Back to Journals » International Journal of Nanomedicine » Volume 19
Advancements in Stimulus-Responsive Co-Delivery Nanocarriers for Enhanced Cancer Immunotherapy
Authors Zhang MR, Fang LL, Guo Y, Wang Q, Li YJ, Sun HF, Xie SY , Liang Y
Received 8 December 2023
Accepted for publication 14 March 2024
Published 8 April 2024 Volume 2024:19 Pages 3387—3404
DOI https://doi.org/10.2147/IJN.S454004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Meng-Ru Zhang,1,2,* Lin-Lin Fang,3,* Yang Guo,1 Qin Wang,1 You-Jie Li,1 Hong-Fang Sun,1 Shu-Yang Xie,1 Yan Liang1
1Department of Biochemistry and Molecular Biology, School of Basic Medicine, Binzhou Medical University, YanTai, ShanDong, 264003, People’s Republic of China; 2Department of Clinical Medicine, Binzhou Medical University, YanTai, ShanDong, 264003, People’s Republic of China; 3RemeGen Co., Ltd, YanTai, ShanDong, 264000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Liang; Shu-Yang Xie, Department of Biochemistry and Molecular Biology, School of Basic Medicine, Binzhou Medical University, YanTai, ShanDong, 264003, People’s Republic of China, Tel/Fax +86 535 6913335 ; +86 535 6913166, Email [email protected]; [email protected]
Abstract: Cancer immunotherapy has emerged as a novel therapeutic approach against tumors, with immune checkpoint inhibitors (ICIs) making significant clinical practice. The traditional ICIs, PD-1 and PD-L1, augment the cytotoxic function of T cells through the inhibition of tumor immune evasion pathways, ultimately leading to the initiation of an antitumor immune response. However, the clinical implementation of ICIs encounters obstacles stemming from the existence of an immunosuppressive tumor microenvironment and inadequate infiltration of CD8+T cells. Considerable attention has been directed towards advancing immunogenic cell death (ICD) as a potential solution to counteract tumor cell infiltration and the immunosuppressive tumor microenvironment. This approach holds promise in transforming “cold” tumors into “hot” tumors that exhibit responsiveness to antitumor. By combining ICD with ICIs, a synergistic immune response against tumors can be achieved. However, the combination of ICD inducers and PD-1/PD-L1 inhibitors is hindered by issues such as poor targeting and uncontrolled drug release. An advantageous solution presented by stimulus-responsive nanocarrier is integrating the physicochemical properties of ICD inducers and PD-1/PD-L1 inhibitors, facilitating precise delivery to specific tissues for optimal combination therapy. Moreover, these nanocarriers leverage the distinct features of the tumor microenvironment to accomplish controlled drug release and regulate the kinetics of drug delivery. This article aims to investigate the advancement of stimulus-responsive co-delivery nanocarriers utilizing ICD and PD-1/PD-L1 inhibitors. Special focus is dedicated to exploring the advantages and recent advancements of this system in enabling the combination of ICIs and ICD inducers. The molecular mechanisms of ICD and ICIs are concisely summarized. In conclusion, we examine the potential research prospects and challenges that could greatly enhance immunotherapeutic approaches for cancer treatment.
Keywords: antitumor therapy, immunogenic cell death, co-delivery, immune-checkpoint inhibitors, stimulus-responsive nanocarriers
Introduction
Cancer, being a substantial global public-health concern, is projected to 28.4 million cases worldwide by the year 2040.1 In recent decades, cancer immunotherapy has made noteworthy strides. In recent decades, cancer immunotherapy has made significant progress. Currently, it predominantly focuses on two domains. The first is the activation of key components within the immune system, including cancer vaccines,2 cytokine therapy,3 and adoptive T-cell therapy.4 The second domain involves the suppression of immune-checkpoint molecules, such as immune-checkpoint blocking (ICB).5,6 Immune-checkpoint molecules refer to the small proteins produced by immune cells in the body that regulate effector function. Tumor cells evade and survive the body’s immune system by overactivating immune-checkpoint molecules. Immune-checkpoint inhibitors (ICIs) can prevent the overactivation of immune checkpoint inhibitors, allowing immune cells to eliminate cancer cells. ICIs exert their antitumor effects mainly through PD-1/PD-L1 inhibitors.7 PD-1/PD-L1 inhibitors include PD-1 antibodies and PD-L1 antibodies, which can bind to PD-1 and PD-L1 respectively, preventing the binding of cancer cells and T cells. Then promoting the T cell-mediated killing and clearance of tumors. However, numerous tumors exhibit a poor response to the use of PD-1/PD-L1 checkpoint inhibitors alone.8–10 This is due to the fact that the tumors are mostly “cold” tumors, which are manifested as a low immunogenicity of the tumor cells. Furthermore, the immunosuppressive tumor microenvironment (ITM) significantly impedes the infiltration and function of cytotoxic lymphocytes (CTLs), which contributes to sustained tumor growth.
Researchers have shown that ICD could activate CD8+T cells, thereby improving the tumor microenvironment.11 ICD represents a type of cell death capable of transforming “cold” tumors into “hot” ones. The process of “cold tumors” becoming “hot tumors” is essentially a process of increasing the immune-inflammatory response to the tumor.12–14 Thus, the combined treatment of ICD inducers with PD-1/PD-L1 inhibitors can enhance the antitumor effects. However, the drug administrations of certain ICD inducers, such as chemotherapy drugs, are commonly delivered systemically, resulting in adverse side effects and limited bioavailability. Thus design targeted approaches for locally stimulating and enhancing tumor immune therapy is necessary. Moreover, the combination of ICD inducers and PD-1/PD-L1 inhibitors faces challenges such as uncontrolled drug release due to the immediate release of drugs upon entering the medium. Hence, there is an urgent need for searching a reliable drug-delivery platform to enhance targeting and achieve controlled drug release.
Nanocarriers possess numerous advantageous attributes, such as their small size, extensive surface area, convenient modifiability and the ability to co-deliver ICD inducers and PD-1/PD-L1 inhibitors. Stimulus-responsive nanocarriers are fabricated by introducing different internal and external stimulus-responsive materials on the basis of the original nanocarriers.15,16 Stimulus-responsive nanocarriers possess favorable attributes for the construction of a drug-delivery platform through the combination of ICD inducers and PD-1/PD-L1 inhibitors. Attributes as follows: (i) introduction of stimulus-responsive materials to reconstruct TEM, which is conducive to the acquisition of long-term immune memory after the release of ICD inducers and PD-1/PD-L1 inhibitors;17 (ii) unifying ICD inducers and PD-1/PD-L1 inhibitors with different distributions and different targets in vivo in nanocarrier to obtain better synergistic antitumor effect;18 (iii) avoiding the systemic toxic side effects of ICD inducers and PD-1/PD-L1 inhibitors alone;19 and (iv) controlling the release of ICD inducers and PD-1/PD-L1 inhibitors and exerting synergistic antitumor effects.20 In the past few years, our group has published several papers in the field of stimulus-responsive nanocarriers and accumulated some experience, which effectively provided technical support for the writing of this review.21,22
This article centers its attention on the progress of stimulus-responsive co-delivery nanocarriers that rely on ICD inducers and PD-1/PD-L1 inhibitors. It provides an overview and analysis of recent advancements in the utilization of these co-delivery nanocarriers within the realm of tumor immunotherapy. Furthermore, novel insights into the realm of efficient tumor immune treatment will be offered.
Overview of ICD and ICIs
ICD
ICD is a process in which tumor cells mediate the generation of adaptive immune responses in the body under external or internal stimuli. Its hallmarks are the release of tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs).23,24 Inducers are required before ICD, as described in detail in the following text.
