Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Adolescents with Type 2 Diabetes: Overcoming Barriers to Effective Weight Management
Authors Salama M, Biggs BK, Creo A, Prissel R , Al Nofal A, Kumar S
Received 25 November 2022
Accepted for publication 9 February 2023
Published 9 March 2023 Volume 2023:16 Pages 693—711
DOI https://doi.org/10.2147/DMSO.S365829
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gian Paolo Fadini
Mostafa Salama,1 Bridget K Biggs,2 Ana Creo,1 Rose Prissel,3 Alaa Al Nofal,1 Seema Kumar1
1Division of Pediatric Endocrinology, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, MN, USA; 2Department of Psychiatry and Psychology, Mayo Clinic, Rochester, MN, USA; 3Division of Endocrinology and Nutrition, Mayo Clinic, Rochester, MN, USA
Correspondence: Seema Kumar, Email [email protected]
Abstract: The prevalence of type 2 diabetes (T2DM) among children and adolescents has remarkably increased in the last two decades, particularly among ethnic minorities. Management of T2DM is challenging in the adolescent population due to a constellation of factors, including biological, socioeconomic, cultural, and psychological barriers. Weight reduction is an essential component in management of T2DM as weight loss is associated with improvement in insulin sensitivity and glycemic status. A family centered and culturally appropriate approach offered by a multidisciplinary team is crucial to address the biological, psychosocial, cultural, and financial barriers to weight management in youth with T2DM. Lifestyle interventions and pharmacotherapy have shown modest efficacy in achieving weight reduction in adolescents with T2DM. Bariatric surgery is associated with excellent weight reduction and remission of T2DM in youth. Emerging therapies for weight reduction in youth include digital technologies, newer GLP-1 agonists and endoscopic procedures.
Keywords: type 2 diabetes mellitus, adolescents, obesity, weight management, youth
Background
The prevalence of type 2 diabetes mellitus (T2DM) in adolescents has doubled in the US over the last few decades1 and this trend has been mirrored by an increase in the prevalence of childhood obesity. The estimates from 2017 to March 2020 on childhood obesity indicated a prevalence of 20.7% among 6- to 11-year-olds, and 22.2% among 12- to 19-year-olds.1–3 Obesity is a major risk factor for T2DM in adolescents and weight management is an integral component of management of T2DM. We review barriers to effective weight management in adolescents with T2DM and discuss various strategies to overcome these barriers.
Epidemiology
In an observational, cross-sectional, multicenter study from the United States, the estimated prevalence of T2DM increased from 0.34 (95% CI, 0.31–0.37) per 1000 youth (aged 10–19 years) in 2001 to 0.67 (95% CI, 0.63–0.70) per 1000 youth in 2017.1 The prevalence of T2DM among youth younger than 20 years varies greatly between ethnic/racial groups. In 2017, the highest prevalence was among Black youth [1.8 (95% CI, 1.62–2.00) per 1000] followed by Native Americans [1.63 (95% CI, 1.32–2.03) per 1000]. Hispanic youth had a prevalence rate of 1.03 (95% CI, 0.94–1.12) per 1000 and White youth had the lowest prevalence of 0.2 (95% CI, 0.17–0.23) per 1000.
