Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Adrenal Artery Ablation for the Treatment of Hypercortisolism Based on Adrenal Venous Sampling: A Potential Therapeutic Strategy
Authors Zhou Q, Liu X, Zhang H, Zhao Z, Li Q, He H, Zhu Z , Yan Z
Received 19 May 2020
Accepted for publication 18 August 2020
Published 6 October 2020 Volume 2020:13 Pages 3519—3525
DOI https://doi.org/10.2147/DMSO.S262092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Konstantinos Tziomalos
Qing Zhou, Xiaoli Liu, Hexuan Zhang, Zhigang Zhao, Qiang Li, Hongbo He, Zhiming Zhu, Zhencheng Yan
Department of Hypertension and Endocrinology, Center for Hypertension and Metabolic Diseases, Chongqing Institute of Hypertension, Daping Hospital and the Research Institute of Surgery, Army Medical University, Chongqing 400042, People’s Republic of China
Correspondence: Zhencheng Yan
Department of Hypertension and Endocrinology, Center for Hypertension and Metabolic Diseases, Chongqing Institute of Hypertension, Daping Hospital and the Research Institute of Surgery, Army Medical University, Chongqing 400042, People’s Republic of China
Tel +86-23-68757883
Email [email protected]
Aim: Hypercortisolism is characterized by metabolic disorders and high mortality rates. Adrenalectomy and medical therapies are considered major treatment options. However, some patients, especially young patients, are strongly against undergoing surgery in case of secondary hypocortisolism or relapses that require replacement supplements or pharmacological interventions. In such cases, alternative therapies are needed to treat hypercortisolism.
Methods: We report a 27-year-old Chinese female with adrenal cortisol-producing adenoma. The patient’s circadian rhythm and concentrations of cortisol were abnormal, accompanying with an increased 24-hour urinary cortisol level. Computed tomography (CT) revealed a nodular soft-tissue mass in the right adrenal gland.
Results: Cortisol hypersecretion from the right adrenal gland was verified by adrenal venous sampling (AVS). Adrenal artery ablation was performed. After ablation, long-term follow-up showed that the patient’s symptoms subsided and abnormal laboratory test results returned to normal without pharmacological treatment.
Conclusion: AVS might be a promising method to aid the diagnosis of cortisol-producing adenoma. Adrenal artery ablation is minimally invasive and may be useful for the treatment of adrenal adenoma or nodular diseases, especially in patients who cannot undergo surgery.
Keywords: adrenal cortisol adenoma, adrenal artery ablation, adrenal venous sampling, Cushing’s syndrome
Introduction
Hypercortisolism, also called Cushing’s syndrome (CS), is characterized by central obesity, hypertension, muscle weakness, secondary diabetes, and osteoporosis. Even with advances in medical technology, the mortality rate of patients with CS is still 1.7–4.8-times higher than that of the healthy population.1,2 Therefore, early diagnosis and treatment, including restoration of cortisol concentrations and successful treatment of hypercortisolism-associated complications, should be emphasized.
As suggested by the Endocrine Society’s Clinical Practice Guidelines,3 the treatment of CS varies by etiology. Initial resection is recommended as a first-line option for adrenal gland-derived hypercortisolism. In addition, multidisciplinary teams of endocrinologists, hypertension experts, and urologists, are necessary to provide individualized treatment strategies to patients.
At present, remission and recurrence rates are still unsatisfactory. In patients with primary aldosteronism (PA), adrenal venous sampling (AVS) is a precise and safe method that can be used to diagnose PA and identify appropriate treatment options; however, AVS is hardly used in the context of cortisol-producing adenoma.
Artery ablation is widely adopted in patients with primary and metastatic carcinoma.4 We have successfully treated a number of patients with primary aldosteronism using catheter-based adrenal artery ablation. Herein, we report a case of adrenal artery ablation to treat unilateral adrenal cortisol-producing adenoma and the associated follow-up observations. Publication of case details was approved by the Ethics Committee of the Daping Hospital, Army Medical University.