Mechanism of ICD
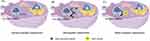
Under external or internal stimuli, certain apoptotic tumor cells can induce immunogenic proteins and then trigger the body’s antitumor immune response. CTLs are activated to more effectively kill tumor cells, finally achieving a more desirable antitumor effect. This phenomenon is known as tumor-cell ICD. Various types of DAMPs are released, including calreticulin protein (CALR) exposed on the cell surface, heat-shock proteins (HSPs), high mobility group box 1 (HMGB1), adenosine triphosphate (ATP), and type I interferons (IFN-I).25–27
CALR is usually located in the endoplasmic reticulum and acts as a multifunctional protein involved in various intracellular immune processes, such as protein folding and maintaining calcium homeostasis.28,29 Diseases such as kidney fibrosis and tumors are closely associated with it.30,31 Following induction by ICD, CALR undergoes translocation and exposure on the cell membrane mediated by EIF2S1 inactivation phosphorylation.32 On the cell membrane, it forms complexes with partner proteins like ERp57, serving as “eat me” signals that promote the engulfment of dying cells by dendritic cells (DCs) and enhance antigen presentation by antigen-presenting cells (APCs), particularly immature DCs.33 ATP is generally located inside cells and is an important component of DAMPs. Extracellular ATP participates in purinergic signaling and inflammatory response and is closely associated with diseases such as tumors, ischemic injury, post-heart transplantation, and rejection reactions34,35 Innate and adaptive immunity are also activated by ATP through a series of reactions.36,37 Karen M Dwyer’s team found that blocking CD39 and CD73 is beneficial for the treatment of acute and chronic kidney diseases.38 HMGB1, located in the cell nucleus, is extensively involved in processes such as inflammation and tumors. It is associated with diabetic nephropathy and primary thrombocytopenia.39,40 Under stimulations by inducers, HMGB1 is released from the cell membrane.41 On one hand, HMGB1 stimulates innate immune cells by binding to TLR2 and TLR4.42 On the other hand, it forms complexes with CXCL12. With the help of chemokine receptor CXCR4, HMGB1 recruits immune cells to tumor cells.43 HSPs could also be released upon external or internal stimuli, and present tumor antigens to T cells, leading to the killing and elimination of tumor cells.44,45
During the ICD, DAMPs and pattern-recognition receptors interact to activate innate immune responses.46 DAMPs can also bind to antigen-presenting cells, promoting the maturation of DCs, phagocytosis of dead-cell antigens, and presentation of TAAs to CTLs and thus activating adaptive immunity.47 In addition to these advantages, pro-inflammatory cytokines are released in ICD, enhancing the activation of T cells and the immune stimulatory effects on tumor infiltration (Figure 1).48,49
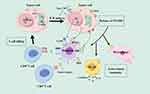 |
Figure 1 Mechanisms of ICD (by Figdraw). |
Different Inducers to Induce ICD
Inducers are required for ICD. Certain chemotherapy drugs (such as doxorubicin (DOX), cyclophosphamide, anthracyclines like mitoxantrone, curcumin, oxaliplatin, and platinum-based chemotherapy drugs like cisplatin),50–52 as well as radiotherapy,53 induce antitumor immune responses by inducing ICD in tumor cells. Several physical stimuli can also trigger ICD, such as photodynamic therapy (PDT)54 and photothermal therapy (PTT), which activate CD8+T cell responses through ER exposure, thereby initiating a cascade of ICD. In addition to these, lysosomes,55 nanopulse stimulation and oncolytic viro-therapy56 can induce ICDs.
Oxaliplatin, a commonly used chemotherapy drug, can increase the infiltration of immune cells such as CD8+T cells and promote DC maturation.57 Li used oxaliplatin as an inducer of ICD, and studied the efficacy of oxaliplatin in the treatment of lung cancer. Results showed that the ICD induced by oxaliplatin provided an immunogenic microenvironment and enhanced the therapeutic effect of Lewis lung cancer.58 PDT primarily induces cellular toxicity through the generation of reactive oxygen species, requiring the synergistic effects of photosensitizers, light, and molecular oxygen. Kensuke Kaneko’s experiments demonstrated that the sole injection of photosensitizer HS201 or laser irradiation does not significantly inhibit the growth of E0771 breast tumors. This finding implied that PDT’s tumor-killing effects required the involvement of photosensitizers and light.58 PTT selectively kills cancer cells by converting light energy into heat using an external light source.59 Wu used iron-based ternary chalcogenide nanoparticles, known as AGFES2, for the treatment of triple-negative breast cancer. AGFES2 acts as an ICD inducer, triggering an immune response by generating heat and promoting the release of tumor-specific antigens.60 In addition, ICD can be induced by radiotherapy. Li developed a nanoscale metal-organic framework that responds to radiation therapy in 2018. The radiodynamic therapy can trigger robust antitumor immune responses. The combination of the framework, X-ray and anti-PD-L1 (aPD-L1) therapy can amplify the antitumor immunotherapy and effectively prevent the growth and metastasis of osteosarcoma.61 ICD can be induced by these inducers in tumor cells to elicit antitumor immune responses.
ICIs
Tumor immunotherapy can be primarily classified into two categories: cellular immunotherapy and ICIs therapy. Currently, available ICIs include CTLA-4 inhibitors and PD-1/PD-L1 inhibitors.62,63 CTLA-4 expression is predominantly found on T cells, while PD-1/PD-L1 expression is more widespread.64,65 In the early stages of immune response, CTLA-4 inhibits T cell activity, whereas PD-1/PD-L1 regulates immune responses in peripheral tissues or tumor sites. CTLA-4 inhibitors, being a pioneering target for immunotherapy, have been extensively researched but still face challenges such as low response rates and the occurrence of autoimmune side effects. Therefore, this section primarily focuses on PD-1/PD-L1 inhibitors.
Mechanism of ICIs
ICIs block immune checkpoints to exert their antitumor effects. Under normal physiological conditions, PD-1 is expressed on activated T cells, B cells, NK cells, monocytes, and DCs. It interacts with PD-L1, PD-L2, and other molecules expressed on the surface of APCs to inhibit the excessive activation of T cells and maintain immune homeostasis in the body.66 In many types of tumors, PD-L1 molecules have abnormally high expression in tumor cell on their surface. These molecules bind to PD-1 molecules on tumor-infiltrating T lymphocytes, thereby inhibiting the normal activation of T cells, evading tumor cell killing by T cells, and ultimately achieving tumor immune escape.67 Anti-PD-1 (aPD-1) or a-PD-L1 antibodies can block the binding between tumor PD-L1 or tumor PD-1, eliminating this immunosuppressive effects. T cells are then reactivated, allowing them to recognize and kill cancer cells.68 At the tumor site, aPD-1 antibodies can also bind to Fcγ receptors on the surface of macrophages, mediating the depletion of regulatory T cells, thereby increasing the proportion of activated T cells and enhancing antitumor ability (Figure 2).69
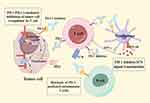 |
Figure 2 Mechanism of PD-1/PD-L1 inhibitors (by Figdraw). |
Type of ICIs
Currently, PD-1/PD-L1 inhibitors can be classified into two distinct categories: PD-1 antibodies and PD-L1 antibodies. The Food and Drug Administration (FDA) has thus far granted approval to 7 ICIs that target the PD1/PD-L1 pathway, consisting of 4 PD-1 blocking antibodies and 3 PD-L1 blocking antibodies.70 The PD-1 antibodies encompass dostarlimab, nivolumab, pembrolizumab, and cemiplimab, while the PD-L1 antibodies comprise atezolizumab, durvalumab, and avelumab.65 As of November 2022, China has a total of 16 ICIs available in the market. Among these, there are 8 domestically developed PD-1 monoclonal antibodies (Toripalimab,71 Sintilimab,72 Camrelizumab,73 Tislelizumab,74 Penpulimab,75 Zimberelimab,76 Srullimab, Puterimab), 2 domestically developed PD-L1 monoclonal antibodies (Envafolimab,77 Suglizumab), and 1 domestically developed dual antibody (PD-1/CTLA4) known as Cadonilimab.78 Additionally, there are 2 imported PD-1 inhibitors (Nivolumab,79 Pembrolizumab80), 2 imported PD-L1 inhibitors (Atelizumab,81 Durvalumab82), and 1 imported CTLA-4 monoclonal antibody (Ipilimumab).83 The availability of these drugs brings newfound optimism for cancer patients in China.
Advantages of Combination of ICD Inducers and PD-1/PD-L1 Inhibitors
ICD inducers and PD-1/PD-L1 inhibitors have the potential to enhance antitumor immunity. ICD inducers themselves present certain limitations, including the occurrence of severe toxic side effects caused by chemotherapy drugs that effectively induce ICD. Moreover, these drugs exhibit inadequate pharmacokinetics and tissue penetration, as well as weak targeting capabilities.84 PD-1/PD-L1 inhibitors encounter challenges associated with low immunogenicity and an immunosuppressive microenvironment.85,86 To solve these issues, the utilization of responsive nanocarriers as co-delivery vehicles can effectively harmonize the physicochemical properties of ICD inducers and PD-1/PD-L1 inhibitors. When PD-1/PD-L1 inhibitors combined with ICD inducers, they can increase CD8+T cell infiltration and convert “cold” tumors, which have low responsiveness to immunotherapy, into “hot” tumors with high immunotherapy responsiveness.
In a systematic review and meta-analysis of randomized controlled trials investigating ICIs monotherapy, ICIs plus chemotherapy, and chemotherapy alone for non-small cell lung cancer (NSCLC), the greatest survival benefit with the highest safety is observed in patients receiving pembrolizumab combined with platinum-based chemotherapy. This finding suggested that chemotherapy, as an ICD inducer, can synergize with PD-1/PD-L1 inhibitors to exert a combined antitumor effect.87 Although promising results have been achieved with the use of ICD inducers in combination with ICIs, many failures still occurred. In a similar trial, 297 patients treated with pembrolizumab and chemotherapy showed no statistically significant improvement.88 Additionally, in a clinical trial that received paclitaxel liposomal chemotherapy in combination with a PD-1/PD-L1 inhibitor as a first-line therapeutic agent, treatment-related adverse events of any grade were still observed in 25 patients, and adverse events of grade 3 or worse were observed in 9 patients.89 Therefore, the cocktail combination of ICD inducers with ICIs are not universally applicable in clinical practice.