The rise in prevalence of T2DM has been more pronounced in youth from ethnic minorities with the highest increase in Non-Hispanic Black and Hispanic youth . For example, the prevalence of T2DM per 1000 youth in Black adolescents increased from 0.95 (0.82–1.10) per 1000 in 2001 to 1.80 (1.62–2.00) in 2017 and in Hispanic youth increased from 0.45 (0.39–0.53) in 2001 to 1.03 (0.94–1.12) in 2017. On the other hand, the prevalence of T2DM in White youth only slightly increased from 0.14 (0.12–0.17) per 1000 in 2001 to 0.2 (0.17–0.23) per 1000 in 2017.1 There has been further increase in frequency of T2DM in adolescents during the COVID-19 pandemic.4
T2DM in youth is a global health problem. In 2021, the estimated number of new cases of adolescents with T2DM worldwide was 41,600 with the highest incidence reported in China, India, and the US.5
Pathophysiology
T2DM develops due to a complex interaction between genetic and environmental factors. Obesity is associated with insulin resistance that leads to beta cell adaptation by increased insulin secretion. Subsequently, insulin resistance overcomes beta cell reserve leading to impaired glucose tolerance and finally overt hyperglycemia.6
Insulin resistance in youth with T2DM is correlated with total body fat mass, especially visceral fat. This is mediated via multiple adipokines including leptin, resistin, Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), in addition to non-esterified fatty acids that lead to insulin resistance by increasing hepatic glucose output and hindering skeletal muscle glucose uptake.6,7
Obesity-related chronic inflammation contributes to both insulin resistance and beta cell dysfunction.8 Additionally, increased visceral fat mass leads to lower adiponectin levels and decreased expression of adiponectin receptors on the cellular surface.9
Risk Factors for T2DM
Obesity
Obesity plays a fundamental role in the pathogenesis of T2DM in adolescents.7 Among the 503 youth from the Pediatric Diabetes Consortium T2D clinic registry from eight medical centers in the United States, median body mass index (BMI) z score was 2.3 (interquartile range 2 to 2.6). 8% were overweight and 90% had obesity.10 In the SEARCH for Diabetes and Youth study, a multicenter cross-sectional population-based study including 2448 adolescents with diabetes, the prevalence of overweight and obesity among adolescents with T2DM was 10.4% and 79.4%, respectively.11 The risk of T2DM tends to increase with increasing BMI percentiles for both sexes.12
In a large German/Austrian multicenter diabetes database of 120,183 pediatric and adult patients with T2DM, higher BMI relative to non-diabetic peers were noted in all age groups. However, the BMI gap was more pronounced in younger patients (<40 years of age) thereby suggesting a stronger contribution of obesity in development of T2DM in youth and young adults.13
Genetic Risk
T2DM is a polygenic disease with each individual gene variant carrying a small but additional risk for diabetes. In the Pediatric Diabetes Consortium T2D clinic registry, approximately 90% of youth with T2DM had positive family history of T2DM at diagnosis.10 Additionally, in a study including 53 pairs of identical twins, 48 pairs developed T2DM, and most twins were living apart with different BMI status. This indicates the strong heritability of T2DM.14 Family history of T2DM is a risk factor for T2DM in youth.15 The estimated lifetime risk for developing T2DM is 40% for individuals who have one parent with T2DM and 70% if both parents are affected.16
The first genome-wide association study of youth-onset T2DM, The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium has led to the identification of seven genome-wide significant loci including rs7903146 in TCF7L2, rs72982988 near MC4R, rs200893788 in CDC123, rs2237892 in KCNQ1, rs937589119 in IGF2BP2, rs113748381 in SLC16A11 and rs2604566 in CPEB2.17
Gender and Puberty
Girls are typically affected more than boys during childhood and adolescence. However, men tend to be more affected than women in adulthood.13 This difference could be related to increase in adiposity than lean body mass and insulin resistance along with decreased level of physical activity in female adolescents.18,19
Ethnicity
In the United States, T2DM is more prevalent in Black, Native American, Hispanic, Asian American and Pacific Islander youth than in White youth.1 In Europe, the prevalence of T2DM is highest among South Asian population followed by Middle Eastern and North African population compared to host European population.20 In the UK, the prevalence of T2DM is higher in the Asian and Black ethnic groups than in the White group.21 In Australia, the prevalence of T2DM is significantly higher among Aboriginal and Torres Strait Islander children than in the non-indigenous population.22
