Back to Journals » Journal of Inflammation Research » Volume 16
Advanced Lung Cancer Inflammation Index for Predicting Prognostic Risk for Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention
Authors Wang X , Wei C , Fan W, Sun L , Zhang Y, Sun Q, Liu Y, Liu J
Received 28 May 2023
Accepted for publication 15 August 2023
Published 23 August 2023 Volume 2023:16 Pages 3631—3641
DOI https://doi.org/10.2147/JIR.S421021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xinchen Wang,1 Chen Wei,1 Wenjun Fan,1 Lixian Sun,1 Ying Zhang,1 Qiyu Sun,2 Yixiang Liu,1 Jingyi Liu1
1Department of Cardiology, The Affiliated Hospital of Chengde Medical University, The Chengde Institute of Cardiovascular Diseases, Chengde, Hebei, 067000, People’s Republic of China; 2Department of Clinical Laboratory, The Affiliated Hospital of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China
Correspondence: Lixian Sun, Department of Cardiology, The Affiliated Hospital of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China, Tel +86 314 227 9016, Fax +86 314 227 4895, Email [email protected]
Purpose: The decreased advanced lung cancer inflammation index (ALI), defined as body mass index (BMI) * albumin (Alb)/neutrophil-to-lymphocyte ratio (NLR), is an independent prognostic risk factor for overall survival in gastric, lung, and colorectal cancers. This study aimed to investigate the value of ALI in predicting the risk of major adverse cardiovascular events (MACEs) in patients with acute coronary syndrome (ACS).
Patients and Methods: A total of 1624 patients with ACS undergoing percutaneous coronary intervention (PCI) were consecutively enrolled between January 2016 and December 2018. Follow-up data were collected at 1, 3, 6, and 12 months and annually thereafter. The primary endpoints were MACEs. All endpoints were defined as all-cause mortality, recurrent angina pectoris, restenosis/intra stent thrombosis, stroke, heart failure, and all-cause bleeding.
Results: The MACEs group and non-MACEs group showed significant differences in patients with age > 65 years (28 [50.0%] vs 319 [23.7%]), history of heart failure (16 [28.6%] vs 127 [9.4%]), history of ischemic stroke (14 [25.0%] vs 186 [13.8%]), history of cardiogenic shock (6 [10.71%] vs 16 [1.19%]), left ventricular ejection fraction < 40% (8 [14.29%] vs 33 [2.46%]), and ALI < 343.96 (44 [78.65%] vs 680 [50.60%]) (all p< 0.001). The optimal cut-off value for ALI was 334.96. The area under the curve (AUC) of the 1-, 2-, 3-, and 5-year was 0.560, 0.577, 0.665, and 0.749, respectively. The survival rate was significantly lower in the low ALI group than in the high ALI group (log-rank p< 0.001). Low ALI was an independent risk factor for the long-term prognosis of patients with ACS after PCI, univariate HR: 3.671, 95% CI: 1.938– 6.953, p< 0.001; multivariate HR: 3.009, 95% CI: 1.57– 5.769, p=0.001.
Conclusion: ALI score less than 334.96 is an independent prognostic risk factor for patients with ACS undergoing PCI and may be a novel marker for clinical practice.