Stimulus-Responsive Nanocarriers
Excellent specificity is possessed by nanocarriers. The passive targeting is achieved through the EPR effect, whereas active targeting is achieved through the surface modification of nanocarriers. Stimulus-responsive nanocarriers regulate drug release in response to exogenous or endogenous stimuli, and achieve rapid and precise responses.90 Exogenous stimulus-responsive and endogenous stimulus-responsive nanocarriers are separated from stimulus-responsive nanocarriers (Table 1). Photothermal stimulation and magnetic fields are exogenous stimuli, whereas redox reactions, pH, and enzymes are endogenous ones.91–93 Dual-stimulus responsive nanocarrier can play a synergistic role in the two stimuli to promote the release of anticancer drugs and improve the therapeutic effect (Table 2). Utilizing stimulus-responsive nanocarriers as drug-delivery vehicles, combined with ICD inducers and ICIs, can maximize the antitumor effects.
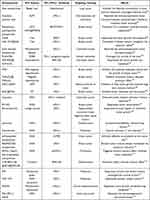 |
Table 1 Latest Progress of Research on the Use of Single Stimulus-Responsive Nanocarriers for the Co-Delivery of ICD Inducers and PD-1/PD-L1 Inhibitors |
 |
Table 2 Latest Progress of Research on the Use of Dual Stimulus-Responsive Nanocarriers for the Co-Delivery of ICD Inducers and PD-1/PD-L1 Inhibitors |
The Benefits of Stimulus-Responsive Nanocarriers in Facilitating the Combination of ICIs and ICD Inducers
Tumor immunotherapy targets a variety of TME components, including tumor cells, tumor-associated macrophages (TAMs), DCs, T cells, and various myeloid suppressor cells.128,129 On-demand release of tumor immunotherapeutic agents is crucial to achieving effective antitumor immunity while minimizing side effects. In recent years, with advances in nanoscience and materials chemistry, “smart” platforms for stimulating responses have shown great potential for addressing cancer immunotherapy (Table 3).130
 |
Table 3 Stimulus Response Sites of Stimulus-Responsive Nanocarriers |
Passive targeting in targeted therapy is mainly realized by adjusting the size, morphology, charge and other physicochemical properties of the carriers, while active targeting is mainly realized by modifying peptides, antibodies or antibody fragments on the surface of the nanocarriers.131 For different drugs and intracellular environments, different types of nanocarriers can be designed according to passive and/or active targeting, and then combined with stimulus-responsive materials to deliver payloads on demand. This greatly improves therapeutic efficacy and reduces adverse effects.
Exogenous Stimulus-Responsive Nanocarriers
Photo-Responsive Nanocarriers
Photo-responsive nanocarriers (Figure 3A) are constructed by combining photosensitizers with PD-1/PD-L1 inhibitors on nanocarriers, inducing potent ICD and enhancing targeting. Photosensitizers are substances that react when exposed to ultraviolet, visible, or infrared light. They are used to construct stimulus-responsive nanocarriers and to induce ICD through PDT. Photo-responsive nanocarriers combine photosensitizers with PD-1/PD-L1 inhibitors to achieve the combined therapy of ICD and immunotherapy.132
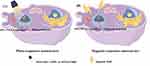 |
Figure 3 Schematic diagram of the release of exogenous stimulus-responsive nanocarriers under stimulus. (A) Photo-responsive nanocarriers. (B) Magnetic-responsive nanocarrier. (by Figdraw). |
Researchers constructed a mesoporous silica nanocarrier (MSNs) capable of responding to light, which loaded with the photosensitizer Rose Bengal, peptide vaccine AL-9, and PD-1/PD-L1 inhibitors for the treatment of bone metastasis in lung cancer in 2021.94 Another research attempted to improve antitumor immunity by further optimizing the nanoparticles size. Researchers developed a photo-sensitive, targeted nuclear nanoparticle delivery system using 5-nm graphene quantum dots to co-deliver the photosensitizer zinc phthalocyanine (ZnPc). This system effectively inhibited distant oral squamous cell carcinoma.95 Tian designed a photothermal-responsive mesoporous polydopamine nanocore with dual-mode magnetic resonance/photoacoustic imaging effect in 2023. It achieved precise release and reversed the immunosuppressive effects of PD-L1/CD47/Tregs.96 Zheng constructed a photo-sensitive, self-delivered nanodrug based on the photosensitizer Ce6, the IDO inhibitor NLG919, and the PD-1/PD-L1 inhibitor BMS-1. The drug platform can activate the cascade immune processes in photodynamic immunotherapy of metastatic tumors. And it was confirmed that the CeNB tremendously suppressed the tumor growth and metastasis.97 Liu designed a serum albumin (SA)-coated thin bauxite scaffold that can be loaded with chlorine e6 (Ce6), photosensitizers, and honey bee venom melittin (MLT). The Ce6/MLT@SAB with PD-1 inhibitor provided more effective control of distant tumors by aPD−1 therapy.98 PTT is also an effective method for tumor treatment. Xiao combined PD-1/PD-L1 inhibitors with photothermal ablation and developed PDA/GNS@aPD-L1 nanoparticles, gold nanostars modified with bio-inspired polydopamine functionalization. Photothermal ablation can induce ICD and reverse the ITM, providing a new strategy for colorectal cancer treatment.99 Yu developed a synthetic photo-sensitive nanoparticle, NP@IR780, which incorporated PD-L1 blockade for the purpose of cancer photothermal-immunotherapy. The utilization of PLGA-PEG nanoparticles loaded with IR780 resulted in significant photothermal effects and the induction of robust antitumor immunity.100 In order to address pancreatic cancer, a combination strategy involving mild hyperthermia and BMS-202 treatment was devised, utilizing size-adjustable thermo- and fibrotic matrix-sensitive liposomes. The results of antitumor analysis proved that subthermal therapy combined with ICB inhibited tumor growth and reduced the risk of tumor metastasis in subcutaneous.101
Magnetic-Responsive Nanocarriers
Magnetic-responsive nanocarriers (Figure 3B) hold great promise for targeted drug delivery, imaging, and chemothermal therapy for cancer.133 The nanoparticles can selectively attach onto functional molecules and allow transportation to target locations under an external magnetic field induced by an electromagnet or permanent magnet.134–136 Magnetothermal therapy (MHT) refers to the induction of tumor cytotoxicity by a magnetic thermal agent. This agent is capable of generating a large amount of heat when exposed to an applied alternating magnetic field (AMF). Compared with other thermotherapy methods, MHT has the unique advantage of high tissue penetration.137 A magnetic hyperthermia-responsive nanocarrier was prepared by Liu using ferromagnetic vortex-like iron oxide (FVIO) nanorings in 2023. FVIO-mediated mild magnetic hyperthermia combined with PD-L1 blockade can inhibit the potential metastatic spread and growth of distant tumors.102 Hu designed a superparamagnetic nanoparticle CoFe2O4@MnFe2O4 nanoparticle by increasing the saturation magnetization value in response to the magnetic environment. This nanoparticle combined magnetic hyperthermia with αPD-L1 treatment. The immunotherapy strategy has shown its efficacy in achieving complete ablation of the primary tumor. Moreover, it has a significant distant antitumor effect on distant simulated metastases.103 Zhang constructed a magnetic field mediated nanoplatform IONC-AAPH. This nanoplatform exhibited the capability to generate localized heat and carbon-centered free radicals. The combination of IONC-AAPH under alternating magnetic field with aPD-1 dramatically suppressed the growth of untreated distant tumors in deep tissue.104 Radiofrequency (RF)-responsive biGCs was synthesized by Zhang activating sequential redox reactions of LA and NaBH4. The integration of RF-responsive biGC@PNA nanoplatforms with decitabine induced a potent ICD effect and significantly inhibited tumor metastasis and recurrence.105
Endogenous Stimulus-Responsive Nanocarriers
Enzyme-Responsive Nanocarriers
Enzymes possess specific substrate selectivity and distinct bio-interactions, making them advantageous biological targets.138 In addition, enzyme-responsive nanocarriers (Figure 4A) can control drug release by targeting bio-enzymes that are only found in specific tissues in the body to achieve drug accumulation at the target site.139 The tumor microenvironment is a very rich environment in which many enzymes are overexpressed, such as matrix metalloproteinase (MMP2 and MMP9), esterase, alpha-amylase and Cathepsin B. Wang’s team constructed an enzyme-responsive camptothecin nanovesicles to the tumor microenvironment. Nanovesicles take advantage of the lipid bilayer cross-over ability of the ICD inducer DOX to coload the indoleamine 2, 3-dioxygenase inhibitor indolemod into their interior, triggering a powerful immune response.106 A prodrug nanoparticles (PD-NPs) that is enzyme sensitive in response to the tumor microenvironment was proposed by Moon, combined with aPD-L1 peptide, cathepsin B-specific cleavable peptide and DOX. DOX can induce ICD and aPD-L1 peptide induces immune-checkpoint blocking. The findings indicated that the utilization of PD-NPs for the targeted delivery of aPD-L1 and DOX effectively suppresses tumor advancement while minimizing adverse effects.107 A λ phage vaccine based on bionanoparticles was administered to mice with syngeneic HCC, in combination with PD-1 inhibition. Compared with control group, combination therapy (Vaccine+PD-1 inhibitor) significantly suppressed primary hepatic or mammary tumor growth.108 Zhu developed a lactate dehydrogenase (LDH)-responsive nanoplatform using TNF-α-loaded liposomes. Then approach that enhanced aPD-1/PD-L1 antitumor immunity.109 Wang developed an enzyme-sensitive nanovesicles as a novel delivery system for DOX and siRNA, achieving synergistic effects between PD-L1 blockade and DOX, finally leading to a potent therapeutic antitumor T cell response in melanoma mice.110