Sedentary Lifestyle
The Treatment Options for T2DM in Adolescents and Youth (TODAY) trial recruited 699 adolescents (age from 10 to 17 years) with T2DM from 15 US clinical centers. When comparing physical activity level in recently diagnosed adolescents with T2DM from the TODAY study relative to subjects with obesity (BMI ≥ 95th percentile) without diabetes in the National Health and Nutrition Examination Survey (NHANES) cohort, TODAY youth were noted to be more sedentary (average difference 56 minutes per day) than the NHANES youth.23 Additionally, time spent engaging in moderate or vigorous physical activity was low in both groups. Younger males in the TODAY group were particularly less active than similarly aged control males from the NHANES cohort. Girls in the TODAY group were also less active than in previous studies.23
Socioeconomic Factors
Low socioeconomic status is very frequently noted in families with adolescents with T2DM. In the TODAY study, 41.5% of patients’ families had annual income less than 25,000 USD and 26.3% of their parents/guardians had an education level less than high school degree.24 In another cohort including 503 children and adolescents with T2DM, the yearly income of 43% of patients’ families was less than 25,000 USD.10
Prenatal Exposure
Low birth weight is associated with higher risk of T2DM.25 Offspring born to mothers with gestational diabetes are also at a higher risk to develop T2DM.26 The risk of T2DM is directly proportional to the degree of hyperglycemia in the third trimester and highest among offspring born to mother carrying the diagnosis of T2DM.26
Diagnosis
Clinical Picture
T2DM is primarily seen in post-pubertal children. In the TODAY study, mean age at diagnosis was 14 years. None of the boys and less than 1% of girls were prepubertal. In the Pediatric Diabetes Consortium, the mean age at diagnosis of T2DM was 13 years.10
Approximately 40% of adolescents with T2DM are asymptomatic at diagnosis and diabetes is diagnosed following screening due to obesity, other risk factors, or signs of insulin resistance.27 Occasionally, the diagnosis is made after detection of glucosuria during a urinalysis. Symptomatic patients typically present with hyperglycemic symptoms including polyuria, polydipsia, nocturia, and sometimes weight loss. Some patients are diagnosed following initial presentation with Candida vulvovaginitis in females and tinea cruris in males. Diabetic ketoacidosis is not a common initial presentation of T2DM in children and adolescents. The reported frequency of diabetic ketoacidosis ranges between 3% and 11% in youth with T2DM at initial presentation, which is much higher compared to adults. However, this percentage increased dramatically during COVID 19 pandemic reaching up to 20%.10,28 Hyperglycemic hyperosmolar state is rare relative to diabetic ketoacidosis among pediatric patients.10
Screening and Diagnosis
Screening for prediabetes or diabetes is recommended at 10 years of age or at the onset of puberty (whichever is earlier) for a child with BMI at or above 85th percentile and one or more additional risk factors for diabetes including strong family history, ethnicity, or signs of insulin resistance.29,30 HbA1c, fasting blood glucose, or oral glucose tolerance test are recommended as initial screening tests.30 In the absence of symptoms, it is recommended that an abnormal result be verified with repeat testing with the same test or another test.31
Health Consequences of T2DM in Adolescents
Cardiovascular Risk
Dyslipidemia is very common in pediatric patients with T2DM. This typically occurs as a component of metabolic syndrome.32,33 Patients with T2DM usually have low high density lipoprotein cholesterol (HDL-C) and elevated levels of triglycerides, non-HDL cholesterol and low-density lipoprotein cholesterol (LDL-C) concentrations. Hypertension is also noted frequently in children and adolescents with T2DM.34,35 In the TODAY study, hypertension was present in 19.2% at baseline and cumulative incidence at 15 years was 67.5%.35
Microvascular Complications
Adolescents with T2DM are at a higher risk for diabetes-related complications relative to patients with type I diabetes (T1DM) and these complications tend to occur earlier.36 In the TODAY cohort, the percentage of patients with microalbuminuria was 6.3% at baseline and rose to 16.6% by the end of the study.35 The cumulative incidence of diabetic nephropathy was 54.8% and of diabetes-related neuropathy and diabetic retinopathy was 32.4% and 51%, respectively, at the end of follow-up with mean time since the diagnosis of diabetes being 13.3±1.8 years.35 This highlights the importance of early intervention to attain better glycemic response to prevent the occurrence of these complications.
