the distribution of the drugs in normal tissue, minimizing the side effects of drugs. For example, Fangyuan Dong40 prepared doxorubicin/zein nanoparticles (DOX-zein-NPs) by self-assembly of zein through hydrophobic interaction and hydrogen bonding, which effectively enhanced the water solubility of doxorubicin. Compared with the non-specific rapid release of free doxorubicin, DOX-zein-NPs slowly released DOX under normal extracellular pH conditions and rapidly released DOX under lower intracellular pH conditions. This phenomenon indicated that zein-based nanoparticles can prolong blood circulation of the drug, reduce cytotoxicity to normal cells, and enhance targeted cytotoxicity to specific tumor cells. Maytansine (DM1) is a potent inhibitor of tubulin polymerization that can effectively treat various malignancies, including breast cancer, melanoma, multiple myeloma, liver cancer, and lung cancer.41–43 However, its clinical application is limited by strong side effects, narrow therapeutic window, and poor water solubility.44 Xianglong Yu45 prepared DM1-loaded ZNPs through hydrophobic interaction. In vitro release experiments showed that the ZNPs released about 20% of DM1 within the first 8 hours and about 40% after the second 24 hours, indicating a good controlled release effect. When fetal bovine serum (FBS) was used to evaluate the stability of ZNPs under physiological conditions, the results showed that only a small amount of DM1 leaked into the serum within 24 hours, and there was no obvious adsorption or precipitation in the reaction solution, indicating that the DM1-loaded ZNPs could remain stable in the serum. In vitro cytotoxicity experiments showed that free DM1 had a dose-dependent antiproliferative activity, while ZNPs loaded with DM1 had no cytotoxicity, indicating that the encapsulation of zein effectively reduced its killing effect on normal cells.
Photosensitizer
Phototherapy is a treatment for tumors that includes reactive oxygen species (ROS)-mediated photodynamic therapy (PDT) and fever-mediated photothermal therapy (PTT). This treatment has several advantages, such as less side effects, less drug resistance, and faster recovery.46 NIR light absorbing dyes like indocyanine, napthalocyanines and porphyrins coordinated with transition metals have been used for light mediated therapeutics.47–52 However, many photosensitizers used in phototherapy have poor selectivity to lesions, poor targeting, poor water solubility, and low bioavailability.46 To overcome these limitations, zein, an amphiphilic nanomaterial, can self-assemble into nanoparticles through hydrophobic interaction to encapsulate photosensitizers, thereby improving their solubility and achieving tumor targeting.
For example, indocyanine green (ICG), as a fluorescent dye approved by FDA for clinical use, has been studied for photodynamic therapy and photothermal therapy of tumors.53–56 However, its water solubility is very poor. Therefore, it is prone to aggregation and precipitation in aqueous solutions, leading to self-quenching and reduced emission intensity. Eun-Hye Lee28 encapsulated it in zein phosphatidylcholine hybrid nanoparticles (Z/PC-NP) nanoparticles. During 10 days of storage in free ICG solution, its absorption peak at 780 nm decreased in a time-dependent manner. At the same time, a new absorption peak appeared and increased at 894 nm, indicating the formation of ICG aggregates. In contrast, Z/PC-NP (zein 3–7 mg) had no 894nm peak during the 10-day incubation period. This indicates that the ICG-zein interaction in Z/PC-NP reduces the interaction between ICG molecules and effectively inhibits the aggregation of ICG. Cytotoxicity experiments showed that free ICG could not significantly inhibit the growth of A253 cells. In contrast, the Z/PC-NP formulation significantly inhibits cell proliferation and retains the photosensitizing activity of ICG.
Integration of diagnosis and treatment is a promising cancer treatment strategy, and gold nanoparticles (GNPs) can be used as a contrast agent and activated by increasing the local temperature to kill tumor cells.57–59 However, low photothermal conversion efficiency and photostability, poor water solubility, and tumor-targeting ability moderate its applicability.60,61 To address these issues, Deepak S. Chauhan et al62 demonstrated the facile and green synthesis of gold deposited zein nanoshells (AuZNS) using environmental benign solvent ethanol (Figure 2). AuZNS was of size around 100 nm. When even given double the therapeutic dosage, AuZNS still showed high inertness and biocompatibility. They used two different cancer cell lines viz. MCF-7 (breast cancer) and C33A (cervical cancer) to evaluate its antitumor effect. The results showed almost equal therapeutic effect. The absorbance was tuned at 808 nm for imaging-guided plasmonic photothermal therapy of cancer. AuZNS also exhibited better X-ray attenuation in comparison to the commercially available iodine-based contrast agent.
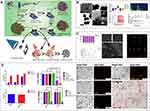 |
Figure 2 (A) Illustration showing the green synthesis and application of AuZNS for imaging-guided plasmonic photothermal therapy. (B) Micrographic images and size distribution; Zeta potential measurement, elemental analysis, absorbance spectrum, and photothermal transduction. (C) Biocompatibility and hemolysis study; X-ray of negative control (top row), AuZNS (middle row) and Omnipaque (bottom row) taken at different conc. (D) Qualitative analysis of non-targeted photothermal therapy on C33A cells using propidium iodide; Uptake study, targeted and non-targeted photothermal therapy. ⁎⁎p ⁎ 0.01, ⁎⁎⁎⁎p ⁎ 0.0001. Reprinted with permission from Chauhan DS, Arunkumar P, Prasad R, et al. Facile synthesis of plasmonic zein nanoshells for imaging-guided photothermal cancer therapy. Mater Sci Eng C Mater Biol Appl. 2018;90:539–548. Copyright 2018. Elsevier.62 |
Combination therapy has been proposed to improve the therapeutic efficacy, including the combination of chemotherapeutic agents, chemo-energy, chemo-gene, chemo-small molecules, and chemo-immunology.14,63 Xianglong Yu14 prepared an “all-in-one” and “one for all” nanoplatform, which combined “chemo−immuno−photo-thermal therapy” (Figure 3). Specifically, Docetaxel (DTX, a chemo-agent) and cynomorium songaricum polysaccharide (CSP, an immunomodulator) were loaded into zein nanoparticles coated by a green tea polyphenols/iron coordination complex (GTP/FeIII, a photothermal agent). This nanoplatform was spherical in morphology with an average particle size of 274 nm, and achieved pH-responsive drug release. In the pharmacodynamic test, it can effectively destroy the tumors, remove the metastatic lesions, and prevent tumor recurrence by inducing the ICD (immunogenic cell death) effect and building long-lived antitumor immune responses.
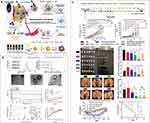 |
Figure 3 (A) Schematic diagram of the “all-in-one” and “one-for-all” nanoplatform for combined “chemo−immuno−photothermal” therapy. (B) Fabrication and characterization of DTX-Loaded Zein/CSP-GTP/FeIII NPs and the in vitro drug release. (C) In vivo therapeutic efficacy of various treatments on 4T1 tumor-bearing mice. *P < 0.05; **P < 0.01; ***P< 0.001 compared to control. #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Reprinted with permission from Yu X, Han N, Dong Z, et al. Combined Chemo-Immuno-Photothermal Therapy for Effective Cancer Treatment via an All-in-One and One-for-All Nanoplatform. ACS Appl Mater Interfaces. 2022;14(38): 42,988–43,009. Copyright 2022, American Chemical Society.14 |
Gene
Gene therapy is a promising new approach for cancer treatment, offering benefits such as gene delivery and gene silencing.64–67 However, free genes can be easily degraded, have difficulty reaching the target site, and can cause toxicity to normal tissues during delivery.68–71 Nanoparticle carriers are effective in controlling drug release, actively and passively targeting tumor sites, protecting the loaded drugs in and out of cells, as well as promoting the cellular uptake or subcellular transport.72 Therefore, they are ideal gene delivery carriers. Zein, a protein containing hydrophobic amino acids and polar glutamine, can self-assemble to form nanoparticles due to its amphiphilicity. Zein’s polar side chain can interact with negatively charged DNA to load genes through electrostatic interaction, making it an ideal gene therapy delivery vehicle.73,74 For instance, Fathia Zaki El Sharkawi75 prepared zein nanoparticles loaded with plasmids encoding PTEN and TRAIL, and the morphology of the prepared DNA-loaded ZNPs was investigated by transmission electron microscopy (TEM). The results show that the prepared ZNPs have solid dense structure, round uniform shape and rough surface, which may be due to the surface-adsorbed DNA on the ZNPs. Compared with the normal control group, the expression level of p53 in the liver cancer-induced animals was significantly decreased (P value < 0.01). However, p53 expression was induced in the PTEN and TRAIL gene-loaded nanoparticles group compared with the untreated group (p<0.0001, p<0.01). VEGF was highly expressed in HCC-induced animals. On the other hand, an anti-angiogenic effect of the genes was observed in the nanoparticle group, with significantly lower expression levels of VEGF compared to untreated animals. In addition, PTEN and TRAIL in ZNPs significantly inhibited liver metastasis, reducing MMP-2 expression in liver homogenates of treated animals compared to untreated animals.
Loading Loading Efficiency and Their Limitations
Table 1 summarized the different loading methods and their different applications. As shown in Table 1, almost all the zein-based nanoparticles have a content encapsulation and loading efficiency. There are even individual nanoparticles with a loading efficiency of over 80%. However, when the encapsulation and loading efficiency is high enough, we can find that the particle size of most of zein-based nanoparticles has been over 150 nm. To reduce systematic clearance and enhanced the penetration and internalization of tumors, the nanoparticles with diameter of 12–50 nm are most appropriate.5 Additionally, monocytes and reticuloendothelial systems can readily remove drug carriers with diameters larger than 200 nm.76 Therefore, in theory, the therapeutic efficacy of these zein-based nanoparticles still needs to be improved. As for the surface charge, while almost all zein-based nanoparticles have a surface charge with an absolute value exceeding 30 mV, making them relatively stable and resistant to aggregation, the charge can also impact their circulation and recognition by the immune system.77,78 Additionally, negatively charged endothelial cells can also cause retention of cationic nanoparticles, thereby reducing their circulation.79,80 Conversely, negatively charged nanoparticles are repelled from the cell-free layer, limiting their extravasation.5 Cationic nanoparticles are more conducive to transvascular transport and tumor penetration.5 Therefore, to optimize the therapeutic efficacy of zein-based nanoparticles, their surface charge needs to be improved to balance their circulation, transvascular transport and tumor penetration, while avoiding recognition and clearance by the immune system.
 |
Table 1 Different Loading Methods and Their Applications About Zein-Based Nanoparticles |
Route of Administration
Currently, the main routes of administration of anticancer drugs include intravenous injection, oral administration, transdermal absorption, intratumoral injection and pulmonary inhalation. However, each of these routes has its own advantages and disadvantages.90 To overcome some of these limitations, zein, with its many excellent characteristics (Figure 4), has been proposed as a drug carrier for various routes of administration. In the following sections, we will discuss the potential applications of zein as a drug carrier in each of these five routes of administration.
 |
Figure 4 Routes of administration of zein carriers and the main properties of zein used in each route of administration. |
Intravenous Injection
Intravenous drug administration is a popular and effective route of administration due to its high bioavailability, minimal irritation to other body parts, and ability to bypass the gastrointestinal environment. However, there are also some disadvantages associated with intravenous injection. For instance, the drug system needs to be highly pure to prevent infections, and the dosage is limited, requiring multiple injections that can decrease patient compliance. Additionally, most anticancer drugs have low water solubility, making them prone to aggregation in the bloodstream or clearance by macrophages and the liver, ultimately reducing their efficacy. Fortunately, these issues can be addressed to some extent by encapsulating the drugs in nanoparticles.