pH-Responsive Nanocarriers
Liposomes possess high biocompatibility and enhance the stability of certain drugs due to the protective effect of the bilayer structure. pH-sensitive nanocarrier systems (Figure 4B) are typically designed with a variety of pH-sensitive chemical bonds or chemical groups that undergo dissociation with changes in environmental pH. Thus, these chemical bonds or groups can release loaded therapeutic drugs in the target tumor area.140,141
Zhu developed amphiphilic DCS nanoparticles through the pH-sensitive linker hydrophobic stearyl chain, which effectively released loaded DOX and D-PPA in response to the slightly acidic tumor environment, blocked PD-1/PD-L1, avoided immune escape, and triggered an enhanced immune response.111 A pH-responsive DOX delivery nanosystem was designed and further combined with DOX and aPD-1 for antitumor. PEG/PEI/CAD nanoparticles can be stable during somatic circulation, and after entering mildly acidic tumors, they can escape the PEG shielding and effectively release the co-delivered drug.112 The researchers first utilized a PEG derivative to encapsulate DOX and second loaded two plasmids to form shPD-L1/Spam1 dual drug-loaded nanoparticles. While under acidic environment, this Schiff base fragment can easily be altered resulting in the dissociation of the nanosystem to release plasmids. This novel therapy effectively increased the degree of T-cell infiltration, triggering an immune memory effect and thus inhibiting tumor metastasis.113 Ge synthesized a pH-sensitive autophagy controlling nanocarrier. After entering tumor cells, nanocarrier induced autophagy and reduced intracellular pH, which in turn promoted the release of curcumin and enhanced autophagic activity. It finally showed satisfactory antitumor effect and strong immune memory effect.114
Redox-Responsive Nanocarriers
By modifying nanocarriers with redox sensitive bonds and/or linkers (such as disulfide bonds), oxidation-reduction reactions can be triggered to release therapeutic drugs encapsulated within the nanocarriers. Redox-responsive nanocarriers (Figure 4C) decorated with disulfide-based bonds and/or linkers can be cleaved by glutathione (GSH), enabling oxidation-reduction reactions for therapeutic responses.142,143 Xu developed a GSH/reactive oxygen species dual-responsive nanogel system (IMS) that actively targeted cancer cells overexpressing mannose receptors. Combined with aPD-1, it can effectively suppress primary and distant tumors and prolong the survival of mice.115 Xie’s group fabricated a phenolic ICD inducer that enhances ROS-dependent cell death, assembling DOX, a phenolic manganese dioxide nanoreactor, iron, and polyethylene glycol polyphenol (MDP NP). It effectively improved the tumor response to PD-1 checkpoint blockade immunotherapy and overcame immunosuppression.116 Jeong developed an oxidative stress-responsive peptide fluorinated and co-treated with αPD-L1. This peptide is able to target mitochondria and trigger mitochondrial dysfunction, thereby inducing oxidative stress-mediated ICD. The experiment significantly regressed tumor growth and prevented lung metastasis.117 A novel strategy was proposed to combine tumor-penetrating peptide iRGD with ROS-sensitive nanoparticles. The ROS-sensitive nanoparticles loaded FGL1 siRNA and PD-L1 siRNA. This project increased infiltration of effector CD8+T cells and effectively alleviated the immunosuppressive microenvironment of the tumor.118
Dual-Stimulus-Responsive Nanocarriers
pH and Redox Dual-Responsive Nanocarriers
pH and redox dual-responsive nanocarriers (Figure 5A), which combine redox-responsive with pH-responsive, playing a synergistic role to amplify the anticancer drug release and ultimately improving the therapeutic effect. The Pickering nanoemulsion (PNE) was designed using a redox-sensitive nanogel. PD-1/PD-L1 inhibitor HY19991, the core of PNE, and shell DOX were effectively released in response to the tumor microenvironment. The result showed that D/HY@PNE enhanced tumor penetration behavior and triggered potent antitumor effects.119 A pH and redox-sensitive nanocarrier was designed utilizing manganese ions and hydrazine functionalized macromolecules. Manganese oxide crosslinked bovine serum albumin/hyaluronic acid nanoparticles (BHM) can effectively alleviate tumor hypoxia and achieve complete eradication of liver cancer by loading DOX and ICG.120 Researchers constructed a fibronectin (FN)-coated Metal Phenolic Networks that combined pH and redox reactions. This system utilized the modification of FN to promote the targeting of drug delivery. Moreover, it achieved enhanced ICD and effective drug aggregation at the tumor site.121 Scientists have designed an upconversion nanoparticle UCNPs@Cu-Cys-GOx (UCCG) based on tumor microenvironment response. This system combined nanozymes to amplify ROS production in situ. Combined treatment with aPD-L1 antibody effectively inhibited the growth of primary tumors and tumor metastasis.122
pH and Light Dual-Responsive Nanocarriers
pH and light dual-responsive nanocarriers (Figure 5B), combining a degradable photosensitizer with a pH stimulator, could achieve the effects of synergistic therapeutic effect. A tumor microenvironment and near-infrared light dual-responsive prodrug hydrogel was developed by Ding. The alginate hydrogels released oxidized iron modified with PPIX and PD-L1 antibody prodrug nanoparticles, which can generate a large amount of reactive oxygen species and effectively treat breast cancer in a 4T1 mouse model.123 Other researchers designed a pH-responsive nanoplatform based on siRNA-loaded layered double hydroxides (LDHs). The photothermal effect generated by LDHs altered the “cold” immune microenvironment, promoting synergistic immunity and effectively inhibited the progression of primary hepatocellular carcinoma.124 Yue designed a light and pH-sensitive mesoporous silica nanocarrier MPSNs@R837. MPSNs@R837 induced high-efficiency ICD and promoted the maturation of dendritic cells, thereby inducing tumor-specific immune responses. Once combined with PD-1/PD-L1 inhibitor, the system significantly inhibited primary and metastatic tumors.125
pH and Enzyme Dual-Responsive Nanocarriers
The combination of pH-sensitive and enzyme-responsive (Figure 5C) have a combined effect on drug release. Su prepared a pH- and enzyme-responsive micellar nanocarrier by PEG layer and matrix protease modification. The weak acidity of the tumor microenvironment and the enriched matrix metalloproteinase-2 (MMP-2) triggered PEG shedding and aPD-1 release, respectively, promoting nanoparticles into cancer cells to play a synergistic antitumor effect.126 Liu constructed polymer-liposomes containing MMP and pH-responsive polymer chains. The polymeric liposomes promoted the release of aPD-L1 peptide and DOX under the stimulation of the tumor microenvironment, effectively disrupting the PD-1/PD-L1 interaction and enhancing the antitumor effect.127
New Horizons
On the basis of stimulus-responsive nanocarriers delivering ICD inducers and PD-1/PD-L1 inhibitors, we proposed some novel strategies to further improve the system, including further refining the nanocarriers, recommending a multimodal synergistic strategy, and taking into account the effects of drug combination ratio and population heterogeneity on experimental results in hopes that it will serve as valuable references for future research.
New Horizons on Nanocarrier Co-Delivery Systems
Considering the complexity of ICD and the synergy of immunotherapy, adjusting the size,144 morphology, surface charge, surface chemical modifications, and surface topological structures of nanocarriers can enhance the cellular uptake and internalization capabilities of co-delivery systems.145–147 This adjustment is also beneficial for in vivo tissue distribution and metabolism.
New Horizons on Multimodal Synergistic Enhancement of Antitumor Immunity
In the face of genetic mutations, immune evasion, invasion, and distant metastasis that may occur in cancer treatment, multimodal synergistic therapy demonstrates tremendous therapeutic potential. Combinatorial approaches involving ICD inducers and PD-1/PD-L1 inhibitors, together with gene therapy, local microwave hyperthermia, immunomodulatory adjuvants, natural products,148 and so forth, might result in remarkable superadditive effects. This may become a new research direction for synergistic therapy.149
New Horizons on Drug-Combination Ratios
Existing studies have indicated that combination anticancer treatments generally depend on the molar ratio of different drugs used in combination. In particular, such effects generally depend on the specific release proportions of these drugs within tumor tissues after systemic administration, which critically influence the therapeutic efficacy.148 So it is important to control precise ratios of ICD inducers and PD-1/PD-L1 inhibitors in nanocarriers to achieve the best antitumor immunity and greatest synergy.