Socioeconomic Impact
With low financial income being common among families with children with T2DM, costs related to medical treatment for diabetes add to the financial burden. In the TODAY study, the estimated annual medical costs in the first year of treatment was $1798 in patients assigned to metformin alone, $2971 to combination drug therapy with metformin plus rosiglitazone and $2092 to metformin plus an intensive lifestyle and behavior change.37 In the TODAY study, low income was not associated with lack of medication adherence. However, the lack of correlation could be attributed to the fact that most families had an estimated annual income of less than 25,000.38
Several studies highlight health inequities related to T2DM among adolescents. In one large retrospective cohort study, the frequency of ophthalmic screening in 6 years post-diabetes diagnosis was lower in Black and Latino groups relative to Whites.39 Also, the frequency of screening was lower in patients whose families had lower financial income. Patients with T2DM with lower education level and family income below federal poverty level have poorer glycemic status and two-fold higher risk of diabetes-related mortality compared to those with higher education level or higher family income.39,40
Psychosocial Health
Children and adolescents with T2DM are at increased risk for depression and other mental health disorders. The estimated prevalence of depression among youth with T2DM is more than 20%.41,42 In a population-based cohort study of 528 youth (7–18 years of age) with T2DM (majority First Nations Heritage), youth with T2DM were more likely to attempt suicide [RR 3.18 (1.30, 7.81) p=0.012] and complete suicide [RR 2.18 (1.32, 3.60) p=0.002] compared to their age, gender, geographic residence and ethnicity matched peers without diabetes.43 In the Pediatric Diabetes consortium, 22% of youth with T2DM had depression and only 9% had been treated by a therapist within the prior 12 months.44
Youth with T2DM also have higher prevalence of other mental health comorbidities. Children with T2DM were more likely to have a mood or anxiety disorder before or after diagnosis in comparison to matched peers without diabetes [RR 2.38 (1.63, 3.48) p<0.001 and 1.70 (1.39, 2.08) p<0.001], respectively.43 Youth with T2DM were also more likely to be prescribed an antipsychotic relative to matched peers without diabetes before or after diagnosis [RR 2.33 (1.23, 4.39) p=0.009 and RR 1.76 (1.23, 2.52) p=0002].43
Mental health and family dynamics have significant impact on weight management and diabetes management. In fact, in patients with T1DM, lower familial involvement without patient’s readiness to take full responsibility to manage their own diabetes is associated with poor glycemic control.45,46 Additionally, patients with poor mental health are at a higher risk for lower medication adherence and poor glycemic control. Adolescents with T2DM exposed to multiple stressful life events demonstrated lower adherence to their prescribed oral medications in addition to impaired quality of life and depressive symptoms.47 Depression and anxiety have been shown to be associated with unhealthy behavior including physical inactivity and unhealthy diet.48
Alcohol and substance use adversely impact self-management behaviors in individuals with a chronic medical illness.49 The Youth Risk Behavior Survey revealed that 29% of school students drink alcohol and 14% reported binge drinking.50,51 In adults, binge drinking has been associated with higher risk of developing T2DM.52 Similarly, active, or passive smoking has been associated with increased risk of T2DM.53 There is scarcity of data on the impact of alcohol and substance use on weight management and glycemic control in youth with T2DM.
Eating Disorders
Eating disorders are prevalent in youth with T2DM and affect adiposity and psychosocial functioning. In the TODAY study, 20% and 6% of youth engaged in subclinical and clinical binge eating, respectively.54 Subclinical and clinical binge eating were associated with higher rates of extreme obesity, global eating disorder, impaired quality of life and depressive symptoms.
In the SEARCH for Diabetes in Youth Study, disordered eating behaviors were noted in 50.3% of youth and young adults with T2DM.55 Presence of disordered eating behaviors was associated with significantly higher BMI Z score, lower insulin sensitivity, poorer quality of life and more depressive symptoms.55 Female gender, a history of dieting, body dissatisfaction, increased body weight, and a history of depression are risk factors for development of eating disorders in adolescents with T2DM.56,57
Management of T2DM in Children and Adolescents
The glycemic target for adolescents with T2DM is HbA1c <7%.31,58 However, lower HbA1c can be targeted (<6.5%) for recently diagnosed patients to preserve their beta cells as chronic hyperglycemic status will exacerbate beta cell toxicity and worsen beta cell secretory function.
A HbA1c <6.5% can be targeted as well in patients who are able to achieve significant weight loss by lifestyle changes and those on metformin.29,31 Adequate glycemic control in children with T2DM is challenging as in children with type 1 diabetes.59
Lifestyle modifications are an essential component of weight management of T2DM in children and adolescents. The guidelines for weight management of youth with T2DM recommend at least 7–10% weight loss in adolescents who have achieved their final adult height and a BMI below the 85th percentile for those who are still growing.30 The weight loss goals, however, should be tailored individually based on patient’s previous weight trajectory and impact of interventions on weight status and glycemic control.
