Back to Journals » International Journal of Nanomedicine » Volume 18
Advances and Prospects in the Treatment of Pancreatic Cancer
Received 21 March 2023
Accepted for publication 11 July 2023
Published 19 July 2023 Volume 2023:18 Pages 3973—3988
DOI https://doi.org/10.2147/IJN.S413496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Huaiyu Duan,1,* Li Li,2,* Shiming He1
1School of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei, People’s Republic of China; 2Department of Hepatobiliary Pancreatic Oncology, Hefei Cancer Hospital, Chinese Academy of Sciences, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shiming He, Email [email protected]
Abstract: Pancreatic cancer is a highly malignant and incurable disease, characterized by its aggressive nature and high fatality rate. The most common type is pancreatic ductal adenocarcinoma (PDAC), which has poor prognosis and high mortality rate. Current treatments for pancreatic cancer mainly encompass surgery, chemotherapy, radiotherapy, targeted therapy, and combination regimens. However, despite efforts to improve prognosis, and the 5-year survival rate for pancreatic cancer remains very low. Therefore, it’s urgent to explore novel therapeutic approaches. With the rapid development of therapeutic strategies in recent years, new ideas have been provided for treating pancreatic cancer. This review expositions the advancements in nano drug delivery system, molecular targeted drugs, and photo-thermal treatment combined with nanotechnology for pancreatic cancer. It comprehensively analyzes the prospects of combined drug delivery strategies for treating pancreatic cancer, aiming at a deeper understanding of the existing drugs and therapeutic approaches, promoting the development of new therapeutic drugs, and attempting to enhance the therapeutic effect for patients with this disease.
Keywords: pancreatic cancer, nano drug delivery system, molecular targeted drugs, photo-thermal treatment
Introduction
Pancreatic cancer is a type of digestive system tumor, the incidence of which has been increasing in recent years with a 5-year survival rate close to 10%.1–3 Generally, obesity and diabetes were known as risk factors for this disease, and newly developed or worsened diabetes may be indicative of PDAC. In addition, genetic changes also played a significant role in the development of PDAC.4,5 At present, surgery remains the primary treatment for pancreatic cancer. For pancreatic cancer patients who were not eligible for surgical resection, chemotherapy, radiotherapy, and immunotherapy may be considered as treatment options. However, their efficacy was not very satisfactory because of drug resistance.6–8 Therefore, it’s pressing to develop novel and more targeted therapies.
With the advancement of modern science and technology, nanoparticles possessed several advantageous characteristics including a large specific surface area, adjustable pore size, high drug loading capacity, excellent biocompatibility, and targeted delivery to tumor tissues with high efficiency.9,10 The co-delivery of gemcitabine and cisplatin via nanoparticles could exert a synergistic effect on PDAC.11 The matrix of PDAC comprises the extracellular matrix, vascular system, and tumor-associated fibroblasts, which can form into a dense tumor mesenchyme that impedes the drug delivery.12 Nano-drug delivery systems have the potential to overcome the tumor interstitial barrier to achieve targeted drug delivery.13
The most frequently overexpressed genes in pancreatic cancer, such as KRAS, CDKN2A, TP53, and SMAD4, which have been identified as potential targets for molecular targeted therapy.14 In recent years, molecular targeted therapy for pancreatic cancer has been rapidly developed, including epidermal growth factor receptor inhibitors, and anti-EGFR antibodies, which can block the activation of downstream tyrosine kinase phosphorylation and subsequently inhibit the proliferation of tumor cells. However, these drugs may generate resistance.15,16 Pancreatic stellate cells were closely associated with drug-resistance of chemotherapy, which could promote the growth, invasion, and metastasis of tumors.17 The precise release of payloads could be achieved through coupling antibodies with nanoparticles.18 The combination of nanotechnology and molecular targeting holds great promise for curing pancreatic cancer in the future.
Photothermal conversion agents or photosensitizers were used in photothermal therapy to absorb energy and generate heat under near-infrared light irradiation of a certain wavelength, thus efficiently eradicating tumor cells. The combination of phototherapy and chemotherapy and phototherapy and gene therapy based on non-viral nanocarriers may be a potential treatment for PDAC.19,20 In spite of the dense tumor interstitium in pancreatic tumor tissue that may obstruct the light irradiation and drug delivery, the application of nano-drug delivery technology assisted with near-infrared light can enhance interstitial penetration and improve drug delivery.21,22 As photothermal receptors, gold nanomaterials showed unique physical properties that enable them to strongly absorb near-infrared light, thus enhancing the cell permeability and inhibiting the growth of tumor cells significantly.23 In plasma photothermal therapy, double peptide-labeled gold nanorods dramatically increase the uptake of pancreatic ductal gland tumor cells and induce the death of pancreatic cancer cells selectively under near-infrared irradiation.24 In brief, photothermal therapy provided a new therapeutic strategy for pancreatic cancer patients.
Although there existed many treatments for pancreatic cancer nowadays, huge challenges were inevitable in curing the tumor. Therefore, it’s imperative to develop innovative and effective therapies for this disease. The purpose of this review was to summarize recent advancements in nano-drug delivery systems, molecular targeted therapy, photothermal therapy, and combination drug administration strategies with the hope of providing novel approaches for treating pancreatic cancer.
The Nano Drug Delivery System
Nano drug delivery system for pancreatic cancer could mitigate side effects and improve therapeutic efficacy, with the size and unique surface properties of nanoparticles playing a pivotal role in regulating drug release (Figure 1).25 Nanoscale drug delivery systems such as liposomes have been widely used in the treatment of pancreatic cancer. Recently, Raza et al systematically reviewed the latest research progress in the diagnosis and treatment of pancreatic cancer based on liposomes, providing a therapeutic direction for the treatment of pancreatic cancer.26 In this section, we reviewed mesoporous silica, Poly (lactic-co-glycolic acid), albumin, natural polymers, and exosome-mediated nano-drug delivery systems.
Mesoporous Silica Nanoparticles
Mesoporous silica nanoparticles (MSNs) have been approved by FDA and attracted wide attention in mediating the drug delivery. They were characterized by a large surface area, high drug loading, and the ability to control the release of bioactive substances. Moreover, the targeting ability of functional groups and ligands might be improved if they were modified.27–29 When using folic acid-modified mesoporous silica nanoparticles, specific targeting to the folic acid receptors on tumor cells could improve the therapeutic effect.30,31 In an in situ K-Ras-dependent pancreatic cancer model, delivery of irinotecan via MSNs elicited a more potent anti-tumor immune response compared to single agent irinotecan or the liposomal formulation Onivyde. Furthermore, the concomitant administration of anti-PD-1 could significantly enhance the survival rate of patients with pancreatic cancer.32 ADAM9 was a protease highly expressed in PDAC. A mesoporous silica-modified ADAM9-mediated drug delivery system might effectively target the delivery of paclitaxel, significantly reduce toxic reactions, and improve therapeutic efficacy.33 The sonic hedgehog pathway (SHh) played a crucial role in mediating the crosstalk between cancer cells and stroma. Tarannum et al developed two versions of MSN-based platforms: Cyclopamine mesoporous silica nanoparticles (CyP-MSNs) which were rich in SHh inhibitors, and PEG-Gem-cisPt-MSNs, composed of chemotherapy drugs gemcitabine and cisplatin, that could reduce tumor matrix and ameliorate the curative effect of PDAC.34
As a promising nano-drug delivery platform, MSNs facilitated drug accumulation in tumor cells while reducing the toxic side effects.35 Therefore, the MSNs-mediated nano-drug delivery system represented a highly promising strategy for the treatment of pancreatic cancer.
The Poly (Lactic-Co-Glycolic Acid) Nanoparticles
Poly (lactic-co-glycolic acid) (PLGA) has been approved by the FDA due to its excellent biodegradability, biocompatibility, surface modification capabilities, and controlled release properties.36 When designed as specific targeting molecules, PLGA nanoparticles could encapsulate chemotherapy drugs that specifically target tumor cells for precise drug delivery.37,38 Curcumin was a natural chemical component extracted from the ginger plant, possessing anti-inflammatory and anti-tumor properties.39,40 In-vivo and in-vitro experiments demonstrated that constructing chitosan and polyethylene glycol (PEG) coated PLGA nanoparticles enriched with curcumin might promote apoptosis of pancreatic cancer cells while enhancing cellular uptake when compared to single curcumin.41 In the in situ and ectopic tumor models, PEG-PLGA nanoparticles coated with neutrophilic cell membranes exhibited specific inhibition of the NF-κB signaling pathway, induction of apoptosis in pancreatic cancer cells, and prolonged the survival rates for tumor-bearing mice.42 These biocompatible PLGA NPs with controlled-release properties offered a novel treatment option for pancreatic cancer.