Keywords: acute coronary syndrome, advanced lung cancer inflammation index, prognosis, percutaneous coronary intervention
Introduction
Recent research shows that more than 7 million people are newly diagnosed with acute coronary syndrome (ACS) each year.1 Although great progress has been made in the diagnosis and treatment of ACSs, such as the development of percutaneous coronary intervention and dual antiplatelet therapy and the high prevalence of coronary computed tomographic angiography, ACS remains the leading cause of death worldwide.2–5 Accurate assessment of prognostic risk and standard follow-up are widely recognized to be important approaches to improving patient survival.6 In recent years, research attention has focused on different kinds of inflammatory indices, as a convenient and noninvasive measure for diagnosing and assessing prognostic risks.7–9
The advanced lung cancer inflammation index (ALI) is a novel index that was firstly reported by Jafri et al in 2013.10 This index is defined as body mass index (BMI) * albumin (Alb)/neutrophil-to-lymphocyte ratio (NLR). BMI is calculated as the height (m)/ weight (kg)^2. The NLR is involved in inflammation, and both BMI and albumin are associated with systemic nutritional status. Numerous studies have shown that inflammation and nutrient status are correlated with coronary heart disease (CAD).11 The derived neutrophil-to-lymphocyte ratio is an novel independent predictor of mortality in patients undergoing PCI.12 In oncology, decreased ALI is an independent prognostic risk factor for overall survival in gastric, lung, and colorectal cancers.13–15 Our previous study using ALI-based nomograms also showed the diagnostic significance of ALI.16
However, the ability of ALI to predict the prognostic risk in patients with ACS undergoing PCI remains unknown. Thus, this study aimed to investigate the value of ALI in predicting the risk of MACEs in patients with ACS undergoing PCI.
Materials and Methods
Study Design and Population
This prospective cohort study was approved by the Ethics Committee of the Affiliated Hospital of Chengde Medical University (Number: LL2021036) and was conducted according to the tenets of the Declaration of Helsinki. All participants provided informed consent.
In this study, 1624 patients with ACS who underwent PCI were consecutively enrolled between January 2016 and December 2018 at the Affiliated Hospital of Chengde Medical University. The inclusion and exclusion criteria were based on our previous study.12 Particularly, age was adjusted to >18 years. Patient data during hospitalization were collected by postgraduates who received professional education, using standard procedures. The diagnostic criteria for hypertension, type 2 diabetes mellitus, smoking, dyslipidemia, and ischemic stroke were as described in our previous study.17 Details about PCI, premedication, and the definition of successful PCI have also been included in our previous study.17 Dual antiplatelet therapy including ticagrelor or clopidogrel and other secondary prevention were administrated for the patients with ACS after PCI at least 12 months as suggested by “2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet, Therapy in Patients With Coronary Artery Disease”. All laboratory data were collected within 24 hours after ACS diagnosis and before PCI. The specifications and models of the testing instruments can also be found in our previous study.17
Follow-Up and Endpoints
Follow-up data were collected via a review of electronic medical records and/or clinic visits at 1, 3, 6, and 12 months and annually thereafter. The primary study endpoints were MACEs, including all-cause mortality and the requirement for rehospitalization with severe heart failure (HF). All-cause mortality was defined as death of any cause. Severe HF was defined as a New York Heart Association classification grade IV. All endpoints were defined as all-cause mortality, ACS recurrence/cardiac ischemia/angina, restenosis/intrastent thrombosis, stroke/transient ischemic attack, heart failure, all-cause bleeding.18
Statistical Analysis
The normality of distribution of continuous variables was confirmed using the Kolmogorov–Smirnov test, and normally and non-normally distributed variables were presented as the mean ± standard deviation and as the median with interquartile range, respectively. Differences in non-normally distributed continuous variables between the MACEs and non-MACEs groups, which was the same as the low and high ALI groups, were analyzed using the Mann–Whitney U-test. Meanwhile, categorical variables were presented as numbers (%) and compared using the χ2 test. Survival was estimated using the Kaplan–Meier method and compared between the groups using the Log rank test. The diagnostic value of ALI was evaluated using receiver operating characteristic (ROC) curves, and the optimal cut-off value was determined using Youden’s index (sensitivity + specificity - 1). Before trend analysis, the ALI index was divided equally into three: T1, T2, and T3. Significant variables in the univariate Cox proportional hazard model (ie, those with P<0.3) were entered into a multivariate Cox hazard proportional model. In the univariate and multivariate Cox hazard proportional hazards models and p for trend, age was divided into four categories (<55, 56–65, 66–75, and >76 years) as ranking variables. The R package time ROC and survival were used to plot time-dependent ROC curves, and the R package rms was used to plot the restricted cubic spline (RCS). All statistical analyses were performed using SPSS (version 26; SPSS Inc., Chicago, IL, USA), GraphPad Prism 8.0 (GraphPad Software Inc, La Jolla, CA, USA) and R 4.2.2. P<0.05 was considered statistically significant.