New Horizon on the Heterogeneity of Patient Populations
The response to synergistic therapy may vary among different types of tumors, different patients with the same type of tumor, and even different regions within the same tumor. Research has found that tumor patients with enhanced oxidative metabolism exhibit poor response to PD-1 blockade therapy, but they are apparently more sensitive to oncolytic virus therapy.150 Thus, integrating these information and using them to design immunotherapy strategies that target a patient’s tumor-specific metabolism is one of the new directions for the future.
Future Prospects and Challenges
The activation and maturation of T-cells are facilitated by ICIs. However, the widespread distribution of T-cell populations throughout organs makes ICIs capable of inducing immune-related adverse events (irAEs) with varying frequencies and severities.151 These adverse events can impact virtually any organs due to the diverse characteristics of T-cells and their ability to infiltrate tissues. To ensure effective management of patients receiving ICIs, it is imperative to implement personalized monitoring strategies that align with the individual risk profile of each patient. This advancement will empower physicians to customize their approach accordingly. Furthermore, the augmented accumulation of immunotherapeutic agents at the tumor site may potentially result in the onset of autoimmune disorders, thereby exacerbating the incidence of irAEs. In addition, the intricate design of responsive drug delivery systems poses significant challenges for pharmaceutical companies in terms of scaling up production and facilitating commercialization. It is anticipated that future research endeavors, encompassing diverse interdisciplinary advancements, will significantly advance the progress of stimulus-responsive nano-co-delivery platforms, ultimately facilitating their translation into clinical practice.
Conclusion
In conclusion, the combination of immune checkpoint inhibitors with ICD represents a novel direction in the realm of immunotherapy. The induction of ICD can enhance the infiltration of tumor cells and modify the immunosuppressive microenvironment of tumors, addressing the limitations of PD-1/PD-L1 therapy. The recent advancements in stimulus-responsive nanocodelivery carriers offer a promising platform for effectively combining ICD inducers and inhibitors. Nevertheless, the successful application of stimulus-responsive nanocarriers in clinical settings is hindered by considerable challenges arising from intricate manufacturing procedures and inevitable drug seepage. Consequently, the development of drug delivery platforms that possess straightforward formulation, well-established preparation techniques, and feasible industrial-scale production is still an urgent problem for researchers to solve. By proposing the effect of modified nanocarrier, multi-modal synergies, drug combination rates, and population heterogeneity on the experimental results, we hope to provide valuable references for future research. This review provides a generic stimulus-responsive nanoplatform for combining ICD inducers and PD-1/PD-L1 inhibitors to achieve enhanced antitumor immunity for cancer immunotherapy. In a word, immunotherapy still has a long way to go in the field of cancer treatment, and stimulus-responsive co-delivery nanocarriers play a crucial role in harnessing their unique advantages.
Acknowledgments
This work was financially supported by the Natural Science Fund of Shandong Province (ZR2022LSW002, ZR2020KH015, ZR2019PH061, ZR2020QH221), the National Natural Science Foundation of China (No. 82002604, 81772281), the Shandong Province Taishan Scholar Project (No. ts201712067), the Support Plan for Youth Entrepreneurship and Technology of Colleges and Universities in Shandong (grant no. 2021KJ101).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li Z, Lai X, Fu S, et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci. 2022;9(22):e2201734. doi:10.1002/advs.202201734
2. Estapé Senti M, García Del Valle L, Schiffelers RM. mRNA delivery systems for cancer immunotherapy: lipid nanoparticles and beyond. Adv Drug Deliv Rev. 2024;206:115190. doi:10.1016/j.addr.2024.115190
3. Leonard WJ, Lin JX. Strategies to therapeutically modulate cytokine action. Nat Rev Drug Discov. 2023;22(10):827–854. doi:10.1038/s41573-023-00746-x
4. Liu Y, Adu-Berchie K, Brockman JM, et al. Cytokine conjugation to enhance T cell therapy. Proc Natl Acad Sci U S A. 2023;120(1):e2213222120. doi:10.1073/pnas.2213222120
5. Shojaie L, Bogdanov JM, Alavifard H, et al. Innate and adaptive immune cell interaction drives inflammasome activation and hepatocyte apoptosis in murine liver injury from immune checkpoint inhibitors. Cell Death Dis. 2024;15(2):140. doi:10.1038/s41419-024-06535-7
6. Zak KM, Grudnik P, Magiera K, Dömling A, Dubin G, Holak TA. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure. 2017;25(8):1163–1174. doi:10.1016/j.str.2017.06.011
7. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–528. doi:10.1038/s41573-021-00345-8
8. Niu C, Zhu K, Zhang J, et al. Analysis of immune-related adverse events in gastrointestinal malignancy patients treated with immune checkpoint inhibitors. Int, J, Cancer. 2024;154(7):1261–1271. doi:10.1002/ijc.34813
9. Desai J, Fong P, Moreno V, et al. A Phase 1/2 study of the PD-L1 inhibitor, BGB-A333, alone and in combination with the PD-1 inhibitor, tislelizumab, in patients with advanced solid tumours. Br J Cancer. 2023;128(8):1418–1428. doi:10.1038/s41416-022-02128-3
10. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi:10.1186/s12943-019-1091-2
11. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–680. doi:10.1002/anie.201804882
12. Kang X, Zhang Y, Song J, et al. A photo-triggered self-accelerated nanoplatform for multifunctional image-guided combination cancer immunotherapy. Nat Commun. 2023;14(1):5216. doi:10.1038/s41467-023-40996-2
13. Wang L, Geng H, Liu Y, et al. Hot and cold tumors: immunological features and the therapeutic strategies. MedComm. 2023;4(5):e343. doi:10.1002/mco2.343
14. Giustarini G, Pavesi A, Adriani G. Nanoparticle-based therapies for turning cold tumors hot: how to treat an immunosuppressive tumor microenvironment. Front Bioeng Biotechnol. 2021;9:689245. doi:10.3389/fbioe.2021.689245
15. Mao L, Ma P, Luo X, et al. Stimuli-responsive polymeric nanovaccines toward next-generation immunotherapy. ACS Nano. 2023;17(11):9826–9849. doi:10.1021/acsnano.3c02273
16. Cui L, Wang X, Liu Z, et al. Metal-organic framework decorated with glycyrrhetinic acid conjugated chitosan as a pH-responsive nanocarrier for targeted drug delivery. Int J Biol Macromol. 2023;240:124370. doi:10.1016/j.ijbiomac.2023.124370
17. Shi Y, Zhang Y, Zhu L, Miao Y, Zhu Y, Yue B. Tailored drug delivery platforms: stimulus-responsive core-shell structured nanocarriers. Adv Healthc Mater. 2024;13(1):e2301726. doi:10.1002/adhm.202301726
18. Batool S, Sohail S, Ud Din F, et al. A detailed insight of the tumor targeting using nanocarrier drug delivery system. Drug Deliv. 2023;30(1):2183815. doi:10.1080/10717544.2023.2183815
19. Mu W, Chu Q, Liu Y, Zhang N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nanomicro Lett. 2020;12(1):142. doi:10.1007/s40820-020-00482-6
20. Shim MK, Song SK, Jeon SI, Hwang KY, Kim K. Nano-sized drug delivery systems to potentiate the immune checkpoint blockade therapy. Expert Opin Drug Deliv. 2022;19(6):641–652. doi:10.1080/17425247.2022.2081683
21. Liang Y, Zhang J, Tian B, Wu Z, Svirskis D, Han J. A NAG-guided nano-delivery system for redox- and pH-triggered intracellularly sequential drug release in cancer cells. Int J Nanomed. 2020;15:841–855. doi:10.2147/IJN.S226249
22. Liang Y, Wang PY, Liu Z, et al. Dual stimuli-responsive micelles for imaging-guided mitochondrion-targeted photothermal/photodynamic/chemo combination therapy-induced immunogenic cell death. Int J Nanomed. 2023;18:4381–4402. doi:10.2147/IJN.S410047