Zein is a natural, biodegradable, and low immunogenicity amphiphilic substance that can be used as a drug carrier material. It is a vegetable protein, which makes it safer and reduces the risk of zoonotic diseases. Zein can assemble into nanoparticles through hydrophobic interaction with drugs, increasing the water solubility of the drugs and reducing their clearance by macrophages or the liver. Zein-based nanoparticles can also effectively control the drug release, prolong the drug’s residence time in the blood circulation, and reduce the number of administrations. So zein is an ideal carrier material for intravenous injection of drugs. 5-Fluorouracil (5-FU) has a long history of use as a chemotherapeutic agent. But less than 20% of an injected dose undergoes enzymatic activation.91 The oral bioavailability of 5-FU is unpredictable due to high variability in enzymatic degradation. In addition, 5-FU has a relatively short half-life period and toxicity to the bone marrow and the gastrointestinal tract.91 To solve above problems, Lai92 prepared zein nanoparticles (ZP) loaded with 5-fluorouracil (5-FU). The optimized ZPs have an average size of 114.9 nm, which can target liver tumors through the EPR effect. Moreover, the ZPs also showed sustained release properties in vitro and effectively prolonged the drug’s residence time (7.2-fold increase) in blood circulation.
Oral Administration
Due to the simple administration method, no direct damage to skin or mucous membrane and relatively low cost of production, oral administration has high patient compliance and is the most common and convenient route of administration.93,94 However, the harsh gastrointestinal environment poses challenges for achieving satisfactory levels of bioavailability through oral administration. Zein, with its resistance to gastric acid and digestive enzymes, hydrophobicity, biodegradability, mucoadhesion, and nontoxicity to enterocytes, offers several advantages that can compensate for these deficiencies.93,95,96 Therefore, encapsulating the drugs into zein-based carrier can effectively increase the bioavailability of the drugs and improve their therapeutic effect. Currently, zein-based oral drug delivery system include oral nanoparticles and oral nanofibers. Details will be given below.
Oral Nanoparticle
Zein’s amphipathic nature allows it to self-assemble into nanoparticles that can be loaded with drugs. Because of its inherent hydrophobicity and resistance to gastrointestinal conditions,93,95,97–99 zein-based nanoparticles can protect drugs and achieve controlled release, without damaging the gastrointestinal tract. This makes zein-based nanoparticles a promising nanocarrier for oral drug delivery. For instance, Shinde100 prepared zein nanoparticles loaded with luteolin, which successfully increased the solubility of luteolin, improved its oral availability, and achieved sustained release of the drug in the intestine. To further improve the stability, penetration, and colon targeting ability of antineoplastic drugs in the gastrointestinal tract, in some cases, zein-based nanoparticle has been proposed to be prepared in combination with other excipients, such as glutaraldehyde,101 chitosan,21,102 carboxymethyl chitosan,103 sodium caseinate,100 soybean lecithin104 and bile salts,105 etc. Phuong H.L. Tran106 has discussed this in detail in his recently published article, so they will not be repeated here. But recently, there was a very interesting study. Lu Liu107 prepared CUR-encapsulated nanoparticles, which were fabricated with zein alone (Zein-CUR) and with zein and a polysaccharide (PS) such as gum Arabic (GA), hyaluronic acid (HA) and pectin (PC), respectively (PS-Zein-CUR) (Figure 5). The results showed the three PS-Zein-CUR formulations had significantly higher (17–22%) CUR encapsulation efficiency (EE) than Zein-CUR. They also effectively inhibited cell viability and colony formation.
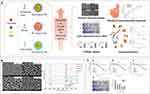 |
Figure 5 (A) Experimental scheme. The evaluation of the potential of PS-Zein-CUR an oral agent for CRC therapy: physicochemical properties, anti-CRC effects, cellular uptake, and gastrointestinal simulated digestion in vitro and pharmacokinetics and tissue distribution in vivo. (B) Spectroscopic analysis of composite nanoparticles. (C) Inhibitory effect of PS-Zein-CUR on CRC (HCT116, HCT8, and HT29) cell growth. Cells were treated with the indicated doses of CUR, PS-Zein, and PS-Zein-CUR for 48 h, respectively. (a–c) Cell viability measured by using a CCK-8 cell counting kit. (d) Left: images of colony formation assay; Right: quantitative analysis of colony formation expressed as percentage relative to vehicle control. Bars marked with different letters indicate significant difference at P < 0.05. Reprinted with permission from Liu L, Yang S, Chen F, et al. Polysaccharide-Zein Composite Nanoparticles for Enhancing Cellular Uptake and Oral Bioavailability of Curcumin: Characterization, Anti-colorectal Cancer Effect, and Pharmacokinetics. Front Nutr. 2022;9: 846,282. Copyright 2022, Frontiers.107 |
Oral Nanofiber
Drug-loaded zein nanofibers is another oral antitumor drug delivery system. Nanofibers prepared by electrospinning technology can achieve efficient drug loading and slow release, and are commonly used in wound healing.108 Moreover, nanofibers can control the drug release rate by modification of the degradation rate of the fibers.108 Until recently, electrospun fibers were tested for oral administration of poorly soluble or unstable drugs.109 It is well known that increasing the contact area between the drug and the solvent can effectively promote the dissolution of the drug. Nanofibers have a larger area, which means can improve the solubility of the drugs. For example, formulation of diosmin or flubendazole in electrospun nanofibers can make the drugs completely amorphizate, thereby resulting in a very rapid release and increasing the bioavailability of the drug. Zein has been widely used as a carrier for preparing nanofibers,110,111 and it is also a good carrier for oral drugs.97,98 Therefore, the use of oral nanofibers prepared with zein has a good application prospect. Francisca Acevedo29 prepared gallic acid (GA)-loaded polyethylene oxide/zein nanofibers by coaxial electrospinning. Under the optimal process conditions, the loading efficiency of GA was as high as 77%. The release of GA was effectively controlled in both acidic and neutral pH media. Compared with free GA, the loading by zein nanofibers effectively increased the cytotoxicity of GA against gallbladder cancer cell lines GB-d1 and NOZ, indicating that zein-based oral nanofibers are a promising drug delivery system.
Transdermal Absorption
Transdermal drug delivery (TDD) offers enormous advantages over oral, nasal, intramuscular and intravenous routes of administration, such as painless administration, good patient compliance, and self-administration.112 Transdermal drug delivery systems (TDDSs) not only facilitate the sustained transport of therapeutic drugs through the skin, but also help drugs overcome certain barriers (such as first-pass metabolism), improving the transport of drugs with low solubility and bioavailability.113 Microneedles, a new type of transdermal drug delivery technology, consist of a series of micron-sized arrays in a patch. Microneedle technology has good patient compliance, great permeability, and better drug efficacy compared to traditional transdermal absorption methods such as subcutaneous injection and patch.114 Microneedle technology has been used in the local treatment of cancer models, including melanoma,115 basal cell carcinoma,115 breast cancer,30 and skin cancer.116 The materials used to prepare microneedles include silicon, metal, ceramics, glass, sugars, polymers, etc. However, these materials have limitations due to low drug loading efficiency, brittleness, easy decomposition at high temperature, and poor biocompatibility.114 Zein, due to its advantages of sufficient mechanical strength, easy castability, high drug loading, and biodegradability, is expected to be a new material for the preparation of microneedles.117 Shubhmita Bhatnagar30 used micromolding technique to prepare zein microneedles co-loaded with gemcitabine and tamoxifen for local treatment of breast cancer. The maximum drug loading of tamoxifen and gemcitabine were 607 ± 21 and 1459 ± 74 μg, showing sufficient mechanical strength to be inserted into pigskin. Although zein is insoluble in aqueous media or skin tissue,117 the experimenters observed that zein microneedles would swell after being cultured in aqueous medium for a long time, which is beneficial for the therapeutic drug to be embedded in the matrix or coated on the surface of the microneedle for transdermal administration.
Intratumoral Injection
Chemotherapy is one of the most important treatments for malignant tumors. However, malignant solid tumors have a high incidence of multiple primary malignant tumors, and their unique microenvironment reduces the clinical efficacy of conventional chemotherapy. Interstitial chemotherapy, first proposed by Brem, provides an alternative to conventional chemotherapy.118 Recently, new formulations for interstitial chemotherapy have been developed, such as gels, microchips, nanoparticles, polymeric wafers, and in situ gels. Among them, in situ gel is gaining popularity as a vehicle for drug delivery systems.119–123 The polymers used for in situ gel are mainly synthetic,124 while the biocompatibility and safety of synthetic materials need to be improved. Zein, a natural plant protein from corn, is a promising alternative due to its low immunogenicity and biodegradability. Moreover, Zein has poor solubility in physiological saline or body fluids due to its hydrophobicity. Therefore, the zein solution can rapidly undergoes a phase transition to be a semi-solid, forming the three-dimensional network gel. And the drug will slowly release from the gel to achieve long-term effects.125–128 So, zein is a good material for preparing tumor in situ hydrogels. Doxorubicin (DOX), a cytotoxic anthracycline, is the first-line treatment option for many hematological diseases and solid tumors.129 Xiaoying Cao31 developed Dox-loaded Zein in situ gel for interstitial chemotherapy. Under the scanning electron microscope, there was no obvious pores on the surface of the zein in situ gel at the first hour. However, at 48 h, the surface of the zein in situ gel was eroded and numerous pores were observed. On the 12th day, the zein in situ gel formed a network structure inside and connected to each other, forming a diffusion barrier for DOX. But its inner porosity was much higher than that of the outer, and the surrounding diffusion barrier area formed a buffer after contacting with water, which would help to modulate the initial burst release of water-soluble drugs. After intratumoral injection of the hydrogel, the system converted from a liquid to a semi-solid. Compared with free doxorubicin solution, due to the continuous release of DOX-loaded Zein in situ gel, the local DOX concentration in solid tumors can be maintained above the anti-tumor threshold concentration for a long time, so as to achieve the purpose of reducing toxicity and improving efficacy.
Pulmonary Inhalation
The administration of drugs through pulmonary inhalation is an attractive drug delivery target due to its non-invasive nature, potential for higher systemic bioavailability, and availability of a large surface area. Moreover, pulmonary inhalation can also avoid the first passage through metabolism and starting therapeutic effects more quickly.130,131 However, drugs that enter the lungs directly can be destroyed by enzymes and expelled by mucous cilia. Therefore, a method must be found to protect the drugs during lung administration. Nanoparticles have been found to encapsulate drugs, protecting them from damage in the pulmonary microenvironment and achieving a relatively uniform distribution of drug doses among the alveoli.132–134 Therefore, the nanoparticles have the huge potential to be a drug carrier in pulmonary inhalation. Zein has amphiphilic properties and can self-assemble into nanoparticles to protect drugs from damage by the pulmonary microenvironment. It is worth noting that, as a protein, it can have a strong affinity with anti-tumor peptides or proteins, which is expected to achieve efficient drug encapsulation. In addition, the lungs have highly expressed neonatal Fc receptor (FcRn), and zein has a modifiable surface which can be linked with the ligands of it, thereby improving delivery efficiency.9 Fatima Hameedat et al9 used insulin as a protein model to prepare zein-based nanoparticles conjugated with ethylene glycol and FcRn-targeted peptides. The experimental results demonstrated the FcRn targeting and the increasing of the permeability of insulin, which fully meet the requirements of the pulmonary drug delivery system. In another study, Nayra M. Kamel et al135 prepared hybrid lipid nanocore-protein shell nanoparticles (HLPNPs) coloaded with all-trans retinoic acid (ATRA) and genistein (GNS), which enabled dual tumor-targeting with biotin and ATRA. The results showed that HLPNPs enhanced the uptake of A549 lung cancer cells and the cytotoxicity of loaded drugs. To improve their deep lung deposition, they fabricated the dual-targeted drug-loaded HLPNP nanocomposites. In vivo, the inhalable nanocomposites were superior to aerosolized or i.v. nanoparticle suspension against lung carcinoma bearing mice.