Albumin Nanoparticles
Albumin was classified into human serum albumin and bovine serum albumin, both of which owned the characteristics of non-toxicity, non-immunogenicity, facile preparation, excellent biocompatibility, and active targeting.
Albumin nanoparticles were utilized for the targeted therapy of PDAC by delivering pre-drug β-lap, nab-(pro-β-lap), with experimental results demonstrating its high safety and anti-tumor efficacy.43 Albendazole was encapsulated in 100 nm-diameter nanoparticles which were conjugated with bovine serum albumin and polycaprolactone (PCL). Experiments showed that 100 nm BSA-PCL nanoparticles could accurately and effectively deliver albendazole to pancreatic multicellular tumor spheroids, which revealed the potential application prospect of BSA-PCL nanoparticles as targeted delivery vectors for albendazole in treating pancreatic cancer.44 To inhibit the tumor microenvironment, hydrophobic celastrol and hydrophilic 1-methyltryptophan were encapsulated in cationic albumin nanoparticles coated with hyaluronic acid. Experiments indicated that these nanoparticles could penetrate into the tumor tissue of mouse xenograft models, accumulate within the tumors, and gradually strengthen to significantly inhibit tumor growth in nude mice.45 Additionally, small-sized albumin nanoparticles containing immune checkpoint inhibitors were encapsulated in size-adjustable thermal fibrosis and fibrosis matrix-sensitive liposomes (HSA-BMS@CAP-ILTSL). The two-pronged treatment significantly enhanced the immunotherapy effect of pancreatic cancer.46 Therefore, the utilization of albumin-based nanoparticles presented a highly promising approach for targeted drug delivery.47,48
Natural Polymers
The application of nanoparticles prepared from natural polymers in the treatment of pancreatic cancer has garnered extensive attention. Marine-derived polymers have emerged as a viable alternative to certain inorganic materials, offering enhanced safety profiles. Fucoidan was a naturally active polysaccharide extracted from brown algae, which exhibited pharmacological effects such as antibacterial, antiviral, and anticancer activities, which could directly inhibit the signal pathway related to cell proliferation, induce cell apoptosis, and suppress cell migration and invasion. Moreover, it had a strong ability to kill pancreatic cancer cells.49,50 To enhance the anti-pancreatic cancer activity of the polymer, a novel fucoidan nanoparticle solution was prepared from a novel fucoidan polymer, which dramatically inhibited the proliferation, migration, and invasion of pancreatic cancer cells.51 Quinacrine was currently used as an anticancer agent.52 Research results showed that fucoidan and lactoferrin could serve as active targeting ligands to fabricate double-targeted nanoparticles containing quinacrine, resulting in a 5.7-fold increase in anti-cancer activity compared to the single-drug approach.53
Lignin was a natural polymer with excellent biodegradability and biocompatibility.54 In the PANC-1 cell line, quinacrine was loaded into lignosulfonate nanoparticles coated with lactoferrin and hyaluronic acid, which restrained the migration and invasion of pancreatic cancer cells while exhibiting no toxicity towards major organs.55
Exosomes
Exosomes were cell-derived nano-vesicles with a diameter ranging from 30 to 150 nm. Due to their good biocompatibility, stability, low immunogenicity, and other characteristics, exosomes have been widely used as nanocarrier platforms for delivering chemotherapy drugs and nucleic acids. As a form of intercellular communication, exosomes can transport molecular substances into target cells and activate signaling pathways.56–59 Exosomes derived from various sources have been widely utilized as drug-delivery vehicles in the treatment of pancreatic cancer (Table 1).
 |
Table 1 Application of Exosome Delivery Drug in the Treatment for Pancreatic Cancer |
Molecular Targeted Therapy
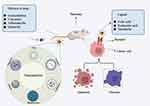
Molecular targeted therapy for pancreatic cancer was mainly carried out based on targeting epidermal growth factor receptor, human epidermal growth factor receptor 2, trophoblast cell-surface antigen 2, and vascular endothelial growth factor. Figure 2 was a schematic diagram of the related pathways involved in molecular targeting strategies for treating tumors.66,67 This section discussed the latest research progress of molecular targeting therapy for pancreatic cancer.
Epidermal Growth Factor Receptor
Epidermal growth factor receptor (EGFR) belongs to the ERBB family of cell surface receptor tyrosine kinases.68 It has the ligand-specific extracellular domain, the transmembrane domain, and an intracellular domain with tyrosine kinase activity. Upon binding to its specific ligand EGF, EGFR can form into homologous or heterodimeric complexes that activate the receptor’s tyrosine kinase through autophosphorylation and trigger the activation of downstream signaling pathways.69,70 Currently, therapies targeting EGFR, such as small-molecule tyrosine kinase inhibitors and monoclonal antibodies, have been utilized in the treatment of pancreatic cancer.71
Small molecule tyrosine kinase inhibitors, such as erlotinib and gefitinib, can selectively bind to overexpressed proteins in tumor cells. They can specifically target the intracellular protein structural domain of EGFR dimer, disrupting adenosine triphosphate (ATP) binding and further inhibiting the phosphorylation of downstream tyrosine kinase. Ultimately, this prevents the growth of tumor cells.72,73 Erlotinib is the sole small-molecule targeted therapy inhibitor approved for treating PDAC. The clinical trials illustrated that the use of erlotinib alone as a monotherapy or in combination with gemcitabine yielded unsatisfactory results.74 Dacomitinib, an irreversible tyrosine kinase inhibitor with a longer half-life and superior bioavailability than erlotinib, has been shown to inhibit the proliferation of PDAC cells and induce apoptosis by suppressing the expression of FOXM1, aurora kinase B and cyclin B1.75,76 Canertinib is another irreversible EGFR inhibitor, which not only inhibits tyrosine phosphorylation but also enhances ubiquitination, and ultimately reduces the proliferation, survival, and migration of pancreatic cancer cells by affecting EGFR family proteins and thereby down-regulating MUC4 mucins.77
Monoclonal antibodies, such as cetuximab, prevent the formation of dimer and inhibit tyrosine kinase activation, ultimately suppressing tumor cell growth.78 In the K-Ras-mutated pancreatic cancer, cetuximab was conjugated with MMAE to form an antibody conjugated (CTX-MMAE), which specifically targeted EGFR and inhibited tumor growth.79 As shown in Table 2, cetuximab-based treatment for pancreatic cancer has been widely used.
 |
Table 2 The Strategy Based on Cetuximab for Pancreatic Cancer |
Human Epidermal Growth Factor Receptor 2
Human epidermal growth factor receptor 2 (HER2), also known as ERBB-2, has been highly expressed in pancreatic cancer, which is a ligand-independent receptor tyrosine kinase encoded by proto-oncogenes that triggers a cascade reaction to promote cell proliferation, survival, and migration.85 Although the combination of HER2 monoclonal antibody trastuzumab and capecitabine has shown promising drug resistance in Phase II clinical trials for pancreatic cancer, it has not yielded satisfactory results in terms of the progression-free survival(PFS) and overall survival(OS).86 In metastatic pancreatic cancer, the efficacy of cetuximab combined with trastuzumab is superior to that of trastuzumab combined with erlotinib.87 However, the triple therapy consisting of trastuzumab, gemcitabine, and erlotinib has demonstrated safety and efficacy in terms of PFS and OS.88 The combination of trastuzumab with other inhibitors has presented a promising prospect for the treatment of pancreatic cancer.