Methods and Results
Medical History and Biochemical Examination
A 27-year-old Chinese female was admitted to hospital after experiencing hypertension for almost two years. The patient’s blood pressure increased up to 150/100 mmHg during pregnancy. The patient did not experience headache, chest pain, nausea, abdominal pain, hyperhidrosis, or emaciation. She had no history of alcohol or drug abuse and had never used steroids. The patient had no family history of hypertension or endocrine disease. The patient was prescribed nifedipine sustained-release tablets; however, medication was discontinued soon after it was prescribed. After discontinuing medication, the patient did not monitor her blood pressure.
In November 2018, the patient experienced dizziness for no obvious reason and was admitted to the local hospital. Upon admission, the patient’s blood pressure was 157/99 mmHg and abdominal ultrasound revealed bilateral adrenal gland enlargement. Therefore, she was admitted to our hospital for further examination. On admission, the patient’s blood pressure, body mass index (BMI), and waist circumference were 164/107 mmHg, 27.1 kg/m2, and 93 cm, respectively. The patient also presented with typical Cushingoid features, such as a flushed and moon face, purple striae, and plethora. Nevertheless, there were no signs of edema in the lower extremities or unusual bruits in the abdominal aorta.
Results of the patient’s biochemical examination are shown in Table 1. Serum potassium and 24-hour urinary potassium concentrations were normal. However, circadian rhythm and concentrations of cortisol and adrenocorticotropic hormone (ACTH) were abnormal, concomitant with an elevated 24-hour urinary free cortisol concentration (>1693.93 nmol/24 h, reference range: 160–1112 nmol/24 h). The patient was administered dexamethasone (1 mg) at midnight and the serum cortisol concentration was assayed before and after performing the suppression test; however, cortisol secretion was not suppressed (before: 798.8 nmol/l; after: 848.55 nmol/l). To exclude the possibility of multiple endocrine neoplasia (MEN), magnetic resonance imaging was performed, which showed no abnormalities. Furthermore, adrenal contrast-enhanced CT revealed a nodular soft-tissue low-density mass in the right adrenal gland (31 × 23 mm; Figure 1). Attenuation values were 30, 53, 77, and 62 HU during the plain scan phase, the arterial phase, the venous phase, and the delayed phase, respectively. Based on these results, AVS was performed. The concentrations of cortisol and aldosterone were measured from the right adrenal vein (RAV), left adrenal vein (LAV), and inferior vena cava (IVC; Table 2). Samples were collected twice and the mean values were calculated. The selectivity index (SI) was calculated for aldosterone, and successful catheterization was defined as an adrenal-to-peripheral venous aldosterone ratio of >2.5 The SI values were 13.29 (RAV:IVC) and 23.86 (LAV:IVC), respectively, which suggested successful sampling. In addition, lateralization index (LI) values were 36.9 (RAV:LAV) and 0.03 (LAV:RAV), which indicated unilateral hypersecretion of cortisol from the right adrenal gland. Based on the aforementioned results, the patient was diagnosed with right-sided adrenal gland cortisol-producing adenoma.
 |
Table 1 Biochemistry Examinations |
 |
Table 2 Adrenal Venous Sampling |
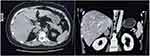 |
Figure 1 Adrenal contrast-enhanced computed tomography at the patient’s first hospital visit. Arrow indicates adrenal adenoma. |
Adrenal Artery Ablation
Initial resection is recommended as the first-line option to treat adrenal adenoma.3 The patient described in this report was strongly against surgical resection as she was concerned about postoperative complications, such as secondary hypocortisolism and life-long replacement. Hence, with the patient’s consent, adrenal artery ablation was performed as described previously6,7 with minor modifications.
After application of local anesthesia at the right radial artery, an introducer sheath was inserted. A multifunctional catheter was positioned and contrast agent was injected at the level of the first lumbar vertebrae in preparation for digital subtraction angiography. Microcatheters were inserted into the inferior branch of the right adrenal artery. The adenoma was identified and approximately 1.0 mL of anhydrous ethanol was injected through the microcatheter. Digital subtraction angiography revealed retention of contrast agent in the inferior branch; therefore, the suprarenal and middle branches were super-selected and 0.75, 1.0 mL of anhydrous ethanol was injected, respectively. During ablation, blood pressure monitoring and electrocardiography were performed, and symptoms of discomfort were closely monitored. A sodium nitroprusside micropump was used to control the increase in blood pressure and pulse rate. After ablation, serum cortisol decreased to 315.1 nmol/L. The patient was treated with amlodipine besylate tablets and metoprolol succinate sustained-release tablets.