Drug Delivery System Functionalization
Currently, many nano-drugs are delivered to the tumor site through the EPR effect. However, this method has some drawbacks, such as easy clearance from the bloodstream, insufficient accumulation at the target site, and unsatisfactory therapeutic effects. Zein, as a protein, contains free carboxyl and amino groups that can be modified and functionalized to improve its nanoparticle drug delivery system. Researchers have developed targeted and microenvironment-sensitive release systems that take into account the unique characteristics of tumor cells, including specific receptor expression, low pH, and high glutathione concentration at the tumor site. Additionally, the surface of zein is PEGylated to prolong the circulation time of nanoparticles, and zein nanoparticles are encapsulated in hydrogels to further enhance their drug protection and release control capabilities. In the following section, we will discuss the functionalization of the zein-based nanoparticle drug delivery system in more detail.
Ligand-Modified
At present, the EPR effect is a relatively recognized way of accumulation of nano-drugs in tumors. However, its accumulation efficiency is low and the specificity of targeting is insufficient. To improve the targeting of nanoparticles to tumor sites and their uptake by tumor cells, many ligands that can specifically bind to receptors expressed on tumor cells have been modified on the surface of nanoparticles.136 The structure of zein contains some free carboxyl groups and amino groups, which can be linked to ligands by amide reaction or other methods. Hyaluronic acid12,137 (Figure 6), chondroitin sulfate,138,139 folic acid,140,141 biotin,135 all-trans retinoic acid,135 etc., are commonly used ligands for zein nanoparticles. These receptors are linked to zein in various ways (such as carbodiimide reaction, electrostatic interaction, ionic hydrogen bonding) and show good tumor targeting ability. The Table 2 shows their current application to zein nanoparticles. The types, receptors, connection mechanism, loaded drugs and achieved effects of tumor-targeting ligands are summarized.
 |
Table 2 The Ligands Used in the Zein-Based Nanoparticles and Achieved Effects |
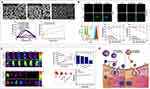 |
Figure 6 (A) SEM images of NGs, photoluminescence of the HA-Zein CRC NGs with varying CRC amounts, and cumulative release percent of CRC from the HA-Zein CRC NGs in two different pH environments. (B) Cellular uptake, cell viability with the HA-Zein-CRC NGs. (C) In vivo imaging and biodistribution analysis of nude mice with CT26 tumors after the tail vein was injected with Zein-IR780 and HA-Zein-IR780 NGs. (D) Schematic illustration of CRC encapsulated HA-Zein NGs for HA receptor targeting against CT26 colorectal cancer cells, and the selective uptake mechanism of HA-Zein NGs for curcumin delivery to HA receptor overexpressing cancer cells. *P < 0.05; **P < 0.01. Reprinted with permission from Seok HY, Sanoj Rejinold N, Lekshmi KM, et al. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J Control Release. 2018;280:20–30. Copyright 2018, Elsevier.137 |
Stimuli-Responsive Release
Table 3 summarized different release methods and their applications about zein-based nanoparticles. The table shows that most of nanoparticles without stimuli-responsive release generally exhibit two-phase release, with the drug being initially released suddenly from within the shell or surface of the nanoparticles, and then gradually released from the drug wrapped inside the particles, which prolongs the blood circulation of the drug.145,146 The isoelectric point of zein is 6.8,92 s which results in different sustained release effects at physiological pH and tumor site pH. However, compared to stimuli-responsive nanoparticles, tumor specific release of normal zein-based nanoparticles is still insufficient. Numerous studies have shown that tumor sites have the characteristics of low pH and high GSH concentration. As shown in Table 3, nanoparticles with additional pH or GSH stimuli-response exhibit significant tumor-microenvironment release, which can reduce the toxicity and side effects of the drug and enhance the efficacy. Therefore, when designing carriers, we can make full use of the easy modification characteristics of zein to functionalize it and achieve precise drug release at tumor site.
 |
Table 3 Different Releasing Methods and Their Applications About Zein-Based Nanoparticles |
pH Sensitive
The tumor microenvironment is characterized by acidosis and hypoxia, which distinguishes it from normal tissues.147 Acidosis results from the production of acidic substances through the fermentation of high sugar during the growth and proliferation of tumor cells. Additionally, hypoxia at the tumor site exacerbates acidosis.148–150 The pH of normal tissues is usually between 7.3–7.4,151 whereas tumor tissues typically have a pH of 6.4–7.152 At the subcellular level, the pH of lysosomes within tumor cells can drop as low as 4.5–5.5.153,154 As a result, many studies are focusing on designing drug delivery systems that can respond specifically to the acidic environment of tumors, enabling targeted drug delivery.155–157
The formation of metal-ligand coordination bonds is well known to be sensitive to external pH conditions.158 Tannic acid (TA), a polyphenol polymer, has a strong metal chelating ability and an affinity for support materials, making it an excellent material for preparing metal-ligand complexes.159,160 Hongshan Liang161 used zein as a carrier to prepare nanoparticles coated with an acid-sensitive membrane formed by the coordination of tannic acid (TA) and metal ions, which simultaneously loaded DOX (Figure 7). The in vitro drug release curve showed that the nanoparticles only with zein as a carrier material released about 80% of DOX within about 8 h at pH 7.4, 6.2, 5.0, and 4.0, while DOX/zein-TA/CuII NPs exhibited inefficient and slow DOX release at pH 7.4 and 6.2. Only about 10% DOX was released within 8h and little further was released within 36h. In contrast, under the acidic conditions of pH 5.0 and pH 4.0, the release of DOX within 8 h was 25% and 55%, respectively. The results showed that the system was responsive to pH stimulation, and the carrier could effectively prevent the release of the loaded drug at pH simulating normal human tissues, but slowly release at the acidic pH simulating the tumor microenvironment. At the same time, the author also used an acid-sensitive film formed by the coordination of tannic acid (TA) and metal ions to coat Nobiletin (NOB)-loaded zein nanoparticles in another paper.162 The nano system also exhibited a lower drug release rate at pH 7.4.
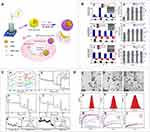 |
Figure 7 (A) Scheme: The preparation of DOX-loaded zein NPs coated by metal-TA films and the proposed model for pH-dependent drug release in tumor cells. (B) Influence of the pH on Particle size, PDI and zeta potential of pre-formed zein NPs (a), zein-TA/CuII NPs (b) and zein-TA/FeIII NPs (c). Particle size and PDI in culture media as a function of time: zein NPs(d), zein-TA/CuII NPs(e) and zein-TA/FeIII NPs (f). (C) Fourier transform infrared spectroscopy (FTIR) spectra of different samples (a). XPS survey spectra of zein(b); zein-TA/CuII NPs (c), (d) and (e); zein-TA/FeIII NPs (f), (g) and (h). (D) TEM image and size distribution of zein NPs(a) and (d), zein-TA/CuII NPs(b) and (e) and zein-TA/FeIII NPs (c) and (f). In vitro release profiles of DOX-loaded zein NPs (g), zein-TA/CuII NPs (h) and zein-TA/FeIII NPs (i) in PBS under different pH conditions. Reprinted with permission from Liang H, Zhou B, Li J, et al. Supramolecular design of coordination bonding architecture on zein nanoparticles for pH-responsive anticancer drug delivery. Colloids Surf B Biointerfaces. 2015;136:1224–1233. Copyright 2015, Elsevier.161 |
Polydopamine (PDA) is a hydrophilic biopolymer,163,164 and its monomeric dopamine hydrochloride can also spontaneously oxidize on various substrate surfaces, indicating good material adhesion. Meanwhile, PDA has good stability in a neutral environment, but is decomposed by protonation in the slightly acidic environment of the tumor site, indicating it has a good pH-responsive function.165,166 Liqiong Zha167 prepared PDA-coated gambogic acid-loaded zein nanoparticles, namely GNA@Zein-PDA NPs. In the in vitro pH-sensitive experiment, the free drug was completely released within 24 h, while both GNA@Zein-PDA NPs and GNA@Zein NPs showed good drug sustained-release properties at pH 7.4, respectively releasing within 72 h. 57% and 72%. However, at pH 6.86, GNA@Zein-PDA NPs showed better drug release ability than GNA@Zein NPs, about 97%, showing a certain pH-sensitive release property.
Hydroxyapatite, which has a composition similar to that of human bone, is a promising bone substitute that is degradable and pH-sensitive.168,169 Liqiong Zha170 prepared hydroxyapatite-coated DOX-loaded zein nanoparticles, namely HA/Zein-DOX NPs, by biomimetic mineralization. In the in vitro release experiments, the drug release rates of free DOX in the first 2 h were both about 92% at pH 7.4 and pH 6.86. The drug release from HA/Zein-DOX NPs showed a high pH dependence. At pH 7.4, only 27.2% of DOX were released from HA/Zein-DOX NPs within 2 h. While at pH 6.86, the release rate of HA/Zein-DOX NPs was as high as 91.8% within 2 h. Although Zein-DOX NPs also showed some acid-sensitive properties at pH 6.86, the drug release reached 59.2% at pH 7.4 within 2 h, indicating that its blocking effect on drugs is not as good as HA/Zein-DOX NPs in normal tissue pH environment. In tissue distribution experiments, HA/Zein-DOX NPs also effectively reduced the distribution of DOX in cardiac tissue and reduced the cardiotoxicity of DOX.
Furthermore, some researchers have designed a drug delivery system that achieves pH sensitivity and receptor targeting due to the highly expressed specific receptors on the surface of tumor cells and the slightly acidic environment of tumor tissues.171–173 Hongdi Wang174 used folic acid as the target and polydopamine as the acid-sensitive material to prepare zein nanoparticles loaded with gold nanoparticles and hydroxycamptothecin, namely HCPT@AuNPs-Zein-PFA. In the cell uptake experiment, the FA-functionalized nanoparticles showed better A549 cell uptake effect. In addition, the tumor targeting experiment and tissue distribution experiment also showed that the nanocomposite had good targeting. In the in vitro release experiments, under the conditions of physiological pH and endosome/lysosome pH, free HCPT nanocrystals released 95.4 ± 4.1% and 73.2 ± 4.2% of the drug within 24 h, respectively. By comparison, the percentage of HCPT release from HCPT@AuNPs-Zein-PFA NCs was found to be 58.4 ± 3.0% after 24h at endosomal/lysosomal pH (~ pH 5.0), which was 3.42-fold higher than the drug released in PBS buffer at physiological pH (pH 7.4). Compared with free HCPT and its non-targeted equivalent, HCPT@AuNPs-Zein-PFA exhibited better tumor-inhibiting ability and lower side effects both in vitro and in vivo.