In the TODAY study, a decrease of ≥7% of the total body weight across treatment arms was associated with significant improvements in multiple cardiovascular risk factors including systolic blood pressure, LDL-C, triglycerides, total cholesterol, and HDL-C over 24 months.60
A multidisciplinary approach is essential in management of adolescents with T2DM due to the biological, socioeconomic, and psychological factors that serve as barriers to effective weight management and glycemic control.
Lifestyle Interventions
The American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes (ISPAD) recommend that children and adolescents with T2DM should be encouraged to participate in at least 60 min of moderate to vigorous physical activity daily (with muscle and bone strength training at least 3 days/week).31,61 Physical activity has been linked to multiple favorable short-term effects in adolescents especially mental health and bone health in addition to other long-term effects throughout adulthood.62
High intensity physical activity has been associated with lower BMI status, lower waist circumference, lower cardiovascular risk, and better cardiovascular fitness.63 These benefits increase with increasing level of physical activity.64 The beneficial effect of physical activity is not only limited to improvement in BMI status and cardiometabolic profile, but it has been also associated with improvement in psychological outcomes.64
Increased waist circumference suggestive of increased visceral fat has been associated with increased insulin resistance.65 Increased physical activity partly improves insulin sensitivity by decreasing visceral fat and waist circumference.63 Additionally, exercise increases expression of GLUT4 receptor expression, thereby increasing peripheral glucose uptake. This effect of improved insulin sensitivity extends 16 hours post exercise in healthy subjects and in patients with T2DM.66 In a cross-sectional study including 164 adolescents with T2DM, adolescents who engaged in vigorous physical activity were able to attain lower HbA1c level and better cardiovascular parameters.67
Patient education regarding healthy dietary habits is crucial for management of T2DM (Table 1). Therefore, every patient with T2DM should receive thorough nutritional education as part of intensive lifestyle therapy.68 Decreased consumption of sugary drinks and increased consumption of whole-grain bread and cereals, fruits, and vegetables are recommended.69,70 Caloric intake should be decreased to achieve weight loss goals.
 |
Table 1 Suggested Dietary Recommendations for Youth with Type 2 Diabetes and Their Families |
The impact of weight loss on insulin sensitivity was demonstrated in children with obesity in whom a decrease in their BMI by ≥ 0.5 SDS over one year was associated with an increase in insulin sensitivity. On the other hand, insulin sensitivity remarkably decreased in those with increase in their BMI SDS over a one year period.71 A systematic review in adults with obesity/overweight and T2DM demonstrated reduction in HbA1c secondary to weight loss in a dose-dependent manner. Each 1 kg of mean weight loss was associated with mean HbA1c reduction by 0.1%. This effect was more prominent in patients with poor glycemic status at baseline.72
Unfortunately, weight loss remains a challenge in the management of youth with T2DM. Obesity is the result of a complex interaction between genetic/epigenetic factors. Underappreciated obesogenic factors sometimes referred to as the “exposome” in conjunction with genetic factors play a major role in the development of obesity as well as the extent of weight loss in response to various treatment strategies.73–75 In the TODAY study, intensive lifestyle interventions in addition to metformin did not result in any improved weight loss (percent overweight from baseline) in comparison to the group that was treated with metformin only.60
Meta-analyses of lifestyle interventions that include behavioral components such as goal setting, self-monitoring, and problem-solving demonstrate significant BMI reductions relative to treatment as usual or passive control, with longer interventions showing greatest benefit.76,77 However, translating the benefits observed in randomized clinical trials to real-world clinical settings has been challenging. In a large multicenter study including around 21,000 children with obesity in which the efficacy of lifestyle interventions was assessed after a 2-year follow-up, only 22% of patients were able to reduce their BMI more than 0.25 SDS in 6 months, 15% after 12 months and 7% after 24 months. Also, less than 25% of the data were available due to high dropout rate and lack of documentation.78 Failure to achieve weight loss in youth without diabetes was also demonstrated in the multicenter Pediatric Obesity Weight Evaluation Registry in the United States.79,80