Post-Ablation Follow Up
One month after ablation, the patient’s BMI decreased to 26.2 kg/m2 and 24-hour ambulatory blood pressure was normal without any medication. The serum cortisol concentration and 24-hour urinary free cortisol were 304 nmol/l and 78.49 nmol/24 h, respectively. The aldosterone-to-renin ratio (ARR), fasting blood glucose (FBG) concentration, kidney function, and serum electrolyte concentrations were normal (Table 1).
Nine months after ablation, BMI, blood pressure, and pulse rate were normal. The circadian rhythm of cortisol and ACTH secretion and the 24-hour urinary free cortisol concentration were normal. In addition, the ARR, FBG, kidney function, and serum electrolytes were within reference ranges (Table 1). Adrenal non-contrast-enhanced CT suggested a decrease in the volume of the cortisol-producing adenoma (24 × 20 mm; Figure 2). In addition, the CT attenuation value declined to 17.5 HU.
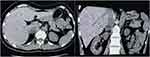 |
Figure 2 Adrenal non-contrast computed tomography after adrenal artery ablation. Arrow indicates adrenal adenoma. |
Discussion
The present paper presents a case of successful remission in a patient with AVS-confirmed right-sided cortisol-producing adenoma after adrenal artery ablation. Adrenal adenoma accounts for the majority of cases of ACTH-independent CS. Biochemistry examinations concerning cortisol secretion and its circadian rhythm, as well as imaging examinations are useful diagnostic tools for patients with adrenal adenoma. However, imaging examinations to localize lesions are not always consistent with pathological changes, which might lead to inappropriate treatment. For example, out of 20 patients diagnosed with unilateral adrenal tumors by CT, 9 had bilateral cortical secretion, which was identified by AVS.8 Cholesterol scintigraphy, which was previously regarded as the gold standard for adrenal cortical evaluation,9 is not widely used nowadays because of its high cost and limited use of imaging agents. Therefore, AVS, which is used for the diagnosis and subtype confirmation of hyperaldosteronism, might be a useful diagnostic tool.5,8,10,11 However, unlike PA,10 the concentration of cortisol in adrenal adenoma is increased. Therefore, adrenal-to-peripheral venous cortisol concentrations cannot be used to confirm a correct catheter position. Both adrenaline and noradrenaline can be measured to confirm successful adrenal vein catheterization.12 However, the use of catecholamines has been questioned due to short-life, unacceptably wide side-to-side differences, and marked inter-individual variation.13,14 In addition, catecholamines are secreted from the adrenal medulla and may not accurately reflect venous drainage from the adrenal cortex.5 Therefore, the AV:IVC aldosterone ratio could be used to confirm catheterization after excluding aldosterone overproduction.15 Consistent with the SI used in PA, successful catheterization is also based on an adrenal-to-peripheral vein ratio of aldosterone of >2.5,16 In addition, cortisol concentrations corrected by aldosterone could diminish asymmetric dilution of different adrenal veins.17 As suggested by the Endocrine Society’s Clinical Practice Guidelines,3 treatment for CS varies according to the etiology and patients’ individual conditions. For adrenal adenomas, unilateral adrenalectomy through transperitoneal or retroperitoneal laparoscopy performed by experienced adrenal surgeons is curative and is therefore recommended as a first-line treatment.3,18 However, intraoperative or postoperative complications may occur, such as the requirement for blood transfusion or organ and tissue injury, especially in older patients, patients with an increased American Society of Anesthesiologists score, and patients with diabetes.19,20 In addition, secondary hypocortisolism might occur, and life-long replacement therapy may be inevitable.21 For patients who cannot undergo resection, medications, such as steroidogenesis inhibitors and mifepristone, are recommended.
The patient in this study was strongly against surgical treatment; therefore, we performed adrenal artery ablation using anhydrous ethanol with the aim of removing the cortisol-producing adenoma. After ablation, the cortisol concentration returned to normal. Although the volume of the cortisol-producing adenoma decreased slightly, circadian rhythm and concentration of cortisol concentration were normal and there were no abnormal signs or symptoms, even without any pharmacological treatment, which suggests that the cortisol-producing adenoma was non-functional.