GSH Sensitive
In addition to having a lower pH than normal tissues,175 the tumor microenvironment contains 100–1000-fold higher levels of reduced GSH than normal body fluids (including extracellular fluid and blood) in the cytoplasm and nucleus.176–178 Furthermore, due to its hypoxic nature, the GSH concentration in tumor tissue is at least 4 times higher than that of normal tissue.179 Therefore, researchers have developed nano-drug delivery systems that can effectively control drug release and respond to stimuli in the tumor microenvironment based on the characteristics of high reduced GSH concentration.180,181 One such system involves the use of disulfide bonds, which can be broken by redox reaction with GSH, to link drugs or carrier materials containing -NH2, -OH, or -COOH via amide or esterification reaction. These materials can form GSH-sensitive self-assembled nanomaterials particles.182,183 Zein, an amphiphilic protein with good chemical modification ability, can self-assemble into nanoparticles that prolong the circulation time of drugs in vivo by 7.2 times.92,184–186 Therefore, zein has great potential to prepare GSH-responsive prodrug nanoparticles. Heting Hou13 prepared a drug delivery system like that. The disulfide bonds were linked to zein and the lipid-soluble anticancer drug paclitaxel by esterification, respectively, and GSH-responsive prodrug nanoparticles (zein-S-S-PTX_NP) were formed by self-assembly (Figure 8). In the in vitro release experiments, zein-S-S-PTX_NP showed almost no release at pH 7.4 without GSH. On the contrary, when 10 mM GSH was added to the buffer, the nanoparticles released 80–90% PTX within 5 min and the release rate reached 95% after 2 h. The results showed that the nanoparticles can be released almost zero under normal body fluid conditions, but can be quickly released under the condition of tumor microenvironment, showing a good controlled release effect. In vitro cell experiments, zein-S-S-PTX_NP showed the same antitumor effect as pure PTX, and showed zero toxicity to NIH/3T3 fibroblasts. In vivo antitumor experiments, the tumor volumes of zein-S-S-PTX_NP, the control, pure PTX, and zein_PTX_NP groups were 50%, 262.6%, 211.7%, and 235.5% of the original size, respectively. The above results showed that zein-S-S-PTX_NP had obvious synergistic and detoxification effect.
 |
Figure 8 (A) Schematic diagram of: I. chemical synthesis and II. self-assembly using zein, disulfide, and PTX to form the zein-S-S-PTX_NP. (B) SEM images of (a) zein-S-S-PTX_NP and (b) zein_PTX_NP. (C) In vitro release of PTX from zein-S-S-PTX_NP and zein_PTX_NP (D) MTS assay of HeLa cell and NIH 3T3 cell (E) Relative tumor volume and body weight ratio: in vivo antitumor activity. The drugs were administrated on days 0, 3, 6, and 9 as indicated by the arrows. *P < 0.05; **P < 0.01; ***P< 0.001. Reprinted with permission from Hou H, Zhang D, Lin J, et al. Zein-Paclitaxel Prodrug Nanoparticles for Redox-Triggered Drug Delivery and Enhanced Therapeutic Efficiency. J Agric Food Chem. 2018;66(44):11,812–11,822. Copyright 2018, American Chemical Society.13 |
Magnetic Targeting
Another approach to achieve targeted therapy for tumors is through magnetic targeting. This involves adding magnetic nanoparticles to drug-loaded nanoparticles to direct their accumulation at tumor sites using an external magnetic field.187 Superparamagnetic iron oxide nanoparticles (SPIONs) are commonly used for this purpose as they are non-toxic and can be synthesized with well-defined dimensions. They are also the only magnetic nanomaterials approved by the US FDA for use in biomedicine.188 However, magnetic targeting alone may not be enough to improve drug uptake by tumor cells. Therefore, researchers often add aptamers to the surface of the carrier material to enhance drug uptake by tumor cells. For example, Kholod A. Elhasany189 used chondroitin sulfate, sulfapyridine, and zein to synthesize amphiphilic fragments that encapsulated SPIONs and celastrol (CST). In in vitro experiments, nanoparticles with chondroitin sulfate targets achieved superior cellular uptake over free RBITC. In the in vitro anti-tumor experiment, the tumor volume of the nanoparticle group without SPIONs increased by 78.24%, while the increase in tumor volume of the nanoparticle group with SPIONs was 36.26%, achieving effective inhibition of tumor proliferation. Jiafeng Pang190 encapsulated SPIONs and gefitinib (GEF) in folic acid-conjugated zein (Fa-zein) nanocomplexes (GEF-fszs). In vitro cell experiments showed that by adjusting the external magnetic field, the ability of the anti-proliferative and in vitro cellular uptake of GEF-fszs in A549 cells was higher than those of free GEF at the same concentration of GEF. Conjugating with FA further promoted the internalization of GEF-FSZs into A549 cells. In addition, Sally A. Sabra191 linked lactoferrin targeting tumor cell surface lactoferrin receptor with zein to form amphiphilic fragments through carbodiimide coupling reaction, and used it to encapsulate SPIONs and dasatinib, which also achieved good tumor targeting effect and tumor cell uptake ability.
PEG-Modified
In terms of the immunogenicity of zein, Hurtado López192 found an interesting phenomenon. Injection of zein microspheres intramuscularly into mice resulted in the production of anti-zein antibodies. Feng Li193 stated that parenteral administration of zein particles can lead to a long-term systemic immune response. Coupled with the strong hydrophobicity of zein, it is easily cleared by the body’s immune system (such as macrophages). PEGylation has been widely used to avoid macrophage uptake and immunogenicity of proteins and nanoparticles.194,195 The PEGylation of zein provides a hydrophilic surface, thus preventing macrophage uptake of zein to generate an immune response. Satheesh Podaralla196 found after subcutaneous injection of PEGylated zein nanoparticles, mice did not produce any anti-zein antibodies. And PEGylation reduced the clearance of zein nanoparticles by macrophages, prolonging the circulation of the drug in the body.
Gel Encapsulation
According to previous introductions, zein has the advantages of hydrophobicity, biocompatibility, and biodegradability, and the nanoparticles formed by it can better enhance the water solubility of drugs. However, objectively speaking, the water solubility of the outer layer, stability and drug release properties of the zein-based nanoparticles need to be further improved. The hydrogel is a three-dimensional soft substance in which polymer chains are cross-linked to form a network in a continuous liquid environment. Encapsulating the drug-loaded nanoparticles into the hydrogel can take advantage of the hydrophilicity of the hydrogel to reduce the possibility that the nanoparticles are cleared by macrophages in the blood circulation. At the same time, due to the porosity and swelling property of the hydrogel itself, it can further enhance the controlled release effect of nanoparticles on drugs.197 Priyanka Kaushik198 prepared Pectin hydrogel-encapsulated doxorubicin-loaded zein nanoparticles without the use of a cross-linking agent. The isoelectric point of zein is 6.2, and pectin is a polyanion, indicating attractive electrostatic interactions between the two biopolymers at pH=2.47. The experimental results showed that the preferential binding of pectin to DOX-loaded zein nanoparticles is indeed achieved through associative electrostatic interactions. At the same time, since the drug formulation exhibits a positive charge as a whole, while the surface of HeLa cells is negatively charged, the electrostatic interaction promotes the cellular uptake of the drug, which is beneficial to enhance the efficacy of the drug. In drug release experiments in vitro, DOX was added to ZP hydrogels, followed by diffusion and swelling controlled release. Under RT conditions, the release of DOX showed a burst first followed by a controlled release. The effectiveness of anticancer therapy depends on the release kinetics of the drug. The slow and sustained release provides prolonged and sustained anticancer therapy. Doxorubicin is an anti-cancerous drug which mediates mitochondrial-dependent apoptosis, and they observed a selective toxicity towards HeLa and not HEK293cells.
Preclinical and Clinical Tests
Based on the information obtained from “ClinicalTrials.gov”, there are currently no drugs that use zein as an anticancer-drug carrier on the market. However, studies have shown that empty zein nanoparticles can lower glucose levels in animals, and clinical trials have been conducted to evaluate the effect of zein nanoparticles on blood glucose control in early diabetes patients. As for the preclinical test, currently, the main focus is on the development of various anticancer drug-loaded zein-based carriers, only evaluating the basic physical and chemical properties, release profile, and therapeutic effects. To the best of our knowledge, a more detailed evaluation of the in vivo metabolism of zein-based carrier loaded with antitumor drugs has not been conducted. To establish a robust zein-based micro/nanomedicine, accumulation rate, release rate, drug metabolism, pharmacokinetic and pharmacodynamic evaluation, and treatment scheduling should be studied.5 Furthermore, the immunogenicity of zein-based carriers also needs to be further studied.
Conclusion
Zein, a plant-derived protein, is a promising and environmentally friendly material for drug delivery. Zein-based carriers have been explored for anticancer drugs through various administration routes, including intravenous injection, oral administration, transdermal absorption, intratumoral injection, and pulmonary inhalation. Zein has multiple methods for drug loading, such as hydrophobic interaction, chemical conjugation, deposition, and electrostatic interaction, but hydrophobic interaction has been more commonly studied for the insoluble small molecules in current anticancer drugs. Currently, nanoparticles loaded with antitumor drugs are the main type of zein-based carriers used for intravenous injection and oral administration. With the development of micro/nanotechnology, zein-based nanofibers, microneedles, and hydrogels have been explored for controlled release and more effective local treatment. Among the various administration routes, the oral route is particularly advantageous due to zein’s good hydrophobicity and resistance to the gastrointestinal environment. Moreover, functionalization of zein-based carriers is crucial in drug delivery, including enhancing targeting and stimulating responsive release. Zein has free amino and hydroxyl groups, which make it easily modifiable and have the potential to be developed as a functional drug carrier. However, clinical transformation of drug-loaded zein-based carriers is still challenging due to the lack of basic research.
Current Challenges and Suggestions
Firstly, the stability. The hydrophobicity of zein not only enhances the solubility of hydrophobic drugs but also decreases the stability of the preparation. Therefore, it is necessary to add stabilizers to form stable zein-based carriers. There are several methods to improve the stability of zein-based carriers. (I) Electrostatic interaction: we can cross-link anionic materials such as anionic polysaccharides with zein by electrostatic interaction to improve its stability. The effects of alginate and hyaluronic acid have been demonstrated.22,199 (II) Surfactants: surfactants like pluronic, tween, and lecithin can be attached to the surface of zein nanoparticles or cross-linked with zein to increase water solubility and stability. (III) Steric repulsions. McClements et al200 developed a series of caseinate-dextran Maillard conjugates to coat resveratrol-loaded ZNs. Caseinate could endow the resultant conjugates with adsorption capacity onto the surface of ZNs whereas the dextran part provided strong steric repulsion to reduce the aggregation of zein-based nanoparticles and make them more stable.