Trophoblast Cell-Surface Antigen 2
Trophoblast cell-surface antigen 2 (TROP2), a transmembrane glycoprotein, is overexpressed in pancreatic cancer and plays a crucial role in the proliferation, migration, and invasion of tumor cells through modulating multiple signaling pathways.89,90 Antibody-drug conjugates represented a novel antitumor agent that targeted cancer cells precisely by covalent linkage of cytotoxic drugs to monoclonal antibodies.91 Sacituzumab govitecan is a monoclonal antibody-drug conjugate that specifically targets TROP2. It consists of a human anti-trop2 monoclonal antibody and SN-38, an active metabolite of irinotecan. IMMU-132 has a significant inhibitory effect on TROP2-overexpressed tumors.92 In another study, TROP2 Fab-DOX was conjugated with doxorubicin and anti-TROP2 Fab antibody, which exhibit potent antitumor activity against pancreatic cancer in vitro and in vivo.93 hIMB1636 is a humanized monoclonal antibody targeting TROP2 with high affinity. In a pancreatic cancer model, 64Cu/177Lu-labeled hIMB1636 demonstrated strong antitumor activity against TROP2-overexpressing tumors.94
Vascular Endothelial Growth Factor
The vascular endothelial growth factor (VEGF) family consists of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and other members, which can bind to the tyrosine kinase receptor VEGFR.95 The overexpression of VEGF-C in cancer is associated with lymphatic vessel invasion and metastasis, which can be detected in 80% of advanced PDAC. A novel circRNA (circNFIB1) may down-regulate the expression of VEGF-C by inhibiting PI3K/Akt pathway; consequently, it inhibits the formation and metastasis of PDAC lymphatic vessels.96–99 Bevacizumab, a monoclonal antibody targeting VEGF, has been widely utilized in a variety of tumors. In the Phase III clinical trial of patients with advanced pancreatic cancer treated with the traditional drug gemcitabine, no significant improvement was observed in median PFS. However, when gemcitabine was combined with the double-targeted drugs cetuximab and bevacizumab, the median PFS was significantly improved. On the contrary, the incidence of adverse reactions also increased.100–102 Cediranib, a pan-VEGF receptor inhibitor, effectively suppresses the migration and invasion of PDAC cells by downregulating EMT markers ZEB1, N-cadherin, and Snail. Moreover, it synergistically enhances the growth inhibition and apoptosis of PDAC cells when combined with gemcitabine and paclitaxel.103
Other
Glycogen synthase kinase 3β (GSK3β) is a highly conserved serine/threonine kinase that regulates cell cycle progression and signal transduction, and its overexpression is closely related to the occurrence and drug resistance of PDAC.104,105 Bruceine A inhibited the growth and induced the apoptosis of human pancreatic cancer cells by restraining the PFKFB4/GSK3β-mediated glycolysis pathway.106 9-ING-41 is a novel GSK3β inhibitor which significantly enhances the sensitivity of gemcitabine by regulating the TopBP1/ATR/Chk1 DNA damage response mediated pathway.107 Some synthetic topological analogues could induce cell apoptosis by suppressing the phosphorylation of GSK3β in PANC-1 cells.108 Long non-coding RNA taurine up-regulated gene 1 (TUG1) was overexpressed in PDAC tissues. In PDAC xenograft mice, the combination of TUG1-specific drug delivery system (TUG1-DDs) and 5-fluorouracil (5-FU) displayed a synergistic effect on chemotherapy compared to 5-FU alone.109 MDM2 or NFAT1 oncogenes are often overexpressed in pancreatic cancer, and the use of lead compound MA242 can bind these two genes and induce protein degradation. MA242 can inhibit the growth and metastasis of pancreatic cancer when it’s used alone or combined with gemcitabine.110 Activation and overexpression of proto-oncogene tyrosine-protein kinase SRC (SRC) can promote the progression of PDAC. Its inhibitor dasatinib has been shown to effectively inhibit the self-renewal and cloning ability in PDAC.111 When combined with erlotinib, the SRC/EGFR inhibitor (dasatinib) could suppress the STAT3 activity, overcome the resistance of gemcitabine, remodel the tumor matrix, and improve the overall survival in mouse models of PDAC.112
Photothermal Therapy
Photothermal therapy (PTT) based on nanotechnology provides promising treatment strategies for pancreatic cancer. Figure 3 illustrates the application of nanotechnology combined with photothermal therapy in treating pancreatic cancer.113 Table 3 summarizes the use of photothermal therapy combined with chemotherapy for pancreatic cancer. This section specifically summarized the various combinations of photothermal therapy and other therapies for pancreatic cancer.
 |
Table 3 Combination of Photothermal Therapy and Chemotherapy for Pancreatic Cancer |
Photothermal Therapy Combined with Gene Therapy
The mutation of K-Ras is prevalent in pancreatic cancer.124 A synergistic therapy utilizing reduced oxidized graphene @gold nanostars and crosslinked with folic acid was combined with a gene targeting G12V mutation of K-Ras. The result illustrated that the combination of photothermal and gene had a significant anti-cancer effect on tumor-bearing mice of pancreatic cancer.125 Multifunctional single-layer oxidized graphene nanosheets could co-delivery HDAC1 and K-Ras siRNAs to induce gene silence, resulting in significant anti-pancreatic cancer efficacy when combined with near-infrared photo hyperthermia.126 When doxorubicin and siRNA were co-carried by graphene quantum dots and biodegradable charged polyester vectors, the release of both agents might be triggered through a photothermal effect under laser irradiation. The anticancer activity of this nano-complex would be greatly enhanced.127
Photothermal Therapy Combined with Immunotherapy
Immunotherapy is considered as a promising approach for treating pancreatic cancer, including the use of immune checkpoint inhibitors, therapeutic vaccines, engineered T cells, etc.128 However, certain challenges also exist in immunotherapy, such as autoimmune response and cytokine syndrome. The combination of immunotherapy and other treatments may regulate the immune responses of tumor cells and produce synergistic therapeutic effects.129 In the treatment of pancreatic cancer with photothermal therapy and local immune adjuvant, 75% of subcutaneous tumors in mice were observed to completely regress, and accompanied by an increase in T cell count that triggered tumor-specific immune memory.130 The combination of DSPE-PEG and indocyanine green coating on amorphous ferric oxide nanoparticles loaded with imiquimod, followed by ion-assisted MRI-guided interventional photothermal therapy (IPTT), could induce in situ death of the immunogenic cells and trigger powerful anti-tumor immunity through local IPTT treatment.131 N/PGEM/dp-5 and N/PGEM/dp-16, which were polydopamine (dp) coated with gemcitabine and NLG919 nanoparticles and had a thick dp coated layer, exhibited dramatic enhancement in inhibiting pancreatic cancer when combined with laser irradiation. This provides a promising approach for designing more effective nanoparticle-based immunochemical photothermal therapy for both early-stage and advanced metastatic tumors.132
Others
Photothermal therapy, when combined with other therapies such as radiotherapy, can not only overcome the resistance of pancreatic tumors to radiation, but also increase the oxygen levels within tumors and regulate the tumor microenvironment, providing a new strategy for curing pancreatic cancer.133–135 A semiconducting polymer nano-radiopharmaceutical labeled with 177Lu (177Lu-SPN-GIP) that possessed photothermal effects has been shown to inhibit the growth of tumor stem cells reverse the epithelial-mesenchymal transition (EMT), and decrease the side effects of radiopharmaceutical drugs.136 A photothermal-based nanoparticle, consisting of anti-urokinase plasminogen activator receptor (uPAR), polyethylene (PEG), and indocyanine green-modified gold nanocapsules, which could improve the median survival rate of complete ablation by 25% with a single intervention when combined with Iodine-125 (125I) interstitial brachytherapy (IBT-125I).137
Under laser irradiation, nano-enzymes with photothermal properties might induce local thermal reactions, deplete GSH, and ultimately trigger apoptosis and ferroptosis in tumor cells.138 The laser could increase the photothermal effect and catalytic capacity of nano-enzymes within the tumor microenvironment. A novel double-enzyme-like active nano-enzyme (PtFe@Fe3O4) has demonstrated effective killing of pancreatic tumor cells in an acidic TME.139