The decreased CT attenuation value after adrenal artery ablation suggested cortisol adenoma necrosis, as microvessel density that determines the attenuation value,22 could be reduced by ethanol. There are also several reports that describe successful ablation with various agents in the treatment of different types of adrenal mass.23–25 During ablation, the feeding arteries are super-selected, which could minimize the risk of secondary hypocortisolism. Nevertheless, hypocortisolism might occur because of long-term suppression of the hypothalamic–pituitary–adrenal axis. Therefore, in line with initial resection, replacement treatment should not be neglected postoperatively and regular follow-up visits are necessary.
Adrenal artery embolization is performed to manage adrenal tumors, acute bleeding from ruptured adrenal tumors, traumatic adrenal injury, and aneurysms.6 Ablation is also an interventional therapy used to target feeding blood vessels; however, the principles and detailed surgical procedures are different. Embolization is mainly centered on destroying the feeding arteries. Nevertheless, ablation might be more efficient than embolization because it targets feeding arteries, lesions, and the associated microvasculature.
Compared with laparoscopy, ablation is minimally invasive with an incision of <3 mm. Local anesthetic with ablation minimizes the risk of anesthesia-related complications; however, there are some limitations of this approach. For patients with anatomical variations in the adrenal branches, ablation may not be appropriate because the microcatheter cannot be targeted. Second, if there are a greater number of feeding arteries, ablation might be incomplete, leading to recurrence. Imaging guided interventional therapies using ethanol is widely applied in multiple tumors, and direct injection of ethanol could result in tissue necrosis through protein denaturation and cytoplasmic dehydration. In addition, ethanol ablation can be locally available and low-cost, and can treat relatively large lesions up to 5 cm in diameter.26,27 Therefore, despite its limitations, ablation could be useful to resolve hypercortisolism in patients who cannot undergo surgery.
Conclusion
The present report describes a patient with CS that underwent adrenal artery ablation based on the results of AVS; therefore, AVS may be useful in patients with adrenal-derived ACTH-independent CS, such as adenoma and nodular hyperplasia. Adrenal artery ablation might be a useful alternative in patients who cannot undergo surgery. The safety and effectiveness of adrenal artery ablation in the treatment of hypercortisolism should be further explored in studies with large sample sizes with the possibility of introducing this approach into the clinic as an alternative conventional treatment for CS.
Abbreviations
ACTH, adrenocorticotropic hormone; ACS, autonomous cortisol secretion; ARR, aldosterone-to-renin ratio; AVS, adrenal venous sampling; BMI, body mass index; CT, computed tomography; CS, Cushing’s syndrome; FBG, fasting blood glucose; IVC, inferior vena cava; LAV, left adrenal vein; LI, lateralization index; PA, primary aldosteronism; RAV, right adrenal vein; SI, selectivity index.
Consent for Publication
Written informed consent was obtained from the patient to publish their clinical details and images.