Secondly, the clinical application. Zein is an alcohol-soluble protein, which means its production process involves the use of alcohol. The removal of organic reagents is difficult, and their excessive residue can also be harmful to the human body. Therefore, it is important to strictly control the time and temperature of ethanol volatilization during the preparation of zein-based carriers to ensure their safety. In addition, compared with human serum albumin, which has been used in Abraxane, (I) Due to the poor water solubility of zein, it is easier to clear in the body, thus reducing the cycle time; (II) Zein is a plant protein, which means its xenogenicity may lead to unpredictable immunogenicity. For the former, encapsulating it into the water-soluble gel and the modification of PEG and various polysaccharide are expected to show better solubilization. Meanwhile, we can also prepare zein-based in situ gel or microneedle, which can also reduce its contact with macrophages. For the latter, in general, from literature, the most frequently reported side effect after injection of a nanotherapeutic agent seems to be immune-mediated side effect.19 So, it’s necessary to make the immunogenicity of zein-based carrier clear. However, at present, the research on immunogenicity of zein-based carrier is mostly confined to mice. We should expand the scope and depth of research to ensure clinical safety.
Lastly, manufacturing issues. Manufacturing of micro/nanomedicine products for commercialization is technically challenging. In large-scale production, due to the polydispersity of micro/nano materials, there will be quality differences between batches.16 In addition, their features (ie, size, shape, cargo loading level, surface property, etc.) also play vital roles in affecting affect traffic journey and bio-distribution of particles and particle-cell interactions.201 Therefore, for the further application of zein-based carrier, we should study how to improve the quality and efficiency of production. Compared with traditional manufacturing methods, we can utilize computer or mechanical-aided systems and resources, or automated material handling systems, to further improve above features. At present, photolithography, soft lithography, nano-imprint lithography, mechanical stretching, and microfluidic fabrication are available.
Data Sharing Statement
Data will be made available on request.
Acknowledgments
This research was funded by Key R&D projects of Sichuan Provincial Department of Science and Technology (No. 2022YFS0390), College Students’ Innovative Entrepreneurial Training Plan Program (No. S202110633042), and the Innovation and Technology Fund, Hong Kong (No. MRP/027/18X).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Kim M, Park SC, Lee DY. Glycyrrhizin as a nitric oxide regulator in cancer chemotherapy. Cancers. 2021;13(22):678. doi:10.3390/cancers13225762
3. Zhang B, Cheng P. Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy. Mol Cancer. 2020;19(1):158. doi:10.1186/s12943-020-01275-6
4. Chakraborty C, Sharma AR, Sharma G, Sarkar BK, Lee -S-S. The novel strategies for next-generation cancer treatment: miRNA combined with chemotherapeutic agents for the treatment of cancer. Oncotarget. 2018;9(11):10164–10174. doi:10.18632/oncotarget.24309
5. Souri M, Soltani M, Kashkooli FM, et al. Towards principled design of cancer nanomedicine to accelerate clinical translation. Materials Today Bio. 2022:13100208. doi:10.1016/j.mtbio.2022.100208
6. Zhang MM, Gao S, Yang DJ, et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharmaceutica Sinica B. 2021;11(8):2265–2285. doi:10.1016/j.apsb.2021.03.033
7. Souri M, Soltani M, Kashkooli FM, Shahvandi MK. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J Controlled Release. 2022;341:227–246. doi:10.1016/j.jconrel.2021.11.024
8. Sindhwani S, Syed AM, Ngai J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566. doi:10.1038/s41563-019-0566-2
9. Hameedat F, Pinto S, Marques J, Dias S, Sarmento B. Functionalized zein nanoparticles targeting neonatal Fc receptor to enhance lung absorption of peptides. Drug Deliv Transl Res. 2023;13(6):1699–1715. doi:10.1007/s13346-022-01286-4
10. Kim KS, Suzuki K, Cho H, Youn YS, Bae YH. Oral Nanoparticles Exhibit Specific High-Efficiency Intestinal Uptake and Lymphatic Transport. Acs Nano. 2018;12(9):8893–8900. doi:10.1021/acsnano.8b04315
11. Lang TQ, Dong XY, Huang Y, et al. Ly6C(hi) Monocytes Delivering pH-Sensitive Micelle Loading Paclitaxel Improve Targeting Therapy of Metastatic Breast Cancer. Adv Funct Mater. 2017;27(26):1701093. doi:10.1002/adfm.201701093
12. Zhang Q, Wang J, Liu D, et al. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr Polym. 2020:240116325. doi:10.1016/j.carbpol.2020.116325
13. Hou HT, Zhang D, Lin JW, et al. Zein-paclitaxel prodrug nanoparticles for redox-triggered drug delivery and enhanced therapeutic efficiency. J Agric Food Chem. 2018;66(44):11812–11822. doi:10.1021/acs.jafc.8b04627
14. Yu XL, Han N, Dong ZY, et al. Combined Chemo-Immuno-Photothermal Therapy for Effective Cancer Treatment via an All-in-One and One-for-All Nanoplatform. ACS Appl Mater Interfaces. 2022;14(38):42988–3009. doi:10.1021/acsami.2c12969
15. Liu GX, Yang LN, Chen G, et al. A review on drug delivery system for tumor therapy. Front Pharmacol. 2021:12735446. doi:10.3389/fphar.2021.735446
16. Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Controlled Release. 2015;200:138–157. doi:10.1016/j.jconrel.2014.12.030
17. He HL, Liu LS, Morin EE, Liu M, Schwendeman A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc Chem Res. 2019;52(9):2445–2461. doi:10.1021/acs.accounts.9b00228
18. Wang ZL. Progress and prospect in the clinical translation of cancer nanomedicine. Acta Pharmaceutica Sinica. 2022;57(1):134–141.
19. Brand W, Noorlander CW, Giannakou C, et al. Nanomedicinal products: a survey on specific toxicity and side effects. Int J Nanomedicine. 2017;12:6107–6129. doi:10.2147/ijn.S139687
20. Li H, Wang DF, Liu CZ, et al. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019;87:342–351. doi:10.1016/j.foodhyd.2018.08.002
21. Pauluk D, Padilha AK, Khalil NM, Mainardes RM. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019;94:411–417. doi:10.1016/j.foodhyd.2019.03.042
22. Zhang C, Wang X, Xiao M, et al. Nano-in-micro alginate/chitosan hydrogel via electrospray technology for orally curcumin delivery to effectively alleviate ulcerative colitis. Mater Des. 2022:221110894. doi:10.1016/j.matdes.2022.110894
23. Zhang C, Chen ZJ, He YN, et al. Oral colon-targeting core-shell microparticles loading curcumin for enhanced ulcerative colitis alleviating efficacy. Chin Med. 2021;16(1):92. doi:10.1186/s13020-021-00449-8
24. Fernandez-Carneado J, Kogan MJ, Castel S, Giralt E. Potential peptide carriers: amphipathic proline-rich peptides derived from the n-terminal domain of gamma-zein. Angewandte Chemie-Int Edition. 2004;43(14):1811–1814. doi:10.1002/anie.200352540
25. Zhang Y, Cui LL, Che XX, et al. Zein-based films and their usage for controlled delivery: origin, classes and current landscape. J Controlled Release. 2015;206:206–219. doi:10.1016/j.jconrel.2015.03.030
26. Labib G. Overview on zein protein: a promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin Drug Deliv. 2018;15(1):65–75. doi:10.1080/17425247.2017.1349752
27. Malekzad H, Mirshekari H, Zangabad PS, et al. Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems. Crit Rev Biotechnol. 2018;38(1):47–67. doi:10.1080/07388551.2017.1312267
28. Lee EH, Lee MK, Lim SJ. Enhanced Stability of Indocyanine Green by Encapsulation in Zein-Phosphatidylcholine Hybrid Nanoparticles for Use in the Phototherapy of Cancer. Pharmaceutics. 2021;13(3):305. doi:10.3390/pharmaceutics13030305
29. Acevedo F, Hermosilla J, Sanhueza C, et al. Gallic acid loaded PEO-core/zein-shell nanofibers for chemopreventive action on gallbladder cancer cells. Eur J Pharm Sci. 2018;119:49–61. doi:10.1016/j.ejps.2018.04.009
30. Bhatnagar S, Kumari P, Pattarabhiran SP, Venuganti VVK. Zein Microneedles for Localized Delivery of Chemotherapeutic Agents to Treat Breast Cancer: drug Loading, Release Behavior, and Skin Permeation Studies. Aaps Pharmscitech. 2018;19(4):1818–1826. doi:10.1208/s12249-018-1004-5
31. Cao XY, Geng JN, Su SW, et al. Doxorubicin-Loaded Zein in Situ Gel for Interstitial Chemotherapy. Chem Pharm Bull. 2012;60(10):1227–1233. doi:10.1248/cpb.c12-00270
32. Zhang YB, Yang GG, Hayat U, et al. Water-responsive 4D printing based on self-assembly of hydrophobic protein “Zein” for the control of degradation rate and drug release. Bioactive Mater. 2023;23:343–352. doi:10.1016/j.bioactmat.2022.11.009
33. Zhang YR, Lin R, Li HJ, He WL, Du JZ, Wang J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Int Rev. 2019;11(1):e1519. doi:10.1002/wnan.1519
34. Wang ZR, He Q, Zhao WG, Luo JW, Gao WP. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J Controlled Release. 2017;264:66–75. doi:10.1016/j.jconrel.2017.08.017
35. Zhang S, Cheng J, Quan CL, et al circCELSR1 (hsa(-)circ(-)0063809) Contributes to Paclitaxel Resistance of Ovarian Cancer Cells by Regulating FOXR2 Expression via miR-1252. Mol Therapy-Nucleic Acids. 2020;19:718–730. doi:10.1016/j.omtn.2019.12.005
36. Botella P, Rivero-Buceta E. Safe approaches for camptothecin delivery: structural analogues and nanomedicines. J Controlled Release. 2017;247:28–54. doi:10.1016/j.jconrel.2016.12.023
37. Enhancing Tumor DC. Cell Response to Multidrug Resistance with pH-Sensitive Quercetin and Doxorubicin Conjugated Multifunctional Nanoparticles. Colloids Surfaces B-Biointerfaces. 2017;156:175–185. doi:10.1016/j.colsurfb.2017.05.012
38. Misra R, Sarkar K, Lee J, et al. Radioluminescent nanoparticles for radiation-controlled release of drugs. J Controlled Release. 2019;303:237–252. doi:10.1016/j.jconrel.2019.04.033
39. Pei Q, Hu XL, Liu S, Li Y, Xie ZG, Jing XB. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Controlled Release. 2017;254:23–33. doi:10.1016/j.jconrel.2017.03.391
40. Dong FY, Dong XL, Zhou LP, et al. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: preparation, in vitro evaluation, and cellular uptake. Colloids Surfaces B-Biointerfaces. 2016;140:324–331. doi:10.1016/j.colsurfb.2015.12.048