Barriers to achieving recommended weight loss in youth with obesity and T2DM are multifactorial (Table 2). While there are intrinsic biological factors that impede weight loss such as genetic predisposition to weight gain, the lack of engagement could be related to family education, motivational factors, availability of social support, and various socioeconomic barriers.78 In the TODAY study, the rate of lifestyle sessions attendance was only 60% of the planned sessions.81 Additionally, multiple health issues associated with obesity can interfere with patients’ daily activities, such as musculoskeletal problems, asthma, and disturbed sleep pattern.82–85 Obstructive sleep apnea (OSA) is commonly present in youth with T2DM. The severity of OSA is directly proportional to the degree of obesity.86 OSA has been associated with worsening in BMI and cardiometabolic status.87 Therefore, screening for OSA is recommended in youth with T2DM.31
 |
Table 2 Psychological and Behavioral Barriers in Adolescent/Caregiver and Potential Behavioral Strategies to Overcome These Barriers |
In a survey administered to physicians and nurses attending a pediatric diabetology conference, that was designed to identify the different barriers that interfere with T2DM management in adolescents, having a family member with unhealthy lifestyle behavior was the most strongly perceived barrier (98%).88 Most (89%) of the participants thought that low motivation to engage in lifestyle modification to T2DM management may be related to family perceptions that risk is low or distal, as the vast majority of patients with T2DM are diagnosed when screened for diabetes without having evidence of life-threatening symptoms. Almost three quarters (71%) perceived behavioral and psychiatric disorders as a barrier and two thirds reported cultural and language barriers. Furthermore, only 37% of the clinics surveyed provided patients with culturally appropriate educational materials tailored to racial and cultural groups. These data highlight the importance of a family centered approach in management of T2DM including weight management and treatment of co-existing mental health disorders in youth with T2DM (Table 2). These also support the importance of recognizing racial, cultural and language differences in managing T2DM in youth.88
In a qualitative pilot study in the United Kingdom of five youth with T2DM and their families, families felt they need more practical recommendations rather than the regular multidisciplinary approach. Also, they felt that attending in groups can be helpful to feel more motivated when sharing their positive experiences with others.89
Emerging and Innovative Lifestyle Change Strategies
Summer camps have been explored as potential strategies for weight loss in children. In one study that evaluated the effectiveness of a residential weight-loss camp in 185 overweight children (mean age 13.9 years), campers who stayed for a mean of 29 days had weight loss of 6 kg, reduction in BMI by 2.4 units and reduction in BMI SD scores by 0.28.90 Other studies showed similar BMI reduction effect, however, long term outcome was questionable.91,92 In a randomized controlled trial including 94 children (age 6–12 years), children who participated in summer camp showed subtle decrease in their BMI by 0.03 despite participating in almost 50% of the summer camp activities, while those who were involved in unstructured summer activities experienced subtle increase in their BMI by 0.07 kg/m2.93 One of the major challenges facing children participating in these residential programs is to implement same healthy lifestyle intervention upon transition to home environment . Cognitive behavioral therapy, weekly lifestyle education and physical activity sessions can reinforce the favorable effect of weight loss summer camps and may establish daily healthy lifestyle routine for favorable long-term outcome.92
Technology-based interventions have been shown to have modest efficacy in weight management in a few studies.94 In a randomized control trial including 77 adolescents with obesity, use of a mobile/tablet-application was associated with lower consumption of fast-food relative to adolescents who received standard care only. However, there were no statistically significant differences in BMI between the groups.95 In another study of 2825 adolescents who participated in a remote weight loss program consisting of weight loss mobile applications, calorie restriction and frequent self-weighing, the mean weight reduction in BMI z score in 120 days post intervention was 0.42 ± 0.03. The percentage of weight loss was higher with higher baseline BMI percentile and increased frequency of use of mobile applications.96
New mobile applications with innovative ideas are now available and look promising to enhance children’s adherence to healthy lifestyle behaviors.97 Some of these applications allow parents to have feedback regarding their child’s physical activity, dietary habits and provide guidance regarding healthy eating habits and recommended daily physical activities.98,99
Video gaming is widely used by the adolescent population. Active video games can be a novel way to enable adolescents to be more physically active. In a systematic review of randomized control trials, active video gaming was associated with reduction in median BMI percentile by −1.77 (95% CI, −2.55 to −0.99; P<0.001).100 This modality is promising as it is widely accepted by adolescents and can be used at school and home.