Photodynamic therapy (PDT) could induce the oxidative stress reaction by photosensitizers, convert photon energy into oxygen molecules and generate reactive oxygen species to effectively eliminate tumor cells.140,141 In order to overcome the challenges of hypoxia and heat shock protein hindrance in pancreatic tumor phototherapy, we developed a photosensitizing agent DCTBT with aggregation-induced emission characteristics. This agent was prepared by an amphiphilic polymer modified with EGFR-targeted peptide, which effectively visualized pancreatic cancer and significantly inhibited tumor growth.142 A novel platform based on gold nanoclusters was employed for confocal laser endoscopy-guided photothermal therapy /photodynamic therapy of PDAC. An enzyme triggered the release of drug 5-ALA and fluorescent dye Cy5.5, resulting in excellent therapeutic effects with minimal side effects.143
Combined Administration Strategy for Pancreatic Cancer
A combined drug administration strategy for pancreatic cancer offers numerous advantages. For example, it may solve the insufficient drug accumulation in tumor tissues, overcome drug resistance and reduce toxicity.144–146
The co-delivery of nanoparticles and molecular targeted drugs have displayed a synergistic anti-tumor effect.147,148 Conjugation of varlitinib with pegylated gold nanoparticles in a controlled-release delivery system alleviated the toxic side effects of the drug while enhancing its efficacy against pancreatic cancer cells.149 Coupling EGFR ligands with nanoparticles could induce apoptosis and increase the drug uptake capacity in target cells.150 The GE11 peptide mixed micellar system, targeted at EGFR, achieved accurate drug release in pancreatic tumor tissues and effectively inhibits the growth of pancreatic tumors when delivering gemcitabine and OMe-PS-miR-519c.151 If EGFR and GE11 peptides self-assembly into amphiphilic peptide nanoparticles with high encapsulation rates and relatively stable properties, they could co-deliver gemcitabine and olaparib to suppress tumor growth and decrease toxic side effects in a mouse model of pancreatic cancer.152 When polyethylene glycol-polyethylenimine-magnetic iron oxide nanoparticles delivered microRNA-21 antisense oligonucleotides and gemcitabine simultaneously, with anti-CD44v6 single chain variable fragment as the targeting moiety, experimental results indicated that co-delivery enhanced the apoptosis and inhibited the growth of pancreatic cancer cells.153
Nanoparticles can serve as the carriers of nucleic acid delivery, which can effectively induce cell apoptosis and overcome drug resistance in heterogeneous tumors.154 PL-1/miR-9 nanoparticles could achieve specific delivery of miR-9, inhibit the expression of eIF5A2, and induce the apoptosis of PDAC cells.155 Using polymeric nanoparticles containing CXCR4 antagonist to deliver anti-miR-210 and siKRASG12D, the combination therapy displayed improved therapeutic effects, including matrix depletion, decreased immunosuppression, and inhibition of metastasis.156 A polymer dual-delivery nanoscale device was utilized to co-deliver gemcitabine and microRNA (miR-345), resulting in a combination therapy that reduced the tumor growth and metastasis to distant organs.157 DODAB: MO (1:2) liposomes were employed as siRNA-lipid complexes prepared by siRNA nano-carriers, which could target FOSL-1 and YAP, leading to significant restrain of tumor growth.158 Experiments revealed that the AuNRs complex could regulate the release of drugs under 665 nm light treatment, and exhibit synergistic antitumor effects when co-delivering siRNA and adriamycin based on gold nanorods.159 A novel delivery system for gemcitabine and miR-21 inhibitors, based on dendritic-embedded gold nanoparticles and ultrasonic targeted microbubble destruction (UTMD) technology, showed great potential in the treatment of pancreatic cancer.160
High-drug-loading AE@NPs prepared by co-loading alantolactone and erlotinib with PLGA nanoparticles restrained the phosphorylation of EGFR and STAT3 simultaneously, activated the ROS-p38 axis, and induced the apoptosis of pancreatic cancer cells, which demonstrated a significant anti-pancreatic cancer effect.161 The use of membrane-coated carriers for chemotherapy drugs provided a new idea in treating pancreatic cancer. The combination of PLGA NPs coated with the macrophage cell membrane and erlotinib might inhibit the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways, which brought about synergistic inhibition of proliferation and angiogenesis in pancreatic cancer cells. This membrane-coated biomimetic nano platform could specifically target pancreatic cancer cells, and dramatically suppress their proliferation and migration when co-delivering gemcitabine, erlotinib, and IRAK4 siRNA.162,163
Magnetic albumin nanosphere containing gemcitabine and the novel cetuximab (C225) was coupled to prepare C225-GEM/MAN, which was used as MRI molecular probes. The results revealed that this dual-targeted thermochemical therapy exhibited the highest targeted killing efficiency on AsPC-1 pancreatic cells.164 In addition, mesoporous silica nanoparticles targeted with cetuximab could specifically deliver photosensitized Zinc Phthalocyanine to pancreatic tumor cells with high expression levels of EGFR and selectively kill pancreatic cancer cells.165 A photoactivable multi-inhibitor nanoliposomes, based on cabozantinib (XL184), achieved the release of XL184 and inhibit metastatic escape in an in-situ pancreatic tumor model.166 Experiment results demonstrated that the nanoPAL-PDT was composed of photo cytotoxic chromophore benzoporphyrin derivative monoacid A (BPD) and bevacizumab, which enhanced drug efficacy by augmenting its cytotoxicity. Moreover, nanoPAL-PDT might dramatically inhibit tumor growth in the in vivo subcutaneous mouse model of pancreatic ductal adenocarcinoma.167
The Future Prospective
Pancreatic cancer is a highly malignant tumor that poses significant challenges to treat. While surgical resection remains the primary approach, its efficacy has not improved over time. Chemotherapy represents the first-line therapy for pancreatic cancer. However, drug resistance, dense tumor interstitium and heterogeneity often limit therapeutic outcomes.
In the field of medicine, nanomaterials have the potential to overcome biological barriers in drug delivery. The platform for nano-drug delivery can specifically target cancer cells and hypoxic microenvironments within tumors, so as to achieve precise targeting and controlled release of drugs. Albumin-bound paclitaxel nanomedicine (Abraxane®) has been approved by the FDA for the treatment of metastatic pancreatic cancer and is currently the only nanomedicine undergoing clinical trials for treating pancreatic cancer. The development of therapeutic drug delivery based on nanobiotechnology may offer a promising avenue for effective treatment.
Molecular targeted drugs have provided a glimmer of hope for the treatment of pancreatic cancer. Furthermore, molecularly-targeted drugs and nanomaterials that are modified with drugs using nanotechnology or small fragments of antibodies with molecular targeting capabilities have demonstrated encouraging results. Aptamer-functionalized nanomaterials have also made significant strides in the treatment of pancreatic cancer. Although some molecular targeted drugs remain in clinical trials, additional targets and novel nano-drug delivery platforms may be selected to design the optimal drug delivery regimen, therapy advancing the development of individualized therapy for pancreatic cancer in the future.
PTT is a promising cancer therapy, but its effectiveness for deep tumors is limited by the poor tissue penetration of light. Imaging-guided localized regional interventional photothermal therapy offers a new direction for treating pancreatic cancer beyond the body’s surface. When PTT therapy is combined with immunotherapy, gene therapy, and nano-enzyme, important breakthroughs have been achieved in the treatment of pancreatic cancer. However, more consideration of how to overcome the tumor stroma and hypoxia within the tumor microenvironment remains necessary.
With the continuous innovation and development of various novel biotechnology, as well as the highly cross-integrated development of medicine, pharmacy, biology, materials science, and other disciplines, we believe that breakthroughs will be achieved in treating pancreatic cancer, so as to benefit a greater number of patients.