41. Issell BF, Crooke ST. Maytansine. Cancer Treat Rev. 1978;5(4):199–207. doi:10.1016/s0305-7372(78)80014-0
42. Wishart DS, Knox C, Guo AC, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–D906. doi:10.1093/nar/gkm958
43. Kusari S, Kusari P, Eckelmann D, Zuhlke S, Kayser O, Spiteller M. Novel insights into plant-endophyte communication: maytansine as an example. Planta Med. 2016;82. doi:10.1055/s-0036-1596123
44. Junttila TT, Li GM, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347–356. doi:10.1007/s10549-010-1090-x
45. Yu XL, Wu HC, Hu HY, et al. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020;27(1):100–109. doi:10.1080/10717544.2019.1704942
46. Xing RR, Liu YM, Zou QL, Yan XH. Self-assembled injectable biomolecular hydrogels towards phototherapy. Nanoscale. 2019;11(46):22182–22195. doi:10.1039/c9nr06266a
47. Chen WR, Adams RL, Heaton S, Dickey DT, Bartels KE, Nordquist RE. Chromophore-Enhanced Laser-Tumor Tissue Photothermal Interaction Using An 808-Nm Diode-Laser. Cancer Lett. 1995;88(1):15–19. doi:10.1016/0304-3835(94)03609-m
48. Chen WR, Adams RL, Bartels KE, Nordquist RE. Chromophore-Enhanced In-Vivo Tumor-Cell Destruction Using An 808-Nm Diode-Laser. Cancer Lett. 1995;94(2):125–131. doi:10.1016/0304-3835(95)03837-m
49. Jori G, Schindl L, Schindl A, Polo L. Novel approaches towards a detailed control of the mechanism and efficiency of photosensitized processes in vivo. J Photochemistry Photobiology Chem. 1996;102(1):101–107. doi:10.1016/s1010-6030(96)04371-7
50. Jori G, Spikes JD. PHOTOTHERMAL SENSITIZERS - POSSIBLE USE IN TUMOR-THERAPY. J Photochem Photobiol B-Biol. 1990;6(1–2):93–101. doi:10.1016/1011-1344(90)85078-b
51. Kumar P, Srivastava R. IR 820 dye encapsulated in polycaprolactone glycol chitosan: poloxamer blend nanoparticles for photo immunotherapy for breast cancer. Materials Sci Eng C-Materials Biol Appl. 2015;57:321–327. doi:10.1016/j.msec.2015.08.006
52. Kumar P, Srivastava R. IR 820 stabilized multifunctional polycaprolactone glycol chitosan composite nanoparticles for cancer therapy. RSC Adv. 2015;5(69):56162–56170. doi:10.1039/c5ra05997f
53. Luo SL, Zhang EL, Su YP, Cheng TM, Shi CM. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32(29):7127–7138. doi:10.1016/j.biomaterials.2011.06.024
54. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10(9):507–518. doi:10.1038/nrclinonc.2013.123
55. Pansare VJ, Hejazi S, Faenza WJ. Review of Long-Wavelength Optical and NIR Imaging Materials: contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chem Materials. 2012;24(5):812–827. doi:10.1021/cm2028367
56. Noh YW, Park HS, Sung MH, Lim YT. Enhancement of the photostability and retention time of indocyanine green in sentinel lymph node mapping by anionic polyelectrolytes. Biomaterials. 2011;32(27):6551–6557. doi:10.1016/j.biomaterials.2011.05.039
57. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int j Angiol. 2013;22(3):149–154. doi:10.1055/s-0033-1348885
58. Reuveni T, Motiei M, Romman Z, Popovtzer A, Popovtzer R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine. 2011;6:2859–2864. doi:10.2147/ijn.S25446
59. Lopes J, Coelho JMP, Vieira PMC, Viana AS, Gaspar MM, Reis C. Preliminary Assays towards Melanoma Cells Using Phototherapy with Gold-Based Nanomaterials. Nanomaterials. 2020;10(8):1536. doi:10.3390/nano10081536
60. Huang XH, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–228. doi:10.1007/s10103-007-0470-x
61. Chen JH, Ma YC, Du W, et al. Furin-Instructed Intracellular Gold Nanoparticle Aggregation for Tumor Photothermal Therapy. Adv Funct Mater. 2020;30(50):2001566. doi:10.1002/adfm.202001566
62. Chauhan DS, Arunkumar P, Prasad R, et al. Facile synthesis of plasmonic zein nanoshells for imaging-guided photothermal cancer therapy. Materials Sci Eng C-Materials Biol Appl. 2018;90:539–548. doi:10.1016/j.msec.2018.04.081
63. Shrestha B, Wang LJ, Brey EM, Uribe GR, Tang L. Smart Nanoparticles for Chemo-Based Combinational Therapy. Pharmaceutics. 2021;13(6):853. doi:10.3390/pharmaceutics13060853
64. Friedmann T. Human gene therapy - An immature genie, but certainly out of the bottle. Nat Med. 1996;2(2):144–147. doi:10.1038/nm0296-144
65. Mady MM. Cationic liposomes as gene delivery system. Af J Pharm Pharmacol. 2011;5(17):2007–2012. doi:10.5897/ajpp11.331
66. Walther W, Schlag PM. Current status of gene therapy for cancer. Curr Opin Oncol. 2013;25(6):659–664. doi:10.1097/cco.0000000000000004
67. Rezvantalab S, Drude NI, Moraveji MK, et al. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol. 2018:91260. doi:10.3389/fphar.2018.01260
68. Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18(1):33–37. doi:10.1038/71889
69. Gao X, Kim KS, Liu DX. Nonviral gene delivery: what we know and what is next. Aaps J. 2007;9(1):E92–E104. doi:10.1208/aapsj0901009
70. Mishra B, Patel BB, Tiwari S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomed Nanotechnol Biol Med. 2010;6(1):9–24. doi:10.1016/j.nano.2009.04.008
71. Mali S. Delivery systems for gene therapy. Indian J Hum Genet. 2013;19(1):3–8. doi:10.4103/0971-6866.112870
72. Kafshdooz T, Kafshdooz L, Akbarzadeh A, Hanifehpour Y, Joo SW. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells, Nanomed Biotechnol. 2016;44(2):581–587. doi:10.3109/21691401.2014.971805
73. Regier MC, Taylor JD, Borcyk T, Yang YQ, Pannier AK. Fabrication and characterization of DNA-loaded zein nanospheres. J Nanobiotechnology. 2012;1044. doi:10.1186/1477-3155-10-44
74. Wang Q, Wang JF, Geil PH, Padua GW. Zein adsorption to hydrophilic and hydrophobic surfaces investigated by surface plasmon resonance. Biomacromolecules. 2004;5(4):1356–1361. doi:10.1021/bm049965r
75. El Sharkawi FZ, Ewais SM, Fahmy RH, Rashed LA. PTEN and TRAIL genes loaded zein nanoparticles as potential therapy for hepatocellular carcinoma. J Drug Target. 2017;25(6):513–522. doi:10.1080/1061186x.2017.1289536
76. Pourhossein A, Rafizadeh M, Chen P. Stimuli-responsive zein-based nanoparticles as a potential carrier for ellipticine: synthesis, release, and in vitro delivery. Polym Adv Technol. 2020;31(9):2007–2019. doi:10.1002/pat.4924
77. Lane LA. Physics in nanomedicine: phenomena governing the in vivo performance of nanoparticles. Appl Phys Rev. 2020;7(1):011316. doi:10.1063/1.5052455
78. Zein R, Sharrouf W, Selting K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J Oncol. 2020;20205194780. doi:10.1155/2020/5194780
79. Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi:10.1038/nnano.2012.45
80. Stylianopoulos T, Soteriou K, Fukumura D, Jain RK. Cationic Nanoparticles Have Superior Transvascular Flux into Solid Tumors: insights from a Mathematical Model. Ann Biomed Eng. 2013;41(1):68–77. doi:10.1007/s10439-012-0630-4
81. Fahmy UA, Ahmed OAA, El-moselhy MA, Asfour HZ, Alhakamy NA. Thymoquinone Loaded Zein Nanoparticles Improves the Cytotoxicity against Breast Cancer Cells. Int J Pharmacol. 2020;16(8):554–561. doi:10.3923/ijp.2020.554.561
82. Kutbi HI, Kammoun AK, El-Telbany DF. Amelioration of Pterostilbene Antiproliferative, Proapoptotic, and Oxidant Potentials in Human Breast Cancer MCF7 Cells Using Zein Nanocomposites. Int J Nanomedicine. 2021;16:3059–3071. doi:10.2147/ijn.S303975
83. Alhakamy NA, Ahmed OAA, Aldawsari HM, et al. Encapsulation of Lovastatin in Zein Nanoparticles Exhibits Enhanced Apoptotic Activity in HepG2 Cells. Int J Mol Sci. 2019;20(22):5788. doi:10.3390/ijms20225788
84. Shi QN, Wang XY, Tang XD, et al. In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci Nutrition. 2021;9(7):3530–3537. doi:10.1002/fsn3.2302
85. Radunz M, Hackbart HCD, Bona NP, et al. Glucosinolates and phenolic compounds rich broccoli extract: encapsulation by electrospraying and antitumor activity against glial tumor cells. Colloids Surfaces B-Biointerfaces. 2020:192111020. doi:10.1016/j.colsurfb.2020.111020
86. Thapa RK, Nguyen HT, Jeong JH, et al. Synergistic anticancer activity of combined histone deacetylase and proteasomal inhibitor-loaded zein nanoparticles in metastatic prostate cancers. Nanomed Nanotechnol Biol Med. 2017;13(3):885–896. doi:10.1016/j.nano.2016.12.010
87. Kamel NM, Helmy MW, Samaha MW, Ragab D, Elzoghby AO. Multicompartmental lipid-protein nanohybrids for combined tretinoin/herbal lung cancer therapy. Nanomedicine. 2019;14(18):2461. doi:10.2217/nnm-2019-0090
88. Liang HS, Huang QR, Zhou B, et al. Self-assembled zein-sodium carboxymethyl cellulose nanoparticles as an effective drug carrier and transporter. J Materials Chem B. 2015;3(16):3242–3253. doi:10.1039/c4tb01920b
89. El-Lakany SA, Elgindy NA, Helmy MW, Abu-Serie MM, Elzoghby AO. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin Drug Deliv. 2018;15(9):835–850. doi:10.1080/17425247.2018.1505858
90. Pang X, Yang XY, Zhai GX. Polymer-drug conjugates: recent progress on administration routes. Expert Opin Drug Deliv. 2014;11(7):1075–1086. doi:10.1517/17425247.2014.912779
91. Tanaka F, Fukuse T, Wada H, Fukushima M. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr Pharm Biotechnol. 2000;1(2):137–164. doi:10.2174/1389201003378979
92. Lai LF, Guo HX. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int J Pharm. 2011;404(1–2):317–323. doi:10.1016/j.ijpharm.2010.11.025
93. Alqahtani MS, Islam MS, Podaralla S, et al. Food Protein Based Core-Shell Nanocarriers for Oral Drug Delivery: effect of Shell Composition on in Vitro and in Vivo Functional Performance of Zein Nanocarriers. Mol Pharm. 2017;14(3):757–769. doi:10.1021/acs.molpharmaceut.6b01017
94. George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan - a review. J Controlled Release. 2006;114(1):1–14. doi:10.1016/j.jconrel.2006.04.017
95. Wang LJ, Hu YQ, Yin SW, Yang XQ, Lai FR, Wang SQ. Fabrication and Characterization of Antioxidant Pickering Emulsions Stabilized by Zein/Chitosan Complex Particles (ZCPs). J Agric Food Chem. 2015;63(9):2514–2524. doi:10.1021/jf505227a
96. Wang X, Gu H, Zhang H, et al. Oral Core-Shell Nanoparticles Embedded in Hydrogel Microspheres for the Efficient Site-Specific Delivery of Magnolol and Enhanced Antiulcerative Colitis Therapy. ACS Appl Mater Interfaces. 2021;13(29):33948–33961. doi:10.1021/acsami.1c09804
97. Shah BM, Palakurthi SS, Khare T, Khare S, Palakurthi S. Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. Int J Biol Macromol. 2020;165:722–737. doi:10.1016/j.ijbiomac.2020.09.214