Results
Patient Characteristics
Among the 1624 patients, 28 patients were excluded due to infectious diseases (n=19), blood system diseases (n=5), malignant tumors (n=4), and hypertrophic cardiomyopathy (n=1). In addition, 195 patients were lost to follow-up. Thus, 1400 patients who completed the follow-up were included in the final analysis. The median follow-up time was 1150 days. There were 56 patients who developed MACEs; among them, 51 patients died and 5 patients required rehospitalization for severe HF (Figure 1). Table 1 shows the characteristics of the patients in the MACEs (n=56) and non-MACEs (n=1344) groups. The MACEs group and non-MACEs group showed significant differences in the proportion of patients with age >65 years (28 [50.0%] vs 319 [23.7%]), history of HF (16 [28.6%] vs 127 [9.4%]), history of ischemic stroke (14 [25.0%] vs 186 [13.8%]), history of cardiogenic shock (6 [10.71%] vs 16 [1.19%]), left ventricular ejection fraction (LVEF) <40% (8 [14.29%] vs 33 [2.46%]), ALI <343.96 (44 [78.65%] vs 680 [50.60%]), in the levels of UA (13 [23.2%] vs 528 [39.3%]), albumin (39.8 [37.63–42.05] vs 41.82 [39.30–44.10]), and creatinine (82.64±30.25 vs 69.29±16.26) (all p<0.001). Patients in the MACEs group tended to be aged >65 years and have a history of HF (HF, ischemic stroke, and cardiogenic shock.
 |
Table 1 Baseline Patient Characteristics of the MACEs and Non-MACEs Groups |
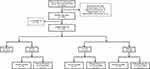 |
Figure 1 Patient selection flowchart. |
The optimal ALI cutoff value was 343.96, and 724 and 676 patients were assigned to the low and high ALI groups, respectively. The groups showed significant differences in the number of patients with male sex (567 [78.31%] vs 479 [70.86%]), dyslipidemia (364 [50.28%] vs 434 [64.20%]), type 2 diabetes mellitus (170 [23.448%] vs 190 [28.11%]), smoking history (401 [55.39%] vs 320 [47.33%]), history of HF (98 [13.54%] vs 45 [6.66%]), family history of CAD (92 [12.71%] vs 114 [16.86%]), UA (141 [19.48%] vs 400 [59.17%]), ST-elevation myocardial infarction (STEMI) (456 [62.98%] vs 168 [24.85%]), and MACEs (44 [6.08%] vs 12 [1.78%]) (all p<0.05). The WBC count (10.06±3.54 vs 7.28±2.18), Alb level (40.4 [37.91–42.80] vs 41.82 [39.30–44.10]), and creatinine (Cr) level (71.26±18.77 vs 68.29±15.26) were also significantly different between the low and high ALI groups (all p<0.05). Low ALI was associated with male sex, smoking, history of HF, family history of CAD, STEMI, high WBC count, high Cr levels, and LVEF <40% (Table 2).
 |
Table 2 Baseline Characteristics of the Low and High ALI Groups |
Receiver Operating Characteristic Curve, Time-Dependent ROC, and Survival Analysis
The AUC for ALI was 0.632 (p =0.001, 95% confidence interval [CI], 0.557–0.707). Based on the Youden’s index, and the optimal diagnostic cutoff value for ALI was 330.49, with a sensitivity of 68.30% and a specificity of 55.50%. Figure 2A shows the time-dependent ROC. The 1-, 2-, 3-, and 5-year AUCs were 0.560, 0.577, 0.665, and 0.749, respectively. As shown in Figure 2B, the AUC and 95% CI tended to increase with time. The time-dependent AUC showed an increasing tendency, suggesting that the diagnostic efficiency of ALI increased with time.
 |
Figure 2 (A) Time-dependent receiver operating characteristic plotted by R. (B) AUC tends to increase with time. |
The Kaplan–Meier curve (Figure 3) showed that compared with the high ALI group, the low ALI group had lower cumulative survival, and the difference was significant (log-rank p < 0.001).