Acknowledgments
This work was supported by the Anhui University of Chinese Medicine Foundation (2023rcZD003).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326(9):851–862. doi:10.1001/jama.2021.13027
3. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.CAN-14-0155
4. Aslanian HR, Lee JH, Canto MI. AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology. 2020;159(1):358–362. doi:10.1053/j.gastro.2020.03.088
5. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502. doi:10.1038/s41575-021-00457-x
6. Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348. doi:10.1038/s41575-018-0005-x
7. Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26. doi:10.1038/s41571-018-0112-1
8. Huang L, Jansen L, Balavarca Y, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut. 2019;68(1):130–139. doi:10.1136/gutjnl-2017-314828
9. Li T, Shi S, Goel S, et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019;89:1–13.
10. Raj S, Khurana S, Choudhari R, et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin Cancer Biol. 2021;69:166–177. doi:10.1016/j.semcancer.2019.11.002
11. Tarannum M, Hossain MA, Holmes B, et al. Advanced nanoengineering approach for target-specific, spatiotemporal, and ratiometric delivery of gemcitabine-cisplatin combination for improved therapeutic outcome in pancreatic cancer. Small. 2022;18(2):e2104449. doi:10.1002/smll.202104449
12. Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487–505. doi:10.1038/s41575-020-0300-1
13. Moradi Kashkooli F, Soltani M, Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J Control Release. 2020;327:316–349. doi:10.1016/j.jconrel.2020.08.012
14. Hayashi A, Hong J, Iacobuzio-Donahue CA. The pancreatic cancer genome revisited. Nat Rev Gastroenterol Hepatol. 2021;18(7):469–481. doi:10.1038/s41575-021-00463-z
15. Roskoski R Jr. ErbB/HER protein-tyrosine kinases: structures and small molecule inhibitors. Pharmacol Res. 2014;87:42–59. doi:10.1016/j.phrs.2014.06.001
16. Vaquero J, Pavy A, Gonzalez-Sanchez E, et al. Genetic alterations shaping tumor response to anti-EGFR therapies. Drug Resist Updat. 2022;64:100863. doi:10.1016/j.drup.2022.100863
17. Haqq J, Howells LM, Garcea G, et al. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. Eur J Cancer. 2014;50(15):2570–2582. doi:10.1016/j.ejca.2014.06.021
18. Greene MK, Nogueira JCF, Tracey SR, et al. Refined construction of antibody-targeted nanoparticles leads to superior antigen binding and enhanced delivery of an entrapped payload to pancreatic cancer cells. Nanoscale. 2020;12(21):11647–11658. doi:10.1039/D0NR02387F
19. Saadh MJ, Baher H, Li Y, et al. The bioengineered and multifunctional nanoparticles in pancreatic cancer therapy: bioresponisive nanostructures, phototherapy and targeted drug delivery. Environ Res. 2023;233:116490. doi:10.1016/j.envres.2023.116490
20. Liu Y, Wu W, Wang Y, et al. Recent development of gene therapy for pancreatic cancer using non-viral nanovectors. Biomater Sci. 2021;9(20):6673–6690. doi:10.1039/D1BM00748C
21. Shanmugam V, Selvakumar S, Yeh CS. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43(17):6254–6287. doi:10.1039/C4CS00011K
22. Li X, Lovell JF, Yoon J, et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
23. Gupta N, Malviya R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188532. doi:10.1016/j.bbcan.2021.188532
24. Patino T, Mahajan U, Palankar R, et al. Multifunctional gold nanorods for selective plasmonic photothermal therapy in pancreatic cancer cells using ultra-short pulse near-infrared laser irradiation. Nanoscale. 2015;7(12):5328–5337. doi:10.1039/C5NR00114E
25. Liu L, Kshirsagar PG, Gautam SK, et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics. 2022;12(3):1030–1060. doi:10.7150/thno.64805
26. Raza F, Evans L, Motallebi M, et al. Liposome-based diagnostic and therapeutic applications for pancreatic cancer. Acta Biomater. 2023;157:1–23. doi:10.1016/j.actbio.2022.12.013
27. Su J, Sun H, Meng Q, et al. Enhanced Blood Suspensibility and Laser-Activated Tumor-specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes. Theranostics. 2017;7(3):523–537. doi:10.7150/thno.17259
28. Hoang Thi TT, Cao VD, Nguyen TNQ, et al. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;99:631–656. doi:10.1016/j.msec.2019.01.129
29. Kankala RK, Han YH, Na J, et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv Mater. 2020;32(23):e1907035. doi:10.1002/adma.201907035
30. Yin F, Zhang B, Zeng S, et al. Folic acid-conjugated organically modified silica nanoparticles for enhanced targeted delivery in cancer cells and tumor in vivo. J Mater Chem B. 2015;3(29):6081–6093. doi:10.1039/C5TB00587F
31. Wang CE, Stayton PS, Pun SH, et al. Polymer nanostructures synthesized by controlled living polymerization for tumor-targeted drug delivery. J Control Release. 2015;219:345–354. doi:10.1016/j.jconrel.2015.08.054
32. Liu X, Jiang J, Liao YP, et al. Combination Chemo-Immunotherapy for Pancreatic Cancer Using the Immunogenic Effects of an Irinotecan Silicasome Nanocarrier Plus Anti-PD-1. Adv Sci. 2021;8(6):2002147. doi:10.1002/advs.202002147
33. Slapak EJ, Kong L, El Mandili M, et al. ADAM9-responsive mesoporous silica nanoparticles for targeted drug delivery in pancreatic cancer. Cancers. 2021;13(13):3321. doi:10.3390/cancers13133321
34. Tarannum M, Holtzman K, Dréau D, et al. Nanoparticle combination for precise stroma modulation and improved delivery for pancreatic cancer. J Control Release. 2022;347:425–434. doi:10.1016/j.jconrel.2022.05.019
35. Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25(11):2265–2284. doi:10.1021/tx300166u
36. Patra A, Satpathy S, Hussain MD. Nanodelivery and anticancer effect of a limonoid, nimbolide, in breast and pancreatic cancer cells. Int J Nanomedicine. 2019;14:8095–8104. doi:10.2147/IJN.S208540
37. Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–231. doi:10.1016/j.colsurfb.2017.07.038
38. Feltrin FDS, Agner T, Sayer C, et al. Curcumin encapsulation in functional PLGA nanoparticles: a promising strategy for cancer therapies. Adv Colloid Interface Sci. 2022;300:102582. doi:10.1016/j.cis.2021.102582
39. Wang Q, Yen YT, Xie C, et al. Combined delivery of salinomycin and docetaxel by dual-targeting gelatinase nanoparticles effectively inhibits cervical cancer cells and cancer stem cells. Drug Deliv. 2021;28(1):510–519. doi:10.1080/10717544.2021.1886378
40. Tomeh MA, Hadianamrei R, Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int J Mol Sci. 2019;20(5):1033. doi:10.3390/ijms20051033
41. Arya G, Das M, Sahoo SK. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed Pharmacother. 2018;102:555–566. doi:10.1016/j.biopha.2018.03.101
42. Cao X, Hu Y, Luo S, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9(3):575–589. doi:10.1016/j.apsb.2018.12.009
43. Dou L, Liu H, Wang K, et al. Albumin binding revitalizes NQO1 bioactivatable drugs as novel therapeutics for pancreatic cancer. J Control Release. 2022;349:876–889. doi:10.1016/j.jconrel.2022.07.033
44. Lu H, Noorani L, Jiang Y, et al. Penetration and drug delivery of albumin nanoparticles into pancreatic multicellular tumor spheroids. J Mater Chem B. 2017;5(48):9591–9599. doi:10.1039/C7TB02902K
45. Hu Y, Chen X, Xu Y, et al. Hierarchical assembly of hyaluronan coated albumin nanoparticles for pancreatic cancer chemoimmunotherapy. Nanoscale. 2019;11(35):16476–16487. doi:10.1039/C9NR03684A
46. Yu Q, Tang X, Zhao W, et al. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021;133:244–256. doi:10.1016/j.actbio.2021.05.002