Financial incentives can be another modality to boost adolescents’ engagement in healthy lifestyle behavior. In one study, using incentives combined with mobile health physical activity program was associated with better acceptability and more program adherence.101 There are ongoing trials to demonstrate the efficacy of financial incentives when used in combination with a structured dietary plan and exercise,102 (National Library of medicine, NCT number: NCT01848353).
Adequate nutrition is fundamental for children and adolescents’ growth and development. Therefore, continuous energy restriction is not reasonable for weight management in children and adolescents. Intermittent energy restriction using very low energy diet (600–700 Kcal/day) 3 days per week was studied in 30 adolescents. After 12 weeks of intermittent energy restriction, the estimated reduction in BMI was 5.6 kg/m2. 21 adolescents elected to continue same dietary regimen for additional 14 weeks. The endpoint result after 26 weeks was reduction in their BMI by 5.1 kg/m2.103
Access to Healthcare
Access to healthcare is one of the challenges for youth with endocrinological disorders, including diabetes.104–106 The dropout rates in children attending weight management programs tend to be high. Reasons for high dropout rates and attrition include lack of children’s motivation for lifestyle change, the program not meeting family’s needs, and not seeing desired outcomes.107 Access to health care was further affected during the COVID 19 pandemic.4 Telehealth has been associated with lower dropout rates and higher satisfaction among children and their families.108 In a systematic review, several clinics that offered both face-to-face visits and telehealth for children and adolescents with overweight and obesity reported improvement in weight outcomes.109 In adults with T2DM, telehealth has been associated with weight reduction and better glycemic control.110
Psychosocial Approach
Psychosocial factors constitute a major challenge in management of T2DM in adolescents. Almost one in five adolescents with T2DM has depression.41 Both the TODAY and the SEARCH study group did not demonstrate a correlation between depression and poor glycemic control.111,112 However, deterioration in quality of life secondary to the psychological burden of diabetes has been associated with worse glycemic control.111 In adults, the estimated prevalence of depression among patients with T2DM is 25% and has been associated with worse glycemic control among those patients.113 Therefore, youth with T2DM should be screened for mental health comorbidities at diagnosis and at every follow-up visit and referred to behavioral health professionals when indicated.30,31
The severity of depression and the timing of these symptoms can have an impact on T2DM outcome.41 In addition to depression, distress related to T2DM diagnosis can be another predisposing factor as well. Also, symptoms associated with diabetes can have an impact on individual’s lifestyle being more sedentary, having disturbed sleep pattern, poor medication adherence and following unhealthy eating habits.41 Several antidepressants and psychotropic medications have been associated with weight gain and therefore it is important to use medications that are weight neutral or are associated with weight loss when treating depression or other mental health in adolescents with T2DM.114
Family readiness and involvement in managing their children with T2DM and obesity is crucial and facilitates engagement with treatment recommendations.45,46 Families of patients with T2DM are subjected to multiple financial and social stressors.115 Therefore, all youth with T2DM and their families should receive thorough education regarding weight management and the education should be culturally appropriate and should address the different socioeconomic, psychosocial and financial barriers30 (Table 2). All these reasons speak to the importance of social work as part of an effective team. Similarly, behaviorally trained professionals such as psychologists and dieticians are essential to modifications in dietary habits and physical activity (Figure 1).
 |
Figure 1 Strategies to address barriers affecting weight management in adolescents with type 2 diabetes. |
Data on the impact of psychological interventions on weight management or glycemic outcomes in children and adolescents with T2DM are lacking. In adults with T2DM, cognitive behavioral therapy along with lifestyle intervention has been associated with better glycemic outcomes.41 A family centered approach that focuses on family’s involvement in the child’s treatment, adaptive and maladaptive interactions secondary to child’s T2DM and obesity can be helpful to overcome these psychosocial barriers.116,117 Also, the home settings play a fundamental role changing child’s daily routine. Involvement of parents is an essential component of disease management in this group of adolescents.118 Supportive family environment includes adherence to dietary recommendations and helping the child with being physically active and engage in less sedentary behavior. Families should receive support about how to manage the anxiety burden of new diabetes diagnosis.116,119
Language differences between providers and patients can be a barrier to effective healthcare delivery. In one study, 65% of pediatric endocrine and diabetes specialists reported difficulties in delivering care to their patients due to language barriers.88 Therefore, having diversity in work force and providing interpreting services in different T2DM pediatric centers are essential to provide proper family education and awareness of their child management plan.