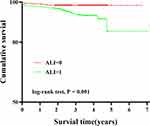 |
Figure 3 Kaplan–Meier curves of cumulative survival by ALI in ACS patients undergoing PCI (log-rank p < 0.001). |
Univariate and Multivariate Cox Hazard Proportional Models
The univariate Cox proportional hazard model showed that a low ALI (<343.96) was an independent risk factor for patients with ACS undergoing PCI (HR: 3.671, 95% CI: 1.938–6.953, P<0.001). Thus, it was entered into the multivariate Cox hazard proportional model. The other significant factors were age category (p<0.001), STEMI diagnosis (p=0.14), history of ischemic stroke (p=0.022), history of HF (p<0.001), family history of CAD (p=0.187), occurrence of cardiogenic shock (p<0.001), left ventricular end-diastolic diameter (LVEDD) >53% for males or LVEDD <50% for females (p=0.225), LVEF <40% (p<0.001). Age, occurrence of cardiogenic shock, LVEF <40%, and ALI <343.96 were finally selected in the multivariate Cox hazard proportional model through adjusted variables, and the variables that effectively influenced the prognosis of ACS patients were selected. The results showed that age category (per 1 category) (HR: 1.59, 95% CI: 1.156–2.187, P=0.004), cardiogenic shock (HR: 6.115, 95% CI: 2.422–15.442, P<0.001), LVEF <40% (HR: 3.626, 95% CI: 1.61–8.168, P=0.002), and ALI <343.96 (HR: 3.009, 95% CI: 1.57–5.769, p=0.001) were independent predictor of MACEs (Table 3, Figure 4). In addition, the multivariate Cox proportional hazards model also demonstrated that a low ALI (<343.96) was an independent risk factor for patients with ACS undergoing PCI.
 |
Table 3 Cox Hazard Proportional Model for Predictive Factors of MACEs |
 |
Figure 4 Forest graphs according to Cox proportional hazards regression model to test the risk factors for MACEs. |
P for Interaction
The independent association between ALI and prognosis was assessed in various subgroups of age (>65 or ≤65 years), cardiogenic shock (yes or no), and LVEF (<40% or ≥40%). The results (Figure 5) were as follows: age >65 years vs age ≤65 years: HR: 3.095 (95% CI: 1.253–7.642) vs HR: 3.919 (95% CI: 1.588–9.672); occurrence of cardiogenic shock vs non-occurrence of cardiogenic shock: HR: 1.438 (95% CI: 0.262–7.876) vs HR: 4.046 (95% CI: 2.022–8.094); LVEF ≥40% vs LVEF <40%: HR: 3.908 (95% CI: 1.946–7.847) vs HR: 1.063 (95% CI: 0.214–5.273), all p for interaction >0.05.
 |
Figure 5 Forest graphs based on subgroup analysis for the effect of different factors in patients with ACS undergoing PCI. |
P for Trend and Restricted Cubic Spline
Patients with low ALI have more adverse events.16,19,20 Therefore, T3 was selected as a reference. Model 1 consisted of only the ALI index, and the results were as follows: T1 vs T3: HR: 3.221, (95% CI:1.514–6.853), p=0.002 and T2 vs T3: HR: 2.255 (95% CI: 1.027–4.952), p=0.043. The p value for trend was 0.01 (Table 4). Model 2 was adjusted for age, cardiogenic shock, and LVEF, and the results were as follows: T1 vs T3: HR: 2.400 (95% CI: 1.001–5.758, p=0.05) and T2 vs T3: HR: 1.012 (95% CI: 0.398–2.575), p=0.980. The p value for trend was 0.026 (Table 4). Two RCS models were generated to visualize the relationship between ALI and the prognostic risk. Model 1 (Figure 6A) was adjusted for ALI, and Model 2 (Figure 6B) was adjusted for age (<55 years=1, 56–65 years=2, 66–75 years=3, >76 years=4), cardiogenic shock (occurrence=1, non-occurrence=0), and LVEF (>40%=0, <40%=1). In both models, the ALI values with an HR close to 1 were 330.49. As shown in the figure, when ALI was <330.49, a low ALI was an independent risk factor in both Models 1 (P nonlinear =0.058) and 2 (P nonlinear = 0.143).