47. Bhushan B, Khanadeev V, Khlebtsov B, et al. Impact of albumin based approaches in nanomedicine: imaging, targeting and drug delivery. Adv Colloid Interface Sci. 2017;246:13–39. doi:10.1016/j.cis.2017.06.012
48. Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–182. doi:10.1016/j.jconrel.2011.07.031
49. Barbosa AI, Costa lima SA, Reis S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules. 2019;24(2):346. doi:10.3390/molecules24020346
50. Wang Y, Xing M, Cao Q, et al. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar Drugs. 2019;17(3):183. doi:10.3390/md17030183
51. Etman SM, Abdallah OY, Elnaggar YSR. Novel fucoidan based bioactive targeted nanoparticles from Undaria Pinnatifida for treatment of pancreatic cancer. Int J Biol Macromol. 2020;145:390–401. doi:10.1016/j.ijbiomac.2019.12.177
52. Oien DB, Pathoulas CL, Ray U, et al. Repurposing quinacrine for treatment-refractory cancer. Semin Cancer Biol. 2021;68:21–30. doi:10.1016/j.semcancer.2019.09.021
53. Etman SM, Mehanna RA, Bary AA, et al. Undaria pinnatifida fucoidan nanoparticles loaded with quinacrine attenuate growth and metastasis of pancreatic cancer. Int J Biol Macromol. 2021;170:284–297. doi:10.1016/j.ijbiomac.2020.12.109
54. Beisl S, Friedl A, Miltner A. Lignin from Micro- to Nanosize: applications. Int J Mol Sci. 2017;18(11):2367. doi:10.3390/ijms18112367
55. Etman SM, Abdallah OY, Mehanna RA, et al. Lactoferrin/Hyaluronic acid double-coated lignosulfonate nanoparticles of quinacrine as a controlled release biodegradable nanomedicine targeting pancreatic cancer. Int J Pharm. 2020;578:119097. doi:10.1016/j.ijpharm.2020.119097
56. Channon LM, Tyma VM, Xu Z, et al. Small extracellular vesicles (exosomes) and their cargo in pancreatic cancer: key roles in the hallmarks of cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188728. doi:10.1016/j.bbcan.2022.188728
57. Namee NM, O’Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer. 2018;1870(2):123–136. doi:10.1016/j.bbcan.2018.07.003
58. Liu J, Ren L, Li S, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11(9):2783–2797. doi:10.1016/j.apsb.2021.01.001
59. Rashed M H, Bayraktar E, Helal G K, et al. Exosomes: from Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017;18(3):538. doi:10.3390/ijms18030538
60. Li YJ, Wu JY, Wang JM, et al. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519–530. doi:10.1016/j.actbio.2019.10.022
61. Xu L, Faruqu FN, Lim YM, et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. 2021;264:120369. doi:10.1016/j.biomaterials.2020.120369
62. Binenbaum Y, Fridman E, Yaari Z, et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78(18):5287–5299. doi:10.1158/0008-5472.CAN-18-0124
63. Zhou W, Zhou Y, Chen X, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. doi:10.1016/j.biomaterials.2020.120546
64. Zhou Y, Zhou W, Chen X, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10(8):1563–1575. doi:10.1016/j.apsb.2019.11.013
65. Jang Y, Kim H, Yoon S, et al. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J Control Release. 2021;330:293–304. doi:10.1016/j.jconrel.2020.12.039
66. Garcia-Sampedro A, Gaggia G, Ney A, et al. The State-of-The-Art of Phase II/III Clinical Trials for Targeted Pancreatic Cancer Therapies. J Clin Med. 2021;10(4):566. doi:10.3390/jcm10040566
67. Wen Y, Ouyang D, Zou Q, et al. A literature review of the promising future of TROP2: a potential drug therapy target. Ann Transl Med. 2022;10(24):1403. doi:10.21037/atm-22-5976
68. R R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi:10.1016/j.phrs.2013.11.002
69. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi:10.1038/nrc1609
70. Eser S, Schnieke A, Schneider G, et al. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111(5):817–822. doi:10.1038/bjc.2014.215
71. Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci. 2017;18(11):2420. doi:10.3390/ijms18112420
72. Lakkakula BVKS, Farran B, Lakkakula S, et al. Small molecule tyrosine kinase inhibitors and pancreatic cancer-Trials and troubles. Semin Cancer Biol. 2019;56:149–167. doi:10.1016/j.semcancer.2018.09.011
73. Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36(7):422–439. doi:10.1016/j.tips.2015.04.005
74. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi:10.1200/JCO.2006.07.9525
75. van Geel RMJM, van Brummelen EMJ, Eskens FALM, et al. Phase 1 study of the pan-HER inhibitor dacomitinib plus the MEK1/2 inhibitor PD-0325901 in patients with KRAS-mutation-positive colorectal, non-small-cell lung and pancreatic cancer. Br J Cancer. 2020;122(8):1166–1174. doi:10.1038/s41416-020-0776-z
76. Momeny M, Esmaeili F, Hamzehlou S, et al. The ERBB receptor inhibitor dacomitinib suppresses proliferation and invasion of pancreatic ductal adenocarcinoma cells. Cell Oncol. 2019;42(4):491–504. doi:10.1007/s13402-019-00448-w
77. Seshacharyulu P, Ponnusamy MP, Rachagani S, et al. Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6(7):5164–5181. doi:10.18632/oncotarget.3286
78. Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7(4):301–311. doi:10.1016/j.ccr.2005.03.003
79. Greene MK, Chen T, Robinson E, et al. Controlled coupling of an ultrapotent auristatin warhead to cetuximab yields a next-generation antibody-drug conjugate for EGFR-targeted therapy of KRAS mutant pancreatic cancer. Br J Cancer. 2020;123(10):1502–1512. doi:10.1038/s41416-020-01046-6
80. Miyamoto R, Oda T, Hashimoto S, et al. Cetuximab delivery and antitumor effects are enhanced by mild hyperthermia in a xenograft mouse model of pancreatic cancer. Cancer Sci. 2016;107(4):514–520. doi:10.1111/cas.12888
81. McMichael EL, Jaime-Ramirez AC, Guenterberg KD, et al. IL-21 Enhances Natural Killer Cell Response to Cetuximab-Coated Pancreatic Tumor Cells. Clin Cancer Res. 2017;23(2):489–502. doi:10.1158/1078-0432.CCR-16-0004
82. Yoshii Y, Matsumoto H, Yoshimoto M, et al. 64Cu-Intraperitoneal Radioimmunotherapy: a Novel Approach for Adjuvant Treatment in a Clinically Relevant Preclinical Model of Pancreatic Cancer. J Nucl Med. 2019;60(10):1437–1443. doi:10.2967/jnumed.118.225045
83. Kim YJ, Jung K, Baek DS, et al. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene. 2017;36(18):2543–2552. doi:10.1038/onc.2016.407
84. Wang J, Chan DKW, Sen A, et al. Tumor Priming by SMO Inhibition Enhances Antibody Delivery and Efficacy in a Pancreatic Ductal Adenocarcinoma Model. Mol Cancer Ther. 2019;18(11):2074–2084. doi:10.1158/1535-7163.MCT-18-0354
85. Yamanaka Y, Friess H, Kobrin MS, et al. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24(10):1127–1134. doi:10.1016/0046-8177(93)90194-L
86. Harder J, Ihorst G, Heinemann V, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106(6):1033–1038. doi:10.1038/bjc.2012.18
87. Larbouret C, Gaborit N, Chardès T, et al. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14(2):121–130. doi:10.1593/neo.111602
88. Assenat E, Mineur L, Mollevi C, et al. Phase II study evaluating the association of gemcitabine, trastuzumab and erlotinib as first-line treatment in patients with metastatic pancreatic adenocarcinoma (GATE 1). Int J Cancer. 2021;148(3):682–691. doi:10.1002/ijc.33225
89. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–29006. doi:10.18632/oncotarget.25615
90. Strop P, Tran TT, Dorywalska M, et al. RN927C, a Site-Specific Trop-2 Antibody-Drug Conjugate (ADC) with Enhanced Stability, Is Highly Efficacious in Preclinical Solid Tumor Models. Mol Cancer Ther. 2016;15(11):2698–2708. doi:10.1158/1535-7163.MCT-16-0431
91. Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394(10200):793–804. doi:10.1016/S0140-6736(19)31774-X
92. Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody-Drug Conjugate: characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug Chem. 2015;26(5):919–931. doi:10.1021/acs.bioconjchem.5b00223
93. Mao Y, Wang X, Zheng F, et al. The tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate in pancreatic cancer. Oncotarget. 2016;7(17):24810–24823. doi:10.18632/oncotarget.8529
94. Li C, Liu J, Yang X, et al. Theranostic application of 64Cu/177Lu-labeled anti-Trop2 monoclonal antibody in pancreatic cancer tumor models. Eur J Nucl Med Mol Imaging. 2022;50(1):168–183. doi:10.1007/s00259-022-05954-y
95. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
96. Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28(15):4843–4850. doi:10.1128/MCB.02214-07
97. Wang CA, Chang IH, Hou PC, et al. DUSP2 regulates extracellular vesicle-VEGF-C secretion and pancreatic cancer early dissemination. J Extracell Vesicles. 2020;9(1):1746529. doi:10.1080/20013078.2020.1746529
98. Tang RF, Itakura J, Aikawa T, et al. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22(3):285–292. doi:10.1097/00006676-200104000-00010
99. Kong Y, Li Y, Luo Y, et al. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol Cancer. 2020;19(1):82. doi:10.1186/s12943-020-01205-6
100. Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385–403. doi:10.1038/nrd.2015.17
101. Tai CJ, Huang MT, Wu CH, et al. Combination of Two Targeted Medications (Bevacizumab Plus Cetuximab) Improve the Therapeutic Response of Pancreatic Carcinoma. Medicine. 2016;95(15):e3259. doi:10.1097/MD.0000000000003259
102. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. doi:10.1200/JCO.2010.28.1386
103. Momeny M, Alishahi Z, Eyvani H, et al. Anti-tumor activity of cediranib, a pan-vascular endothelial growth factor receptor inhibitor, in pancreatic ductal adenocarcinoma cells. Cell Oncol. 2020;43(1):81–93. doi:10.1007/s13402-019-00473-9
104. Pecoraro C, Faggion B, Balboni B, et al. GSK3β as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist Updat. 2021;58:100779. doi:10.1016/j.drup.2021.100779
105. McCubrey JA, Davis NM, Abrams SL, et al. Diverse roles of GSK-3: tumor promoter-tumor suppressor, target in cancer therapy. Adv Biol Regul. 2014;54:176–196. doi:10.1016/j.jbior.2013.09.013
106. Zhang P, Tao W, Lu C, et al. Bruceine A induces cell growth inhibition and apoptosis through PFKFB4/GSK3β signaling in pancreatic cancer. Pharmacol Res. 2021;169:105658. doi:10.1016/j.phrs.2021.105658
107. Ding L, Madamsetty VS, Kiers S, et al. Glycogen Synthase Kinase-3 Inhibition Sensitizes Pancreatic Cancer Cells to Chemotherapy by Abrogating the TopBP1/ATR-Mediated DNA Damage Response. Clin Cancer Res. 2019;25(21):6452–6462. doi:10.1158/1078-0432.CCR-19-0799
108. Carbone D, Parrino B, Cascioferro S, et al. 1,2,4-Oxadiazole Topsentin Analogs with Antiproliferative Activity against Pancreatic Cancer Cells, Targeting GSK3β Kinase. ChemMedChem. 2021;16(3):537–554. doi:10.1002/cmdc.202000752
109. Tasaki Y, Suzuki M, Katsushima K, et al. Cancer-Specific Targeting of Taurine-Upregulated Gene 1 Enhances the Effects of Chemotherapy in Pancreatic Cancer. Cancer Res. 2021;81(7):1654–1666. doi:10.1158/0008-5472.CAN-20-3021
110. Wang W, Qin JJ, Voruganti S, et al. Discovery and Characterization of Dual Inhibitors of MDM2 and NFAT1 for Pancreatic Cancer Therapy. Cancer Res. 2018;78(19):5656–5667. doi:10.1158/0008-5472.CAN-17-3939
111. Alcalá S, Mayoral-Varo V, Ruiz-Cañas L, et al. Targeting SRC Kinase Signaling in Pancreatic Cancer Stem Cells. Int J Mol Sci. 2020;21(20):7437. doi:10.3390/ijms21207437
112. Dosch AR, Dai X, Reyzer ML, et al. Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer. Mol Cancer Res. 2020;18(4):623–631. doi:10.1158/1541-7786.MCR-19-0741
113. Dwivedi P, Kiran S, Han S, et al. Magnetic Targeting and Ultrasound Activation of Liposome-Microbubble Conjugate for Enhanced Delivery of Anticancer Therapies. ACS Appl Mater Interfaces. 2020;12(21):23737–23751. doi:10.1021/acsami.0c05308
114. Zhan X, Nie X, Gao F, et al. An NIR-activated polymeric nanoplatform with ROS- and temperature-sensitivity for combined photothermal therapy and chemotherapy of pancreatic cancer. Biomater Sci. 2020;8(21):5931–5940. doi:10.1039/D0BM01324B
115. Teng T, Lin R, Lin Z, et al. Photothermal augment stromal disrupting effects for enhanced Abraxane synergy chemotherapy in pancreatic cancer PDX mode. Biomater Sci. 2020;8(12):3278–3285. doi:10.1039/D0BM00549E
116. Thapa RK, Nguyen HT, Gautam M, et al. Hydrophobic binding peptide-conjugated hybrid lipid-mesoporous silica nanoparticles for effective chemo-photothermal therapy of pancreatic cancer. Drug Deliv. 2017;24(1):1690–1702. doi:10.1080/10717544.2017.1396382
117. Banstola A, Pham TT, Jeong J-H, et al. Polydopamine-tailored paclitaxel-loaded polymeric microspheres with adhered NIR-controllable gold nanoparticles for chemo-phototherapy of pancreatic cancer. Drug Deliv. 2019;26(1):629–640. doi:10.1080/10717544.2019.1628118
118. Yu Q, Qiu Y, Li J, et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Control Release. 2020;321:564–575. doi:10.1016/j.jconrel.2020.02.040
119. Xu K, Jin L, Xu L, et al. IGF1 receptor-targeted black TiO2 nanoprobes for MRI-guided synergetic photothermal-chemotherapy in drug resistant pancreatic tumor. J Nanobiotechnology. 2022;20(1):315. doi:10.1186/s12951-022-01525-3
120. Poudel BK, Gupta B, Ramasamy T, et al. PEGylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids Surf B Biointerfaces. 2017;160:73–83. doi:10.1016/j.colsurfb.2017.09.010
121. Zhao R, Han X, Li Y, et al. Photothermal Effect Enhanced Cascade-Targeting Strategy for Improved Pancreatic Cancer Therapy by Gold Nanoshell@Mesoporous Silica Nanorod. ACS Nano. 2017;11(8):8103–8113. doi:10.1021/acsnano.7b02918
122. Jin Y, Schladetsch MA, Huang X, et al. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917. doi:10.1016/j.pharmthera.2021.107917
123. Thapa RK, Ku SK, Choi HG, et al. Vibrating droplet generation to assemble zwitterion-coated gold-graphene oxide stealth nanovesicles for effective pancreatic cancer chemo-phototherapy. Nanoscale. 2018;10(4):1742–1749. doi:10.1039/C7NR07603G
124. Lin G, Hu R, Law WC, et al. Biodegradable nanocapsules as siRNA carriers for mutant K-Ras gene silencing of human pancreatic carcinoma cells. Small. 2013;9(16):2757–2763. doi:10.1002/smll.201201716
125. Jia X, Xu W, Ye Z, et al. Functionalized Graphene@Gold Nanostar/Lipid for Pancreatic Cancer Gene and Photothermal Synergistic Therapy under Photoacoustic/Photothermal Imaging Dual-Modal Guidance. Small. 2020;16(39):e2003707. doi:10.1002/smll.202003707
126. Yin F, Hu K, Chen Y, et al. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics. 2017;7(5):1133–1148. doi:10.7150/thno.17841
127. Yang C, Chan KK, Xu G, et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2019;11(3):2768–2781. doi:10.1021/acsami.8b16168
128. Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4(6):418–428. doi:10.1016/j.trecan.2018.04.001
129. Zhao Z, Zheng L, Chen W, et al. Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. 2019;12(1):126. doi:10.1186/s13045-019-0817-3
130. Zhou F, Yang J, Zhang Y, et al. Local Phototherapy Synergizes with Immunoadjuvant for Treatment of Pancreatic Cancer through Induced Immunogenic Tumor Vaccine. Clin Cancer Res. 2018;24(21):5335–5346. doi:10.1158/1078-0432.CCR-18-1126