Author Contributions
All authors made substantial contributions to conception, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC No.81870641).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Dekkers OM, Horváth-Puhó E, Jørgensen JO, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. doi:10.1210/jc.2012-3582
2. Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand [published correction appears in Clin Endocrinol (Oxf). Clin Endocrinol. 2011;75(4):436–442. doi:10.1111/j.1365-2265.2011.04124.x
3. Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing’s Syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi:10.1210/jc.2015-1818
4. Inoue H, Nakajo M, Miyazono N, Nishida H, Ueno K, Hokotate H. Transcatheter arterial ablation of aldosteronomas with high-concentration ethanol: preliminary and long-term results. AJR Am J Roentgenol. 1997;168(5):1241–1245. doi:10.2214/ajr.168.5.9129420
5. Papakokkinou E, Jakobsson H, Sakinis A, et al. Adrenal venous sampling in patients with ACTH-independent hypercortisolism. Endocrine. 2019;66(2):338–348. doi:10.1007/s12020-019-02038-0
6. Fowler AM, Burda JF, Kim SK. Adrenal artery embolization: anatomy, indications, and technical considerations. AJR Am J Roentgenol. 2013;201(1):190–201. doi:10.2214/AJR.12.9507
7. Hokotate H, Inoue H, Baba Y, Tsuchimochi S, Nakajo M. Aldosteronomas: experience with superselective adrenal arterial embolization in 33 cases. Radiology. 2003;227(2):401–406. doi:10.1148/radiol.2272011798
8. Ueland GÅ, Methlie P, Jøssang DE, et al. Adrenal venous sampling for assessment of autonomous cortisol secretion. J Clin Endocrinol Metab. 2018;103(12):4553–4560. doi:10.1210/jc.2018-01198
9. Avram AM, Fig LM, Gross MD. Adrenal gland scintigraphy. Semin Nucl Med. 2006;36(3):212–227. doi:10.1053/j.semnuclmed.2006.03.004
10. Young WF
11. Acharya R, Dhir M, Bandi R, Yip L, Challinor S. Outcomes of adrenal venous sampling in patients with bilateral adrenal masses and ACTH-independent cushing’s syndrome. World J Surg. 2019;43(2):527–533. doi:10.1007/s00268-018-4788-2
12. Martins RG, Agrawal R, Berney DM, et al. Differential diagnosis of adrenocorticotropic hormone-independent Cushing syndrome: role of adrenal venous sampling. Endocr Pract. 2012;18(6):e153e157. doi:10.4158/EP12136.CR
13. Freel EM, Stanson AW, Thompson GB, et al. Adrenal venous sampling for catecholamines: a normal value study. J Clin Endocrinol Metab. 2010;95(3):1328–1332. doi:10.1210/jc.2009-2253
14. Baba Y, Nakajo M, Hayashi S. Adrenal venous catecholamine concentrations in patients with adrenal masses other than pheochromocytoma. Endocrine. 2013;43(1):219–224. doi:10.1007/s12020-012-9792-y
15. Lu Z, Wei X, Sun F, et al. Non-insulin determinant pathways maintain glucose homeostasis upon metabolic surgery. Cell Discov. 2018;4(58). doi:10.1038/s41421-018-0062-x
16. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi:10.1210/jc.2015-4061
17. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227–1235. doi:10.1016/j.surg.2004.06.051
18. Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg. 1999;23(4):389–396. doi:10.1007/pl00012314
19. Chen Y, Scholten A, Chomsky-Higgins K, et al. Risk factors associated with perioperative complications and prolonged length of stay after laparoscopic adrenalectomy. JAMA Surg. 2018;153(11):1036–1041. doi:10.1001/jamasurg.2018.2648
20. Kazaure HS, Roman SA, Sosa JA. Adrenalectomy in older Americans has increased morbidity and mortality: an analysis of 6416 patients. Ann Surg Oncol. 2011;18(10):2714–2721. doi:10.1245/s10434-011-1757-5
21. Alexandraki KI, Kaltsas GA, Isidori AM, et al. Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur J Endocrinol. 2013;168(4):639–648. doi:10.1530/EJE-12-0921
22. Jinzaki M, Tanimoto A, Mukai M, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr. 2000;24(6):835–842. doi:10.1097/00004728-200011000-00002
23. Venugopal H, Griffin K, Amer S. A case of severe ectopic ACTH syndrome from an occult primary - diagnostic and management dilemmas. Endocrinol Diab Metab Case Rep. 2015;2015:150099. doi:10.1530/EDM-15-0099
24. Liang H-L, Pan H-B, Lee Y-H, et al. Small functional adrenal cortical adenoma: treatment with ct-guided percutaneous acetic acid injection—report of three cases. Radiology. 1999;213(2):612–615. doi:10.1148/radiology.213.2.r99nv10612
25. Xiao YY, Tian JL, Li JK, Yang L, Zhang JS. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008;190(1):105–110. doi:10.2214/AJR.07.2145
26. Kuang M, Lu MD, Xie XY, et al. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253(2):552–561. doi:10.1148/radiol.2532082021
27. Morhard R, Nief C, Barrero Castedo C, et al. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci Rep. 2017;7(1):8750. doi:10.1038/s41598-017-09371-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