 |
Table 4 Cox Hazard Proportional Models of MACEs Risk According to Tertiles of ALI |
 |
Figure 6 Restricted cubic spline (RCS). (A) Model 1 is adjusted for ALI. (B) Model 2 is adjusted for CKD stage, age stage, cardiogenic shock, and LVEF. |
Discussion
The predictive ability of ALI for the prognostic risk in patients with ACS undergoing PCI remains unknown. The main findings of our research were as follows. First, low ALI was correlated with poor prognosis and was an independent risk factor for ACS patients undergoing PCI. Second, patients with ACS who underwent PCI had low ALI and a lower cumulative survival rate than those in the control group. Third, the diagnostic efficiency of ALI increased with time. Fourth, ALI was a better predictor of MACEs in patients with ACS who underwent PCI. Finally, low ALI was significantly associated with male sex, smoking, history of HF, family history of CAD, STEMI, higher WBC count, higher Cr level, and LVEF <40%. To the best of our knowledge, this is the first study to analyze the correlation between this novel index and prognosis in patients with ACS who underwent PCI.
The ALI index combines both inflammation and nutritional status,21 and includes BMI, serum albumin levels, and NLR. Nutrition plays an important part in CAD.22,23 This index was first used to assess the degree of systemic inflammation in non-small cell lung cancer. A previous study using propensity score matching showed great prognostic value of ALI in gastric cancer and renal cell carcinoma.24,25 Compared with other indices, ALI has better predictive performance for the MACEs risk in patients with ACS undergoing PCI because it combines anthropometric, nutritional, and inflammatory status.21 Atherosclerosis is the original pathological change in CAD and is regarded as inflammatory and oxidative stress.26 Many studies have confirmed the significance of anti-inflammatory therapy, such as canakinumab and low-dose methotrexate, in CAD patients.27–29 Clinical trials have suggested that inflammation plays an important role in CAD. Particularly, neutrophils, neutrophil extracellular traps, and lymphocytes are correlated with atherosclerosis.30 The disturbed equilibrium among lipid accumulation, immune responses, and clearance is regulated by leukocyte trafficking and homeostasis controlled by chemokines and their receptors. Animal experiments have shown that CD4+ T cells are commonly found in atherosclerotic plaques.31 Therefore, NLR is regarded as an independent prognostic factor for coronary artery disease.32,33
We used several approaches to investigate the correlation between ALI values and prognostic risk. The results of the multivariate Cox proportional hazards model showed that ALI, as a novel index to predict MACEs risk, had the same efficiency as other classical prognostic risk factors (HR: 3.009, 95% CI: 1.57–5.769). In addition, age, occurrence of cardiogenic shock, and LVEF <40% were the main factors influencing the prognosis. Particularly, the risk of MACEs increased per 1 category increase in age. The p value for the interaction analysis method was used to identify the bias produced by different variables. Classical factors, such as age (>65 or ≤65 years), cardiogenic shock (yes or no), and LVEF (<40% or ≥40%), were included in our study. The results showed no significant differences in age, cardiogenic shock, or left heart function. This indicated that ACS patients undergoing PCI with a low ALI had an increased incidence of MACEs regardless of age (>65 or ≤65 years), occurrence or non-occurrence of cardiogenic shock, and LVEF <40% or ≥40%.