23. Kepp O, Kroemer G. Immunogenic cell stress and death sensitize tumors to immunotherapy. Cells. 2023;12(24):2843. doi:10.3390/cells12242843
24. Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280(1):126–148. doi:10.1111/imr.12574
25. Luan Y, Luan Y, Jiao Y, et al. Broadening horizons: exploring mtDAMPs as a mechanism and potential intervention target in cardiovascular diseases. Aging Dis. 2023;1130. doi:10.14336/AD.2023.1130
26. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi:10.1038/nrc3380
27. Birmpilis AI, Paschalis A, Mourkakis A, et al. Immunogenic cell death, DAMPs and prothymosin α as a putative anticancer immune response biomarker. Cells. 2022;11(9):1415. doi:10.3390/cells11091415
28. Luo W, Yu Z. Calreticulin (CALR) mutation in myeloproliferative neoplasms (MPNs). Stem Cell Investig. 2015;2:16. doi:10.3978/j.issn.2306-9759.2015.08.01
29. Zhang M, Xiao J, Liu J, et al. Calreticulin as a marker and therapeutic target for cancer. Clin Exp Med. 2023;23(5):1393–1404. doi:10.1007/s10238-022-00937-7
30. Politis PK, Charonis AS. Calreticulin in renal fibrosis: a short review. J Cell Mol Med. 2022;26(24):5949–5954. doi:10.1111/jcmm.17627
31. Liu X, Xie P, Hao N, et al. HIF-1-regulated expression of calreticulin promotes breast tumorigenesis and progression through Wnt/β-catenin pathway activation. Proc Natl Acad Sci U S A. 2021;118(44):e2109144118. doi:10.1073/pnas.2109144118
32. Fucikova J, Spisek R, Kroemer G, Galluzzi L. Calreticulin and cancer. Cell Res. 2021;31(1):5–16. doi:10.1038/s41422-020-0383-9
33. Kielbik M, Szulc-Kielbik I, Klink M. Calreticulin-multifunctional chaperone in immunogenic cell death: potential significance as a prognostic biomarker in ovarian cancer patients. Cells. 2021;10(1):130. doi:10.3390/cells10010130
34. Bai X, Li Q, Peng X, et al. P2X7 receptor promotes migration and invasion of non-small cell lung cancer A549 cells through the PI3K/Akt pathways. Purinergic Sig. 2023. doi:10.1007/s11302-023-09928-z
35. Jiang Y, Lin J, Zheng H, Zhu P. The role of purinergic signaling in heart transplantation. Front Immunol. 2022;13:826943. doi:10.3389/fimmu.2022.826943
36. Kepp O, Bezu L, Yamazaki T, et al. ATP and cancer immunosurveillance. EMBO J. 2021;40(13):e108130. doi:10.15252/embj.2021108130
37. Pavelec CM, Young AP, Luviano HL, et al. Pannexin 1 channels control cardiomyocyte metabolism and neutrophil recruitment during non-ischemic heart failure. Preprint bioRxiv. 2023;573679. doi:10.1101/2023.12.29.573679
38. Dwyer KM, Kishore BK, Robson SC. Conversion of extracellular ATP into adenosine: a master switch in renal health and disease. Nat Rev Nephrol. 2020;16(9):509–524. doi:10.1038/s41581-020-0304-7
39. Yuan Y, Sun M, Jin Z, Zheng C, Ye H, Weng H. Dapagliflozin ameliorates diabetic renal injury through suppressing the self-perpetuating cycle of inflammation mediated by HMGB1 feedback signaling in the kidney. Eur J Pharmacol. 2023;943:175560. doi:10.1016/j.ejphar.2023.175560
40. Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol. 2015;21(5):1424–1435. doi:10.3748/wjg.v21.i5.1424
41. Chen R, Zou J, Kang R, Tang D. The redox protein HMGB1 in cell death and cancer. Antioxid Redox Signal. 2023;2. doi:10.1089/ars.2023.0007
42. Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. 2020;13(1):116. doi:10.1186/s13045-020-00950-x
43. Chen S, Tang W, Yu G, Tang Z, Liu E. CXCL12/CXCR4 axis is involved in the recruitment of NK cells by HMGB1 contributing to persistent airway inflammation and AHR during the late stage of RSV infection. J Microbiol. 2023;61(4):461–469. doi:10.1007/s12275-023-00018-8
44. Wang Q, Liu P, Wen Y, et al. Metal-enriched HSP90 nanoinhibitor overcomes heat resistance in hyperthermic intraperitoneal chemotherapy used for peritoneal metastases. Mol Cancer. 2023;22(1):95. doi:10.1186/s12943-023-01790-2
45. Hu C, Yang J, Qi Z, et al. Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm. 2022;3(3):e161. doi:10.1002/mco2.161
46. Sakai S, Shichita T. Role of alarmins in poststroke inflammation and neuronal repair. Semin Immunopathol. 2023;45(3):427–435. doi:10.1007/s00281-022-00961-5
47. Bai S, Yang LL, Wang Y, et al. Prodrug-based versatile nanomedicine for enhancing cancer immunotherapy by increasing immunogenic cell death. Small. 2020;16(19):e2000214. doi:10.1002/smll.202000214
48. Workenhe ST, Pol J, Kroemer G. Tumor-intrinsic determinants of immunogenic cell death modalities. Oncoimmunology. 2021;10(1):1893466. doi:10.1080/2162402X.2021.1893466
49. Zhang R, Kang R, Tang D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther. 2021;6(1):208. doi:10.1038/s41392-021-00613-4
50. Wang H, Chen Y, Wei R, et al. Synergistic chemoimmunotherapy augmentation via sequential nanocomposite hydrogel-mediated reprogramming of cancer-associated fibroblasts in osteosarcoma. Adv Mater. 2023;9591. doi:10.1002/adma.202309591
51. Wang A, Yang X, Li R, et al. Immunomodulator-mediated suppressive tumor immune microenvironment remodeling nanoplatform for enhanced immuno/chemo/photothermal combination therapy of triple negative breast cancer. ACS Appl Mater Interfaces. 2023;15(46):53318–53332. doi:10.1021/acsami.3c14137
52. Duo Y, Chen Z, Li Z, et al. Combination of bacterial-targeted delivery of gold-based AIEgen radiosensitizer for fluorescence-image-guided enhanced radio-immunotherapy against advanced cancer. Bioact Mater. 2023;30:200–213. doi:10.1016/j.bioactmat.2023.05.010
53. Liu YB, Chen XY, Yu BX, et al. Chimeric peptide-engineered self-delivery nanomedicine for photodynamic-triggered breast cancer immunotherapy by macrophage polarization. Small. 2023;94. doi:10.1002/smll.202309994
54. Ji S, Li J, Duan X, et al. Targeted enrichment of enzyme-instructed assemblies in cancer cell lysosomes turns immunologically cold tumors hot. Angew Chem Int Ed Engl. 2021;60(52):26994–27004. doi:10.1002/anie.202110512
55. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–4865. doi:10.1111/jcmm.14356
56. Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol Dordr. 2020;43(6):1203–1214. doi:10.1007/s13402-020-00552-2
57. Chang X, Bian M, Liu L, et al. Induction of immunogenic cell death by novel platinum-based anticancer agents. Pharmacol Res. 2023;187:106556. doi:10.1016/j.phrs.2022.106556
58. Kaneko K, Acharya CR, Nagata H, et al. Combination of a novel heat shock protein 90-targeted photodynamic therapy with PD-1/PD-L1 blockade induces potent systemic antitumor efficacy and abscopal effect against breast cancers. J Immunother Cancer. 2022;10(9):e004793. doi:10.1136/jitc-2022-004793
59. Wu Q, Li Z, Zhou X, et al. Photothermal ferrotherapy-induced immunogenic cell death via iron-based ternary chalcogenide nanoparticles against triple-negative breast cancer. Small. 2023;766. doi:10.1002/smll.202306766
60. Li T, Gao M, Wu Z, et al. Tantalum-zirconium co-doped metal-organic frameworks sequentially sensitize radio-radiodynamic-immunotherapy for metastatic osteosarcoma. Adv Sci. 2023;10(10):e2206779. doi:10.1002/advs.202206779
61. Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther. 2022;7(1):331. doi:10.1038/s41392-022-01136-2
62. Wang R, Kumar P, Reda M, et al. Nanotechnology applications in breast cancer immunotherapy. Small. 2023. doi:10.1002/smll.202308639
63. Kim GR, Choi JM. Current understanding of cytotoxic T lymphocyte antigen-4 (CTLA-4) signaling in T-cell biology and disease therapy. Mol Cells. 2022;45(8):513–521. doi:10.14348/molcells.2022.2056
64. Naimi A, Mohammed RN, Raji A, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal. 2022;20(1):44. doi:10.1186/s12964-022-00854-y
65. Chi Z, Lu Y, Yang Y, Li B, Lu P. Transcriptional and epigenetic regulation of PD-1 expression. Cell Mol Life Sci. 2021;78(7):3239–3246. doi:10.1007/s00018-020-03737-y
66. Pang K, Shi ZD, Wei LY, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist Updat. 2023;66:100907. doi:10.1016/j.drup.2022.100907
67. Pimenta J, Prada J, Pires I, Cotovio M. Programmed cell death-ligand 1 (PD-L1) immunohistochemical expression in equine melanocytic tumors. Animals. 2023;14(1):48. doi:10.3390/ani14010048
68. Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2(10):1001–1012. doi:10.1038/s42255-020-00280-9
69. Chen Y, Zhi S, Ou J, et al. Cancer cell membrane-coated nanoparticle co-loaded with photosensitizer and toll-like receptor 7 agonist for the enhancement of combined tumor immunotherapy. ACS Nano. 2023;17(17):16620–16632. doi:10.1021/acsnano.3c02724
70. Luo C, Chen H, Wu H, Liu Y, Li G, Lun W. Case report: toripalimab: a novel immune checkpoint inhibitor in advanced nasopharyngeal carcinoma and severe immune-related colitis. Front Immunol. 2023;14:1298902. doi:10.3389/fimmu.2023.1298902
71. Zeng TM, Yang G, Lou C, et al. Clinical and biomarker analyses of sintilimab plus gemcitabine and cisplatin as first-line treatment for patients with advanced biliary tract cancer. Nat Commun. 2023;14(1):1340. doi:10.1038/s41467-023-37030-w
72. Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international Phase 3 study. Lancet. 2023;402(10408):1133–1146. doi:10.1016/S0140-6736(23)00961-3
73. Ding K, Liu H, Ma J, et al. Tislelizumab with gemcitabine and oxaliplatin in patients with relapsed or refractory classic Hodgkin lymphoma: a multicenter Phase II trial. Haematologica. 2023;108(8):2146–2154. doi:10.3324/haematol.2022.282266
74. Shi Y, Gao L, Tian Y, et al. Penpulimab combined with anlotinib in patients with R/M HNSCC after failure of platinum-based chemotherapy: a single-arm, multicenter, phase II study. ESMO Open. 2023;8(6):102194. doi:10.1016/j.esmoop.2023.102194
75. Xia L, Wang J, Wang C, et al. Efficacy and safety of zimberelimab (GLS-010) monotherapy in patients with recurrent or metastatic cervical cancer: a multicenter, single-arm, phase II study. Int J Gynecol Cancer. 2023;33(12):1861–1868. doi:10.1136/ijgc-2023-004705
76. Markham A. Envafolimab: first approval. Drugs. 2022;82(2):235–240. doi:10.1007/s40265-022-01671-w
77. Keam SJ. Cadonilimab: first approval. Drugs. 2022;82(12):1333–1339. doi:10.1007/s40265-022-01761-9
78. Provencio M, Nadal E, González-Larriba JL, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2023;389(6):504–513. doi:10.1056/NEJMoa2215530
79. Lim SM, Peters S, Ortega Granados AL, et al. Dostarlimab or pembrolizumab plus chemotherapy in previously untreated metastatic non-squamous non-small cell lung cancer: the randomized PERLA phase II trial. Nat Commun. 2023;14(1):7301. doi:10.1038/s41467-023-42900-4
80. Schmid P, Turner NC, Barrios CH, et al. First-line ipatasertib, atezolizumab, and taxane triplet for metastatic triple-negative breast cancer: clinical and biomarker results. Clin Cancer Res. 2023. doi:10.1158/1078-0432.CCR-23-2084
81. Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the Phase III POSEIDON study. J Clin Oncol. 2023;41(6):1213–1227. doi:10.1200/JCO.22.00975
82. Rohaan MW, Borch TH, van den Berg JH, et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. 2022;387(23):2113–2125. doi:10.1056/NEJMoa2210233
83. Guo J, Zou Y, Huang L. Nano delivery of chemotherapeutic ICD inducers for tumor immunotherapy. Small Methods. 2023;7(5):e2201307. doi:10.1002/smtd.202201307
84. Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):24. doi:10.1186/s13045-022-01242-2
85. Han X, Li H, Zhou D, Chen Z, Gu Z. Local and targeted delivery of immune checkpoint blockade therapeutics. Acc Chem Res. 2020;53(11):2521–2533. doi:10.1021/acs.accounts.0c00339
86. Jelinek T, Mihalyova J, Kascak M, Duras J, Hajek R. PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology. 2017;152(3):357–371. doi:10.1111/imm.12788
87. Verschueren MV, Peters BJ, Bloem LT, et al. Pembrolizumab plus chemotherapy per PD-L1 stratum in patients with metastatic non-small cell lung cancer: real-world effectiveness versus trial efficacy. Clin Lung Cancer. 2023. doi:10.1016/j.cllc.2023.12.011
88. Li R, Liang H, Li J, et al. Paclitaxel liposome (lipusu) based chemotherapy combined with immunotherapy for advanced non-small cell lung cancer: a multicenter, retrospective real-world study. BMC Cancer. 2024;24(1):107. doi:10.1186/s12885-024-11860-3
89. Luo S, Lv Z, Yang Q, Chang R, Wu J. Research progress on stimulus-responsive polymer nanocarriers for cancer treatment. Pharmaceutics. 2023;15(7):1928. doi:10.3390/pharmaceutics15071928
90. Majumder J, Minko T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin Drug Deliv. 2021;18(2):205–227. doi:10.1080/17425247.2021.1828339
91. Yan Z, Liu Y, Zhao L, et al. In situ stimulus-responsive self-assembled nanomaterials for drug delivery and disease treatment. Mater Horiz. 2023;10(9):3197–3217. doi:10.1039/d3mh00592e
92. Karimi M, Ghasemi A, Sahandi Zangabad P, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45(5):1457–1501. doi:10.1039/c5cs00798d
93. Kaushik N, Borkar SB, Nandanwar SK, Panda PK, Choi EH, Kaushik NK. Nanocarrier cancer therapeutics with functional stimuli-responsive mechanisms. J Nanobiotechnol. 2022;20(1):152. doi:10.1186/s12951-022-01364-2
94. Wang Z, Chen L, Ma Y, et al. Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal. J Nanobiotechnol. 2021;19(1):243. doi:10.1186/s12951-021-00975-5
95. Zhang X, Yi C, Zhang L, et al. Size-optimized nuclear-targeting phototherapy enhances the type I interferon response for “cold” tumor immunotherapy. Acta Biomater. 2023;159:338–352. doi:10.1016/j.actbio.2023.01.023
96. Tian Y, Younis MR, Zhao Y, et al. Precision delivery of dual immune inhibitors loaded nanomodulator to reverse immune suppression for combinational photothermal-immunotherapy. Small. 2023;19(21):e2206441. doi:10.1002/smll.202206441
97. Zheng RR, Zhao LP, Yang N, et al. Cascade immune activation of self-delivery biomedicine for photodynamic immunotherapy against metastatic tumor. Small. 2023;19(3):e2205694. doi:10.1002/smll.202205694
98. Liu H, Hu Y, Sun Y, et al. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano. 2019;13(11):12638–12652. doi:10.1021/acsnano.9b04181
99. Xiao Y, Zhu T, Zeng Q, Tan Q, Jiang G, Huang X. Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy. Acta Biomater. 2023;157:451–466. doi:10.1016/j.actbio.2022.11.043
100. Yu Y, Li J, Song B, et al. Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials. 2022;280:121312. doi:10.1016/j.biomaterials.2021.121312
101. Yu Q, Tang X, Zhao W, et al. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021;133:244–256. doi:10.1016/j.actbio.2021.05.002
102. Liu X, Zheng J, Sun W, et al. Ferrimagnetic vortex nanoring-mediated mild magnetic hyperthermia imparts potent immunological effect for treating cancer metastasis. ACS Nano. 2019;13(8):8811–8825. doi:10.1021/acsnano.9b01979
103. Pan J, Hu P, Guo Y, et al. Combined magnetic hyperthermia and immune therapy for primary and metastatic tumor treatments. ACS Nano. 2020;14(1):1033–1044. doi:10.1021/acsnano.9b08550
104. Zhang L, Zhang Q, Hinojosa DT, et al. Multifunctional magnetic nanoclusters can induce immunogenic cell death and suppress tumor recurrence and metastasis. ACS Nano. 2022;16(11):18538–18554. doi:10.1021/acsnano.2c06776
105. Zhang Q, Shi D, Guo M, Zhao H, Zhao Y, Yang X. Radiofrequency-activated pyroptosis of bi-valent gold nanocluster for cancer immunotherapy. ACS Nano. 2023;17(1):515–529. doi:10.1021/acsnano.2c09242
106. Wang Z, Little N, Chen J, et al. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat Nanotechnol. 2021;16(10):1130–1140. doi:10.1038/s41565-021-00950-z
107. Moon Y, Shim MK, Choi J, et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics. 2022;12(5):1999–2014. doi:10.7150/thno.69119
108. Bai X, Zhou Y, Yokota Y, et al. Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J Exp Clin Cancer Res. 2022;41(1):132. doi:10.1186/s13046-022-02307-3
109. Xia GQ, Lei TR, Yu TB, Zhou PH. Nanocarrier-based activation of necroptotic cell death potentiates cancer immunotherapy. Nanoscale. 2021;13(2):1220–1230. doi:10.1039/d0nr05832g
110. Wang C, Shi X, Song H, et al. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials. 2021;268:120579. doi:10.1016/j.biomaterials.2020.120579
111. Zhu W, Bai Y, Zhang N, et al. A tumor extracellular pH-sensitive PD-L1 binding peptide nanoparticle for chemo-immunotherapy of cancer. J Mater Chem B. 2021;9(20):4201–4210. doi:10.1039/d1tb00537e