98. Wang CY, Chen X, Nakamura Y, Yu CX, Qi H. Fucoxanthin activities motivate its nano/micro-encapsulation for food or nutraceutical application: a review. Food Funct. 2020;11(11):9338–9358. doi:10.1039/d0fo02176h
99. Wu AX, Chen CH, Lu J, et al. Preparation of Oral Core-Shell Zein Nanoparticles to Improve the Bioavailability of Glycyrrhizic Acid for the Treatment of Ulcerative Colitis. Biomacromolecules. 2022;23(1):210–225. doi:10.1021/acs.biomac.1c01233
100. Shinde P, Agraval H, Singh A, Yadav UCS, Kumar U. Synthesis of luteolin loaded zein nanoparticles for targeted cancer therapy improving bioavailability and efficacy. J Drug Deliv Sci Technol. 2019;52:369–378. doi:10.1016/j.jddst.2019.04.044
101. Elzoghby AO, El-Lakany SA, Helmy MW, Abu-Serie MM, Elgindy NA. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine. 2017;12(24):2785–2805. doi:10.2217/nnm-2017-0247
102. Baspinar Y, Ustundas M, Bayraktar O, Sezgin C. Curcumin and piperine loaded zein-chitosan nanoparticles: development and in-vitro characterisation. Saudi Pharmaceutical J. 2018;26(3):323–334. doi:10.1016/j.jsps.2018.01.010
103. Luo YC, Wang TTY, Teng Z, Chen P, Sun JH, Wang Q. Encapsulation of indole-3-carbinol and 3,3 ‘-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013;139(1–4):224–230. doi:10.1016/j.foodchem.2013.01.113
104. Shinde P, Agraval H, Srivastav AK, Yadav UCS, Kumar U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int J Pharm. 2020;588119795. doi:10.1016/j.ijpharm.2020.119795
105. Alhakamy NA, Caruso G, Al-Rabia MW, et al. Piceatannol-Loaded Bilosome-Stabilized Zein Protein Exhibits Enhanced Cytostatic and Apoptotic Activities in Lung Cancer Cells. Pharmaceutics. 2021;13(5):638. doi:10.3390/pharmaceutics13050638
106. Tran PHL, Duan W, Lee BJ, Tran TTD. Drug stabilization in the gastrointestinal tract and potential applications in the colonic delivery of oral zein-based formulations. Int J Pharm. 2019;569118614. doi:10.1016/j.ijpharm.2019.118614
107. Liu L, Yang SF, Chen F, Cheng KW. Polysaccharide-Zein Composite Nanoparticles for Enhancing Cellular Uptake and Oral Bioavailability of Curcumin: characterization, Anti-colorectal Cancer Effect, and Pharmacokinetics. Front Nutrition. 2022;9846282. doi:10.3389/fnut.2022.846282
108. Tabakoglu S, Kolbuk D, Sajkiewicz P. Multifluid electrospinning for multi-drug delivery systems: pros and cons, challenges, and future directions. Biomater Sci. 2022;11(1):37–61. doi:10.1039/d2bm01513g
109. Ignatious F, Sun LH, Lee CP, Baldoni J. Electrospun Nanofibers in Oral Drug Delivery. Pharm Res. 2010;27(4):576–588. doi:10.1007/s11095-010-0061-6
110. Ullah S, Hashmi M, Khan MQ, et al. Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv. 2019;9(1):268–277. doi:10.1039/c8ra09082c
111. Moradkhannejhad L, Abdouss M, Nikfarjam N, Mazinani S, Heydari V. Electrospinning of zein/propolis nanofibers; antimicrobial properties and morphology investigation. J Materials Sci Mater Med. 2018;29(11):165. doi:10.1007/s10856-018-6174-x
112. Szunerits S, Heat: BR. A Highly efficient Skin enhancer for Transdermal Drug Delivery. Front Bioengineering Biotechnol. 2018;615. doi:10.3389/fbioe.2018.00015
113. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi:10.1038/nbt.1504
114. Waghule T, Singhvi G, Dubey SK, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi:10.1016/j.biopha.2018.10.078
115. Wang C, Ye YQ, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16(4):2334–2340. doi:10.1021/acs.nanolett.5b05030
116. Ye YQ, Wang C, Zhang XD, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692. doi:10.1126/sciimmunol.aan5692
117. Bhatnagar S, Chawla SR, Kulkarni OP, Venuganti VVK. Zein Microneedles for Transcutaneous Vaccine Delivery: fabrication, Characterization, and in Vivo Evaluation Using Ovalbumin as the Model Antigen. Acs Omega. 2017;2(4):1321–1332. doi:10.1021/acsomega.7b00343
118. Qi ST, Qiu BH. 手术切除联合间质内缓释化疗治疗复发性脑胶质瘤 [Treatment of recurrent malignant brain gliomas by surgical excision combined with biodegradable polymers of interstitial chemotherapy]. Zhonghua zhong liu za zhi. 2004;26(1):58–61. Chinese.
119. Won YW, Kim JK, Cha MJ, Hwang KC, Choi D, Kim YH. Prolongation and enhancement of the anti-apoptotic effects of PTD-Hsp27 fusion proteins using an injectable thermo-reversible gel in a rat myocardial infarction model. J Controlled Release. 2010;144(2):181–189. doi:10.1016/j.jconrel.2010.02.014
120. Lee JS, Zhou W, Meng FH, Zhang DW, Otto C, Feijen J. Thermosensitive hydrogel-containing polymersomes for controlled drug delivery. J Controlled Release. 2010;146(3):400–408. doi:10.1016/j.jconrel.2010.06.002
121. Censi R, Fieten PJ, Di Martino P, Hennink WE, Vermonden T. In-situ forming hydrogels by simultaneous thermal gelling and Michael addition reaction between methacrylate bearing thermosensitive triblock copolymers and thiolated hyaluronan. J Controlled Release. 2010;148(1):E28–E29. doi:10.1016/j.jconrel.2010.07.039
122. Jin R, Dijkstra PJ, Feijen J. Rapid gelation of injectable hydrogels based on hyaluronic acid and poly(ethylene glycol) via Michael-type addition. J Controlled Release. 2010;148(1):E41–E43. doi:10.1016/j.jconrel.2010.07.049
123. Park MR, Chun CJ, Ahn SW, Ki MH, Cho CS, Song SC. Sustained delivery of human growth hormone using a polyelectrolyte complex-loaded thermosensitive polyphosphazene hydrogel. J Controlled Release. 2010;147(3):359–367. doi:10.1016/j.jconrel.2010.07.126
124. Al-Abd AM, Hong KY, Song SC, Kuh HJ. Pharmacokinetics of doxorubicin after intratumoral injection using a thermosensitive hydrogel in tumor-bearing mice. J Controlled Release. 2010;142(1):101–107. doi:10.1016/j.jconrel.2009.10.003
125. Tu JW, Wang HJ, Li HW, Dai KR, Wang JY, Zhang XL. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials. 2009;30(26):4369–4376. doi:10.1016/j.biomaterials.2009.04.054
126. Wang HJ, Gong SJ, Lin ZX, et al. In vivo biocompatibility and mechanical properties of porous zein scaffolds. Biomaterials. 2007;28(27):3952–3964. doi:10.1016/j.biomaterials.2007.05.017
127. Gong SJ, Wang HJ, Sun QS, Xue ST, Wang JY. Mechanical properties and in vitro biocompatibility of porous zein scaffolds. Biomaterials. 2006;27(20):3793–3799. doi:10.1016/j.biomaterials.2006.02.019
128. Dong J, Sun QS, Wang JY. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials. 2004;25(19):4691–4697. doi:10.1016/j.biomaterials.2003.10.084
129. Kopecka J, Campia I, Olivero P, et al. A LDL-masked liposomal-doxorubicin reverses drug resistance in human cancer cells. J Controlled Release. 2011;149(2):196–205. doi:10.1016/j.jconrel.2010.10.003
130. Yang W, Peters JI, Williams RO. Inhaled nanoparticles - A current review. Int J Pharm. 2008;356(1–2):239–247. doi:10.1016/j.ijpharm.2008.02.011
131. Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74. doi:10.1038/nrd2153
132. Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–570. doi:10.1016/j.tibtech.2007.09.005
133. Bailey MM, Berkland CJ. Nanoparticle Formulations in Pulmonary Drug Delivery. Med Res Rev. 2009;29(1):196–212. doi:10.1002/med.20140
134. Mansour HM, Rhee YS, Wu XA. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 2009;4:299–319. doi:10.2147/IJN.S4937
135. Kamel NM, Helmy MW, Abdelfattah EZ, et al. Inhalable Dual-Targeted Hybrid Lipid Nanocore-Protein Shell Composites for Combined Delivery of Genistein and All-Trans Retinoic Acid to Lung Cancer Cells. Acs Biomaterials Sci Eng. 2020;6(1):71–87. doi:10.1021/acsbiomaterials.8b01374
136. Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharmaceutics. 2015;93:52–79. doi:10.1016/j.ejpb.2015.03.018
137. Seok HY, Rejinold NS, Lekshmi KM, Cherukula K, Park IK, Kim YC. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: in vitro and in vivo evaluation. J Controlled Release. 2018;280:20–30. doi:10.1016/j.jconrel.2018.04.050
138. Gaber M, Elhasany KA, Sabra S, et al. Co-Administration of Tretinoin Enhances the Anti-Cancer Efficacy of Etoposide via Tumor-Targeted Green Nano-Micelles. Colloids Surfaces B-Biointerfaces. 2020:192110997. doi:10.1016/j.colsurfb.2020.110997
139. Lee HS, Kang NW, Kim H, et al. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr Polym. 2021:253117187. doi:10.1016/j.carbpol.2020.117187
140. Liu GJ, Pang JF, Huang YN, Xie QL, Guan GQ, Jiang YB. Self-Assembled Nanospheres of Folate-Decorated Zein for the Targeted Delivery of 10-Hydroxycamptothecin. Ind Eng Chem Res. 2017;56(30):8517–8527. doi:10.1021/acs.iecr.7b01632
141. Hou HT, Zhang D, Zeng J, et al. Bilayer Nanocarriers with Protein-Acid Conjugation for Prolonged Release and Enhanced Anticancer Effects. Langmuir. 2019;35(10):3710–3716. doi:10.1021/acs.langmuir.8b02882
142. Sabra SA, Sheweita SA, Haroun M, et al. Magnetically Guided Self-Assembled Protein Micelles for Enhanced Delivery of Dasatinib to Human Triple-Negative Breast Cancer Cells. J Pharm Sci. 2019;108(5):1713. doi:10.1016/j.xphs.2018.11.044
143. Sabra SA, Elzoghby AO, Sheweita SA, et al. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–169. doi:10.1016/j.ejpb.2018.04.023
144. El-Lakany SA, Elgindy NA, Helmy MW, Abu-Serie MM, Elzoghby AO. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin Drug Deliv. 2018;15(9):835. doi:10.1080/17425247.2018.1505858
145. Jain A, Sharma G, Kushwah V, et al. Beta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: a preclinical study for breast cancer. Artif Cells, Nanomed Biotechnol. 2018;46:S402–S412. doi:10.1080/21691401.2018.1428811
146. Sabra SA, Elzoghby AO, Sheweita SA, et al. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharmaceutics. 2018;128:156–169. doi:10.1016/j.ejpb.2018.04.023
147. Yang S, Gao HL. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108. doi:10.1016/j.phrs.2017.05.004
148. Romero-Garcia S, Lopez-Gonzalez JS, Baez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H. Tumor cell metabolism An integral view. Cancer Biol Ther. 2011;12(11):939–948. doi:10.4161/cbt.12.11.18140
149. Moellering RE, Black KC, Krishnamurty C, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008;25(4):411–425. doi:10.1007/s10585-008-9145-7
150. Martinez D, Vermeulen M, Trevani A, et al. Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol. 2006;176(2):1163–1171. doi:10.4049/jimmunol.176.2.1163
151. Boedtkjer E, Pedersen SF. The Acidic Tumor Microenvironment as a Driver of Cancer. In: Nelson MT, Walsh K, editors. Annual Review of Physiology. Vol. 82. 2020:103–126.
152. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65(1–2):271–284. doi:10.1016/s0168-3659(99)00248-5
153. Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. EXTRACELLULAR PH DISTRIBUTION IN HUMAN TUMORS. Int J Hyperthermia. 1995;11(2):211–216. doi:10.3109/02656739509022457
154. Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly (ethylene glycol)-b-poly(L-histidine). J Controlled Release. 2007;123(1):19–26. doi:10.1016/j.jconrel.2007.08.006
155. Xu JX, Fang Q, Yang LS, et al pH-sensitive deoxycholic acid dimer for improving doxorubicin delivery and antitumor activity in vivso. Colloids Surfaces B-Biointerfaces. 2020:196111319. doi:10.1016/j.colsurfb.2020.111319
156. Hou WX, Zhao X, Qian XQ, et al pH-Sensitive self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale. 2016;8(1):104–116. doi:10.1039/c5nr06842h
157. Zhang XY, Zhao MY, Cao N, et al. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater Sci. 2020;8(7):1885–1896. doi:10.1039/c9bm01927h
158. Zheng HQ, Gao CB, Peng BW, Shu MH, Che SN. pH-Responsive Drug Delivery System Based on Coordination Bonding in a Mesostructured Surfactant/Silica Hybrid. J Phys ChemC. 2011;115(15):7230–7237. doi:10.1021/jp110808f
159. Rahim MA, Ejima H, Cho KL, et al. Coordination-Driven Multistep Assembly of Metal-Polyphenol Films and Capsules. Chem Materials. 2014;26(4):1645–1653. doi:10.1021/cm403903m
160. Ye Q, Zhou F, Liu WM. Bioinspired catecholic chemistry for surface modification. Chem Soc Rev. 2011;40(7):4244–4258. doi:10.1039/c1cs15026j
161. Liang HS, Zhou B, Li J, et al. Supramolecular design of coordination bonding architecture on zein nanoparticles for pH-responsive anticancer drug delivery. Colloids Surfaces B-Biointerfaces. 2015;136:1224–1233. doi:10.1016/j.colsurfb.2015.09.037
162. Huang YN, Wu D, Bao MT, Li B, Liang HS. Coordination driven self-assembly for enhancing the biological stability of nobiletin. J Mol Liq. 2019;292111420. doi:10.1016/j.molliq.2019.111420
163. Coyne KJ, Qin XX, Waite JH. Extensible collagen in mussel byssus: a natural block copolymer. Science. 1997;277(5333):1830–1832. doi:10.1126/science.277.5333.1830
164. Waite JH, Qin XX. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry. 2001;40(9):2887–2893. doi:10.1021/bi002718x
165. Bernsmann F, Ball V, Addiego F, et al. Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir. 2011;27(6):2819–2825. doi:10.1021/la104981s
166. Chang DF, Gao YF, Wang LJ, et al. Polydopamine-based surface modification of mesoporous silica nanoparticles as pH-sensitive drug delivery vehicles for cancer therapy. J Colloid Interface Sci. 2016;463:279–287. doi:10.1016/j.jcis.2015.11.001
167. Zha LQ, Qian JJ, Wang BL, et al. In vitro/in vivo evaluation of pH-sensitive Gambogenic acid loaded Zein nanoparticles with polydopamine coating. Int J Pharm. 2020:587119665. doi:10.1016/j.ijpharm.2020.119665
168. Yang YH, Liu CH, Liang YH, Lin FH, Wu KCW. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J Materials Chem B. 2013;1(19):2447–2450. doi:10.1039/c3tb20365d
169. Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomaterialia. 2011;7(7):2769–2781. doi:10.1016/j.actbio.2011.03.019
170. Zha LQ, Wang BL, Qian JJ, et al. Preparation, characterization and preliminary pharmacokinetic study of pH-sensitive Hydroxyapatite/Zein nano-drug delivery system for doxorubicin hydrochloride. J Pharm Pharmacol. 2020;72(4):496–506. doi:10.1111/jphp.13223
171. Huang GL, Huang HL. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J Controlled Release. 2018;278:122–126. doi:10.1016/j.jconrel.2018.04.015
172. Ryu TK, Baek SW, Lee GJ, Rhee CK, Choi SW. Targeted Tumor Therapy Based on Nanodiamonds Decorated with Doxorubicin and Folic Acid. Macromol Biosci. 2017;17(2):1600180. doi:10.1002/mabi.201600180
173. Kolosenko I, Avnet S, Baldini N, Viklund J, De Milito A. Therapeutic implications of tumor interstitial acidification. Semin Cancer Biol. 2017;43:119–133. doi:10.1016/j.semcancer.2017.01.008
174. Wang HD, Zhu W, Huang YN, Li ZX, Jiang YB, Xie QL. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomaterialia. 2017;61:88–100. doi:10.1016/j.actbio.2017.04.017
175. Jin HJ, Wang N, Wang C, Qin WX. MicroRNAs in hypoxia and acidic tumor microenvironment. Chine Sci Bulletin. 2014;59(19):2223–2231. doi:10.1007/s11434-014-0273-y
176. Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55(2):199–215. doi:10.1016/s0169-409x(02)00179-5
177. Meng FH, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–2198. doi:10.1016/j.biomaterials.2009.01.026
178. Cheng R, Feng F, Meng FH, Deng C, Feijen J, Zhong ZY. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Controlled Release. 2011;152(1):2–12. doi:10.1016/j.jconrel.2011.01.030
179. Kuppusamy P, Li HQ, Ilangovan G, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62(1):307–312.
180. Liu P, Zhang RN. Polymer microspheres with high drug-loading capacity via dual-modal drug-loading for modulating controlled release property in pH/reduction dual-responsive tumor-specific intracellular triggered doxorubicin release. Coll Surfaces Phys Eng Aspects. 2019;577:291–295. doi:10.1016/j.colsurfa.2019.05.089
181. Wang LJ, Du JS, Han X, Dou J, Shen J, Yuan J. Self-crosslinked keratin nanoparticles for pH and GSH dual responsive drug carriers. J Biomaterials Sci Polymer Edition. 2020;31(15):1994–2006. doi:10.1080/09205063.2020.1788371
182. Ling X, Tu JS, Wang JQ, et al. Glutathione-Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. Acs Nano. 2019;13(1):357–370. doi:10.1021/acsnano.8b06400
183. Cao YW, Gao M, Chen C, et al. Triggered-release polymeric conjugate micelles for on-demand intracellular drug delivery. Nanotechnology. 2015;26(11):115101. doi:10.1088/0957-4484/26/11/115101
184. Zhang W, Sangtong V, Peterson J, Scott MP, Messing J. Divergent properties of prolamins in wheat and maize. Planta. 2013;237(6):1465–1473. doi:10.1007/s00425-013-1857-5
185. Wang Y, Padua GW. Formation of Zein Microphases in Ethanol-Water. Langmuir. 2010;26(15):12897–12901. doi:10.1021/la101688v
186. Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi:10.1126/science.1074972
187. Polyak B, Friedman G. Magnetic targeting for site-specific drug delivery: applications and clinical potential. Expert Opin Drug Deliv. 2009;6(1):53–70. doi:10.1517/17425240802662795
188. Xie J, Huang J, Li X, Sun S, Chen X. Iron Oxide Nanoparticle Platform for Biomedical Applications. Curr Med Chem. 2009;16(10):1278–1294. doi:10.2174/092986709787846604
189. Elhasany KA, Khattab SN, Bekhit AA, et al. Combination of magnetic targeting with synergistic inhibition of NF-kappa B and glutathione via micellar drug nanomedicine enhances its anti-tumor efficacy. Eur J Pharm Biopharmaceutics. 2020;155:162–176. doi:10.1016/j.ejpb.2020.08.004
190. Pang JF, Li ZX, Li SM, et al. Folate-conjugated zein/Fe3O4 nanocomplexes for the enhancement of cellular uptake and cytotoxicity of gefitinib. J Mater Sci. 2018;53(21):14907–14921. doi:10.1007/s10853-018-2684-7
191. Sabra SA, Sheweita SA, Haroun M, et al. Magnetically Guided Self-Assembled Protein Micelles for Enhanced Delivery of Dasatinib to Human Triple-Negative Breast Cancer Cells. J Pharm Sci. 2019;108(5):1713–1725. doi:10.1016/j.xphs.2018.11.044
192. Hurtado-Lopez P, Murdan S. An investigation into the adjuvanticity and immunogenicity of zein microspheres being researched as drug and vaccine carriers. J Pharm Pharmacol. 2006;58(6):769–774. doi:10.1211/jpp.58.6.0007
193. Li F, Chen Y, Liu SB, et al. The Effect of Size, Dose, and Administration Route on Zein Nanoparticle Immunogenicity in BALB/c Mice. Int J Nanomedicine. 2019;14:9917–9928. doi:10.2147/ijn.S226466
194. Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi:10.1016/j.ijpharm.2005.10.010
195. Veronese FM, Mero A. The impact of PEGylation on biological therapies. Biodrugs. 2008;22(5):315–329. doi:10.2165/00063030-200822050-00004
196. Podaralla S, Averineni R, Alqahtani M, Perumal O. Synthesis of Novel Biodegradable Methoxy Poly(ethylene glycol)-Zein Micelles for Effective Delivery of Curcumin. Mol Pharm. 2012;9(9):2778–2786. doi:10.1021/mp2006455
197. Wang WW, Song HJ, Zhang J, et al. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J Controlled Release. 2015;203:57–66. doi:10.1016/j.jconrel.2015.02.015
198. Kaushik P, Priyadarshini E, Rawat K, Rajamani P, Bohidar HB. pH responsive doxorubucin loaded zein nanoparticle crosslinked pectin hydrogel as effective site-specific anticancer substrates. Int J Biol Macromol. 2020;152:1027–1037. doi:10.1016/j.ijbiomac.2019.10.190
199. Chen YF, Peng JC, Wang YQ, et al. Development, Characterization, Stability and Bioaccessibility Improvement of 7,8-Dihydroxyflavone Loaded Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles: comparison of Sodium Alginate and Sodium Carboxymethyl Cellulose. Foods. 2021;10(11):2629. doi:10.3390/foods10112629
200. Davidov-Pardo G, Joye IJ, Espinal-Ruiz M, McClements DJ. Effect of Maillard Conjugates on the Physical Stability of Zein Nanoparticles Prepared by Liquid Antisolvent Coprecipitation. J Agric Food Chem. 2015;63(38):8510–8518. doi:10.1021/acs.jafc.5b02699
201. Zhang PP, Xia JF, Luo SD. Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery. Materials. 2018;11(4):623. doi:10.3390/ma11040623
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