131. Wang M, Li Y, Wang M, et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022;138:453–462. doi:10.1016/j.actbio.2021.10.048
132. Sun J, Wan Z, Xu J, et al. Tumor size-dependent abscopal effect of polydopamine-coated all-in-one nanoparticles for immunochemo-photothermal therapy of early- and late-stage metastatic cancer. Biomaterials. 2021;269:120629. doi:10.1016/j.biomaterials.2020.120629
133. Li L, Dai K, Li J, et al. A Boron-10 nitride nanosheet for combinational boron neutron capture therapy and chemotherapy of tumor. Biomaterials. 2021;268:120587. doi:10.1016/j.biomaterials.2020.120587
134. Ferreira CA, Ehlerding EB, Rosenkrans ZT, et al. 86/90Y-Labeled Monoclonal Antibody Targeting Tissue Factor for Pancreatic Cancer Theranostics. Mol Pharm. 2020;17(5):1697–1705. doi:10.1021/acs.molpharmaceut.0c00127
135. Horsman MR, Vaupel P. Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front Oncol. 2016;6:66. doi:10.3389/fonc.2016.00066
136. Shi X, Li Q, Zhang C, et al. Semiconducting polymer nano-radiopharmaceutical for combined radio-photothermal therapy of pancreatic tumor. J Nanobiotechnology. 2021;19(1):337. doi:10.1186/s12951-021-01083-0
137. Hu Y, Chi C, Wang S, et al. A Comparative Study of Clinical Intervention and Interventional Photothermal Therapy for Pancreatic Cancer. Adv Mater. 2017;29(33):1700448. doi:10.1002/adma.201700448
138. Yuan H, Xia P, Sun X, et al. Photothermal nanozymatic nanoparticles induce ferroptosis and apoptosis through tumor microenvironment manipulation for cancer therapy. Small. 2022;18(41):e2202161. doi:10.1002/smll.202202161
139. Li S, Shang L, Xu B, et al. A nanozyme with photo-enhanced dual enzyme-like activities for deep pancreatic cancer therapy. Angew Chem Int Ed Engl. 2019;58(36):12624–12631. doi:10.1002/anie.201904751
140. Donohoe C, Senge MO, Arnaut LG, et al. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188308. doi:10.1016/j.bbcan.2019.07.003
141. Kolemen S, Ozdemir T, Lee D, et al. Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods: towards a paradigm change in photodynamic therapy. Angew Chem Int Ed Engl. 2016;55(11):3606–3610. doi:10.1002/anie.201510064
142. Li D, Chen X, Wang D, et al. Synchronously boosting type-I photodynamic and photothermal efficacies via molecular manipulation for pancreatic cancer theranostics in the NIR-II window. Biomaterials. 2022;283:121476. doi:10.1016/j.biomaterials.2022.121476
143. Li H, Wang P, Deng Y, et al. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials. 2017;139:30–38. doi:10.1016/j.biomaterials.2017.05.030
144. Jaaks P, Coker EA, Vis DJ, et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature. 2022;603(7899):166–173. doi:10.1038/s41586-022-04437-2
145. Plana D, Palmer AC, Sorger PK. Independent drug action in combination therapy: implications for precision oncology. Cancer Discov. 2022;12(3):606–624. doi:10.1158/2159-8290.CD-21-0212
146. Li X, Dowling EK, Yan G, et al. Precision combination therapies based on recurrent oncogenic coalterations. Cancer Discov. 2022;12(6):1542–1559. doi:10.1158/2159-8290.CD-21-0832
147. Yang A, Sheng S, Bai Y, et al. Hydrogel/nanoparticles-mediated cooperative combination of antiangiogenesis and immunotherapy. Acta Biomater. 2022;153:124–138. doi:10.1016/j.actbio.2022.09.060
148. Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. doi:10.1016/j.phrs.2010.03.005
149. Coelho SC, Reis DP, Pereira MC, et al. Gold nanoparticles for targeting varlitinib to human pancreatic cancer cells. Pharmaceutics. 2018;10(3):91. doi:10.3390/pharmaceutics10030091
150. Grapa CM, Mocan T, Gonciar D, et al. Epidermal growth factor receptor and its role in pancreatic cancer treatment mediated by nanoparticles. Int J Nanomedicine. 2019;14:9693–9706. doi:10.2147/IJN.S226628
151. Xin X, Kumar V, Lin F, et al. Redox-responsive nanoplatform for codelivery of miR-519c and gemcitabine for pancreatic cancer therapy. Sci Adv. 2020;6(46):eabd6764. doi:10.1126/sciadv.abd6764
152. Du C, Qi Y, Zhang Y, et al. Epidermal Growth Factor Receptor-Targeting Peptide Nanoparticles Simultaneously Deliver Gemcitabine and Olaparib To Treat Pancreatic Cancer with Breast Cancer 2 (BRCA2) Mutation. ACS Nano. 2018;12(11):10785–10796. doi:10.1021/acsnano.8b01573
153. Li Y, Chen Y, Li J, et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017;108(7):1493–1503. doi:10.1111/cas.13267
154. Vaughan HJ, Green JJ, Tzeng SY. Cancer-targeting nanoparticles for combinatorial nucleic acid delivery. Adv Mater. 2020;32(13):e1901081. doi:10.1002/adma.201901081
155. Wu Y, Tang Y, Xie S, et al. Chimeric peptide supramolecular nanoparticles for plectin-1 targeted miRNA-9 delivery in pancreatic cancer. Theranostics. 2020;10(3):1151–1165. doi:10.7150/thno.38327
156. Xie Y, Hang Y, Wang Y, et al. Stromal Modulation and Treatment of Metastatic Pancreatic Cancer with Local Intraperitoneal Triple miRNA/siRNA Nanotherapy. ACS Nano. 2020;14(1):255–271. doi:10.1021/acsnano.9b03978
157. Uz M, Kalaga M, Pothuraju R, et al. Dual delivery nanoscale device for miR-345 and gemcitabine co-delivery to treat pancreatic cancer. J Control Release. 2019;294:237–246. doi:10.1016/j.jconrel.2018.12.031
158. Diego-González L, Fernández-Carrera A, Igea A, et al. Combined Inhibition of FOSL-1 and YAP Using siRNA-Lipoplexes Reduces the Growth of Pancreatic Tumor. Cancers. 2022;14(13):3102. doi:10.3390/cancers14133102
159. Yin F, Yang C, Wang Q, et al. A Light-Driven Therapy of Pancreatic Adenocarcinoma Using Gold Nanorods-Based Nanocarriers for Co-Delivery of Doxorubicin and siRNA. Theranostics. 2015;5(8):818–833. doi:10.7150/thno.11335
160. Lin L, Fan Y, Gao F, et al. UTMD-Promoted Co-Delivery of Gemcitabine and miR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics. 2018;8(7):1923–1939. doi:10.7150/thno.22834
161. Bao S, Zheng H, Ye J, et al. Dual Targeting EGFR and STAT3 With Erlotinib and Alantolactone Co-Loaded PLGA Nanoparticles for Pancreatic Cancer Treatment. Front Pharmacol. 2021;12:625084. doi:10.3389/fphar.2021.625084
162. Cai H, Wang R, Guo X, et al. Combining gemcitabine-loaded macrophage-like nanoparticles and erlotinib for pancreatic cancer therapy. Mol Pharm. 2021;18(7):2495–2506. doi:10.1021/acs.molpharmaceut.0c01225
163. Tang H, Xue Y, Li B, et al. Membrane-camouflaged supramolecular nanoparticles for co-delivery of chemotherapeutic and molecular-targeted drugs with siRNA against patient-derived pancreatic carcinoma. Acta Pharm Sin B. 2022;12(8):3410–3426. doi:10.1016/j.apsb.2022.02.007
164. Wang L, An Y, Yuan C, et al. GEM-loaded magnetic albumin nanospheres modified with cetuximab for simultaneous targeting, magnetic resonance imaging, and double-targeted thermochemotherapy of pancreatic cancer cells. Int J Nanomedicine. 2015;10:2507–2519. doi:10.2147/IJN.S77642
165. Er Ö, Colak SG, Ocakoglu K, et al. Selective photokilling of human pancreatic cancer cells using cetuximab-targeted mesoporous silica nanoparticles for delivery of zinc phthalocyanine. Molecules. 2018;23(11):2749. doi:10.3390/molecules23112749
166. Spring BQ, Bryan Sears R, Zheng LZ, et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat Nanotechnol. 2016;11(4):378–387. doi:10.1038/nnano.2015.311
167. Tangutoori S, Spring BQ, Mai Z, et al. Simultaneous delivery of cytotoxic and biologic therapeutics using nanophotoactivatable liposomes enhances treatment efficacy in a mouse model of pancreatic cancer. Nanomedicine. 2016;12(1):223–234. doi:10.1016/j.nano.2015.08.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.