112. Jiang M, Chen W, Yu W, et al. Sequentially pH-responsive drug-delivery nanosystem for tumor immunogenic cell death and cooperating with immune checkpoint blockade for efficient cancer chemoimmunotherapy. ACS Appl Mater Interfaces. 2021;13(37):43963–43974. doi:10.1021/acsami.1c10643
113. Wu J, Chen J, Feng Y, et al. An immune cocktail therapy to realize multiple boosting of the cancer-immunity cycle by combination of drug/gene delivery nanoparticles. Sci Adv. 2020;6(40):eabc7828. doi:10.1126/sciadv.abc7828
114. Ge YX, Zhang TW, Zhou L, et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials. 2022;282:121407. doi:10.1016/j.biomaterials.2022.121407
115. Xu J, Qiu W, Liang M, et al. Dual-stimulus phototherapeutic nanogel for triggering pyroptosis to promote cancer immunotherapy. J Control Release. 2023;358:219–231. doi:10.1016/j.jconrel.2023.04.030
116. Xie L, Wang G, Sang W, et al. Phenolic immunogenic cell death nanoinducer for sensitizing tumor to PD-1 checkpoint blockade immunotherapy. Biomaterials. 2021;269:120638. doi:10.1016/j.biomaterials.2020.120638
117. Jeong SD, Jung BK, Ahn HM, et al. Immunogenic cell death inducing fluorinated mitochondria-disrupting helical polypeptide synergizes with PD-L1 immune checkpoint blockade. Adv Sci. 2021;8(7):2001308. doi:10.1002/advs.202001308
118. Wan WJ, Huang G, Wang Y. Coadministration of iRGD peptide with ROS-sensitive nanoparticles co-delivering siFGL1 and siPD-L1 enhanced tumor immunotherapy. Acta Biomater. 2021;136:473–484. doi:10.1016/j.actbio.2021.09.040
119. Jia L, Pang M, Fan M, et al. A pH-responsive Pickering nanoemulsion for specified spatial delivery of immune checkpoint inhibitor and chemotherapy agent to tumors. Theranostics. 2020;10(22):9956–9969. doi:10.7150/thno.46089
120. Hou G, Qian J, Guo M, et al. Hydrazide-manganese coordinated multifunctional nanoplatform for potentiating immunotherapy in hepatocellular carcinoma. J Colloid Interface Sci. 2022;628(Pt B):968–983. doi:10.1016/j.jcis.2022.08.091
121. Xu Y, Guo Y, Zhang C, et al. Fibronectin-coated metal-phenolic networks for cooperative tumor chemo-/chemodynamic/Immune therapy via enhanced ferroptosis-mediated immunogenic cell death. ACS Nano. 2022;16(1):984–996. doi:10.1021/acsnano.1c08585
122. Wang M, Chang M, Li C, et al. Tumor-microenvironment-activated reactive oxygen species amplifier for enzymatic cascade cancer starvation/chemodynamic/immunotherapy. Adv Mater. 2022;34(4):e2106010. doi:10.1002/adma.202106010
123. Ding M, Fan Y, Lv Y. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022;149:334–346. doi:10.1016/j.actbio.2022.06.041
124. Lu YF, Zhou JP, Zhou QM. Ultra-thin layered double hydroxide-mediated photothermal therapy combine with asynchronous blockade of PD-L1 and NR2F6 inhibit hepatocellular carcinoma. J Nanobiotechnol. 2022;20(1):351. doi:10.1186/s12951-022-01565-9
125. Yue J, Mei Q, Wang P, Miao P, Dong WF, Li L. Light-triggered multifunctional nanoplatform for efficient cancer photo-immunotherapy. J Nanobiotechnol. 2022;20(1):181. doi:10.1186/s12951-022-01388-8
126. Su Z, Xiao Z, Wang Y. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-Sensitive micelles for enhanced tumor chemoimmunotherapy. Small. 2020;16(7):e1906832. doi:10.1002/smll.201906832
127. Liu Y, Chen XG, Yang PP, Qiao ZY, Wang H. Tumor microenvironmental pH and enzyme dual responsive polymer-Liposomes for synergistic treatment of cancer immuno-chemotherapy. Biomacromolecules. 2019;20(2):882–892. doi:10.1021/acs.biomac.8b01510
128. Dahri M, Beheshtizadeh N, Seyedpour N, et al. Biomaterial-based delivery platforms for transdermal immunotherapy. Biomed Pharmacother. 2023;165:115048. doi:10.1016/j.biopha.2023.115048
129. Rui R, Zhou L, He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. 2023;14:1212476. doi:10.3389/fimmu.2023.1212476
130. Li L, Yang Z, Chen X. Recent advances in stimuli-responsive platforms for cancer immunotherapy. Acc Chem Res. 2020;53(10):2044–2054. doi:10.1021/acs.accounts.0c00334
131. Vincent MP, Navidzadeh JO, Bobbala S, Scott EA. Leveraging self-assembled nanobiomaterials for improved cancer immunotherapy. Cancer Cell. 2022;40(3):255–276. doi:10.1016/j.ccell.2022.01.006
132. Shin Y, Husni P, Kang K. Recent advances in pH- or/and photo-responsive nanovehicles. pharmaceutics. 2021;13(5):725. doi:10.3390/pharmaceutics13050725
133. Chauhan M, Basu SM, Qasim M, Giri J. Polypropylene sulphide coating on magnetic nanoparticles as a novel platform for excellent biocompatible, stimuli-responsive smart magnetic nanocarriers for cancer therapeutics. Nanoscale. 2023;15(16):7384–7402. doi:10.1039/d2nr05218k
134. Yusefi M, Shameli K, Jahangirian H. How magnetic composites are effective anticancer therapeutics? A comprehensive review of the literature. Int J Nanomed. 2023;18:3535–3575. doi:10.2147/IJN.S375964
135. Zhang K, Qi C, Cai K. Manganese-based tumor immunotherapy. Adv Mater. 2023;35(19):e2205409. doi:10.1002/adma.202205409
136. Eslami P, Albino M, Scavone F. Smart magnetic nanocarriers for multi-stimuli on-demand drug delivery. Nanomaterials. 2022;12(3):303. doi:10.3390/nano12030303
137. Ge J, Yang N, Yang Y. The combination of eddy thermal effect of biodegradable magnesium with immune checkpoint blockade shows enhanced efficacy against osteosarcoma. Bioact Mater. 2023;25:73–85. doi:10.1016/j.bioactmat.2023.01.008
138. Kapalatiya H, Madav Y, Tambe VS, Wairkar S. Enzyme-responsive smart nanocarriers for targeted chemotherapy: an overview. Drug Deliv Transl Res. 2022;12(6):1293–1305. doi:10.1007/s13346-021-01020-6
139. Zhao N, Zhu L, Liu M, He L, Xu H, Jia J. Enzyme-responsive lignin nanocarriers for triggered delivery of abamectin to control plant root-knot nematodes (Meloidogyne incognita). J Agric Food Chem. 2023;71(8):3790–3799. doi:10.1021/acs.jafc.2c07466
140. Jia N, Gao Y, Li M. Metabolic reprogramming of proinflammatory macrophages by target delivered roburic acid effectively ameliorates rheumatoid arthritis symptoms. Signal Transduct Target Ther. 2023;8(1):280. doi:10.1038/s41392-023-01499-0
141. Ding H, Tan P, Fu S. Preparation and application of pH-responsive drug delivery systems. J Control Release. 2022;348:206–238. doi:10.1016/j.jconrel.2022.05.056
142. Lee CG, Kwon TH. Controlling morphologies of redox-responsive polymeric nanocarriers for a smart drug delivery system. Chemistry. 2023;29(34):e202300594. doi:10.1002/chem.202300594
143. Mollazadeh S, Mackiewicz M, Yazdimamaghani M. Recent advances in the redox-responsive drug delivery nanoplatforms: a chemical structure and physical property perspective. Mater Sci Eng C Mater Biol Appl. 2021;118:111536. doi:10.1016/j.msec.2020.111536
144. Zhang W, Zhu D, Tong Z, et al. Influence of surface ligand density and particle size on the penetration of the blood-brain barrier by porous silicon nanoparticles. Pharmaceutics. 2023;15(9):2271. doi:10.3390/pharmaceutics15092271
145. Nezhadi S, Dorkoosh FA. Co-delivery systems: hope for clinical application? Drug Deliv Transl Res. 2022;12(6):1339–1354. doi:10.1007/s13346-021-01041-1
146. Chen D, Liu X, Lu X, Tian J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Front Pharmacol. 2023;14:1111991. doi:10.3389/fphar.2023.1111991
147. Al Bostami RD, Abuwatfa WH, Husseini GA. Recent advances in nanoparticle-based co-delivery systems for cancer therapy. Nanomaterials. 2022;12(15):2672. doi:10.3390/nano12152672
148. Liang Y, Liu ZY, Wang PY, Li YJ, Wang RR, Xie SY. Nanoplatform-based natural products co-delivery system to surmount cancer multidrug-resistant. J Control Release. 2021;336:396–409. doi:10.1016/j.jconrel.2021.06.034
149. Qi J, Jin F, You Y, et al. Synergistic effect of tumor chemo-immunotherapy induced by leukocyte-hitchhiking thermal-sensitive micelles. Nat Commun. 2021;12(1):4755. doi:10.1038/s41467-021-24902-2
150. DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21(12):785–797. doi:10.1038/s41577-021-00541-y
151. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi:10.1038/s41571-019-0218-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.