Both the p-value for trend and RCS methods were used to analyze the correlation between ALI and MACEs, and the dependent and independent variables were the J shape, U shape, or linear shape. When performing p for trend, we used model 1 (adjusted only for ALI) and model 2 (adjusted for age category, cardiogenic shock, and LVEF) in the analysis. Interestingly, the results showed that the association between ALI and MACEs initially decreased and then increased. Model 1 suggested that from T1 to T2, HR presented a decreasing tendency, while in model 2, T1 still presented the same results. Both models demonstrated that a low ALI was a dependent risk factor for prognosis in patients with ACS undergoing PCI. The RCS plot indicated similar conclusions that a low ALI of <330.49 was a dependent risk factor. Although when RCS was performed (Figure 6, model 1 adjusted for ALI alone, model 2 adjusted for age, cardiogenic shock, and LVEF), the curve showed that the HR of patients with ACS undergoing PCI increased sharply when the ALI value was < 330.49. This supported that patients with ACS undergoing PCI who have an ALI value <330.49 have a high prognostic risk. Further studies are needed to elucidate the association between ALI and ACS.
Limitations
Firstly, our data were obtained from a single center in China, and the sample size was relatively small. Therefore, multicenter studies with larger sample size are needed. Secondly, the correlation between the ALI index and MACEs requires further investigation using the RCS analysis method to further examine whether the associated is linear, J shaped, or U shaped. Thirdly, future studies are needed to compare the prognosis among different subtypes of the ACS. Finally, there was great heterogeneity in antiplatelet therapy including clopidogrel and ticagrelor, so the conclusion may be conservative in the present study.
Conclusion
ALI, as a novel inflammation index, is independently associated with a higher risk of all-cause mortality and severe HF requiring rehospitalization in patients with ACS undergoing PCI. This index combines inflammatory and nutritional statuses, is more convenient and effective, and can be widely used to predict the risk of MACEs in patients with ACS undergoing PCI.
Abbreviations
ACS, acute coronary syndrome; ALI, advanced lung cancer inflammation index; AUC, area under the curve; BMI, body mass index; CAD coronary heart disease; Cr, creatinine; HF, heart failure; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiovascular events; NLR, neutrophil-to-lymphocyte ratio; PCI, percutaneous coronary intervention; RCS, restricted cubic spline; ROC, receiver operating characteristic; STEMI, ST-elevation myocardial infarction.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
The authors would like to thank the doctors and nurses of the Cardiology Research Team at the Affiliated Hospital of Chengde Medical University for their assistance.
Funding
This study was supported by the Natural Science Foundation of Hebei Province (grant number H2021406071) to Dr. Lixian Sun.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–675. doi:10.1001/jama.2022.0358
2. Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020;106(18):1380–1386. doi:10.1136/heartjnl-2019-315707
3. Kamran H, Jneid H, Kayani WT, et al. Oral antiplatelet therapy after acute coronary syndrome: a review. JAMA. 2021;325(15):1545–1555. doi:10.1001/jama.2021.0716
4. Serruys PW, Hara H, Garg S, et al. Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC state-of-The-art review. J Am Coll Cardiol. 2021;78(7):713–736. doi:10.1016/j.jacc.2021.06.019
5. Awesat J, Abitbol M, Vons S, Eisen A, Porter A. Current challenges in the diagnosis and management of acute coronary syndromes in women. Kardiol Pol. 2022;80(11):1084–1093. doi:10.33963/KP.a2022.0254
6. Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718–730. doi:10.1001/jamacardio.2016.2049
7. Han K, Shi D, Yang L, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. 2022;54(1):1667–1677. doi:10.1080/07853890.2022.2083671
8. Syed Soffian SS, Mohammed Nawi A, Hod R, et al. Meta-analysis of the association between dietary inflammatory index (DII) and colorectal cancer. Nutrients. 2022;14(8):1555. doi:10.3390/nu14081555
9. Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. doi:10.1038/s41598-019-39150-0
10. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13(1):158. doi:10.1186/1471-2407-13-158
11. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. 2022;23(21):12906. doi:10.3390/ijms232112906
12. Fan W, Zhang Y, Gao X, et al. The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. 2021;27:107602962110345. doi:10.1177/10760296211034579
13. Liu XR, Wang LL, Zhang B, et al. The advanced lung cancer inflammation index is a prognostic factor for gastrointestinal cancer patients undergoing surgery: a systematic review and meta-analysis. World J Surg Oncol. 2023;21(1):81. doi:10.1186/s12957-023-02972-4
14. Zhang Y, Chen B. Prognostic value of the advanced lung cancer inflammation index in patients with lung cancer: a meta-analysis. Dis Markers. 2019;2019:2513026. doi:10.1155/2019/2513026
15. Xie H, Huang S, Yuan G, et al. The advanced lung cancer inflammation index predicts short and long-term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ. 2020;8:e10100. doi:10.7717/peerj.10100
16. Fan W, Zhang Y, Liu Y, et al. Nomograms based on the advanced lung cancer inflammation index for the prediction of coronary artery disease and calcification. Clin Appl Thromb Hemost. 2021;27:107602962110604. doi:10.1177/10760296211060455
17. Fan W, Liu Y, Zhang Y, et al. Prognostic value of a novel dNLR-PNI score in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Perfusion. 2022;38(5):973–982. doi:10.1177/02676591221090620
18. Locuratolo N, Scicchitano P, Antoncecchi E, et al. Follow-up of patients after an acute coronary event: the Apulia PONTE-SCA program. G Ital Cardiol. 2022;23(1):63–74. doi:10.1714/3715.37064
19. Zhang L, Zhao K, Kuang T, et al. The prognostic value of the advanced lung cancer inflammation index in patients with gastrointestinal malignancy. BMC Cancer. 2023;23(1):101. doi:10.1186/s12885-023-10570-6
20. Chen H, Zhang F, Luo D, et al. Advanced lung cancer inflammation index predicts the outcomes of patients with non-metastatic gastric cancer after radical surgical resection. J Gastrointest Oncol. 2023;14(1):85–96. doi:10.21037/jgo-22-657
21. Song M, Zhang Q, Song C, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. 2022;13(5):2504–2514. doi:10.1002/jcsm.13032
22. Houston M. The role of noninvasive cardiovascular testing, applied clinical nutrition and nutritional supplements in the prevention and treatment of coronary heart disease. Ther Adv Cardiovasc Dis. 2018;12(3):85–108. doi:10.1177/1753944717743920
23. Renaud S, de Lorgeril M. Nutrition, atherosclerosis and coronary heart disease. Reprod Nutr Dev. 1994;34(6):599–607. doi:10.1051/rnd:19940606
24. Mao W, Wang K, Wu Y, et al. Prognostic significance of modified advanced lung cancer inflammation index in patients with renal cell carcinoma undergoing laparoscopic nephrectomy: a multi-institutional, propensity score matching cohort study. Front Nutr. 2021;8:781647. doi:10.3389/fnut.2021.781647
25. Yin C, Toiyama Y, Okugawa Y, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr. 2021;40(3):1130–1136. doi:10.1016/j.clnu.2020.07.018
26. Poznyak AV, Grechko AV, Orekhova VA, Khotina V, Ivanova EA, Orekhov AN. NADPH oxidases and their role in atherosclerosis. Biomedicines. 2020;8(7):206. doi:10.3390/biomedicines8070206
27. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. doi:10.1056/NEJMoa1809798
28. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
29. Ma J, Chen X. Anti-inflammatory therapy for coronary atherosclerotic heart disease: unanswered questions behind existing successes. Front Cardiovasc Med. 2020;7:631398. doi:10.3389/fcvm.2020.631398
30. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–743. doi:10.1161/CIRCRESAHA.116.309692
31. Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401. doi:10.1038/s41569-020-0352-5
32. Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22(5):405–411. doi:10.1177/1076029615569568
33. Marin BS, Cesena F, Laurinavicius AG, Santos RD, Bittencourt MS. Neutrophil-to-lymphocyte ratio and abdominal aortic atherosclerosis among asymptomatic individuals. Arq Bras Cardiol. 2022;118(4):729–734. doi:10.36660/abc.20201163
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