Pharmacological Interventions
Pharmacologic therapy in conjunction with lifestyle modifications is recommended in all adolescents at the time of diagnosis of T2DM. The decision regarding which medication should be initiated depends on patient’s metabolic status.30 Metformin and glucagon like peptide −1 (GLP-1) agonists are the medications approved by the Food and Drug Administration for adolescents with T2DM that do not present with ketosis or ketoacidosis at the time of diagnosis.120,121 Insulin (basal or short acting) is recommended in those that present with ketosis/ketoacidosis or those with an HbA1c greater than 8.5%. Table 3 details the weight outcomes with various medications used for weight loss in adolescents with obesity.
 |
Table 3 Pharmacologic Agents Used for Weight Management in Children and Adolescents with Obesity |
Metformin
Metformin is the first-line therapy for treatment of T2DM in metabolically stable patients. It is often initiated as monotherapy in asymptomatic patients with HbA1c < 8.5% or in combination with a GLP-1 analog or basal insulin for symptomatic patients with HbA1c ≥ 8.5% in the absence of ketosis or ketoacidosis.30
In children and adolescents with obesity, metformin may have modest weight reduction effect in a subgroup of patients. In a systematic review, metformin was associated with decrease in BMI by 1.16 kg/m² after 6 months treatment duration.120 The weight loss effect was noted to be small but significant in children and adolescents of Hispanic ethnicity, those with acanthosis nigricans, those with baseline BMI below 35 kg/m2, and those with failure of diet and exercise programs.120 In the TODAY study, the percentage of patients who were able to achieve weight loss target while on metformin plus lifestyle interventions group was 31.2% versus 24.3% for those who were on metformin alone.122 The change in percent overweight from baseline was not different between the metformin and the metformin plus lifestyle group suggesting that metformin itself may have a beneficial effect on weight status in adolescents with T2DM. Metformin is not approved by FDA for weight management in children. However, it has been widely used as off-label for weight reduction in children and adolescents with obesity.123 The most common side effects of metformin are gastrointestinal disturbances, such as diarrhea, nausea, and vomiting. In a systematic review, 26% reported gastrointestinal side effects relative to 13% in the control group. However, no serious side effects have been reported.120
GLP-1 Agonists
Both, daily GLP-1 agonist liraglutide or weekly GLP-1 agonist exenatide have been approved by the United States Food and Drug Administration for treatment of T2DM in children and adolescents above 10 years of age. As the post-prandial insulin release is usually blunted or impaired in patients with T2DM, GLP-1 agonists improve glycemic control by enhancing post-prandial insulin release. Additionally, these drugs delay gastric emptying and increase beta cell life span by inhibiting their apoptosis. GLP-1 agonists also improve insulin sensitivity by increasing peripheral glucose uptake by muscles and decreasing hepatic glucose output. An indirect mechanism to improve insulin sensitivity is weight reduction. The weight loss effect of these medications is mainly attributed to delayed gastric emptying and appetite suppression through direct effect on the hypothalamus.124 Liraglutide is also approved for weight loss in adolescents 12 years of age or older. However, the approved dose for weight management is higher (3 mg daily) than the dose for T2DM management (1.8 mg daily). Semaglutide, another GLP-1 agonist that is given subcutaneously once a week was recently approved by the FDA for weight management in children 12 years of age and older.
In a systematic review of nine randomized controlled trials including 286 children under 18 years of age with obesity, prediabetes or T2DM that were treated with GLP-1 agonists, the use of a GLP-1 agonist was associated with an average weight reduction of 2.7 kg. This effect was observed more in patients with higher BMI status.125
The favorable effect of GLP-1 agonists on weight in adolescents with T2DM when used with metformin has been shown in a randomized control trial including 134 children and adolescents (age 10–17 years) with T2DM. The liraglutide group had a weight loss of 2.3 kg at week 26 relative to weight loss of 0.99 kg with placebo. However, the weight loss was only maintained in the liraglutide group at week 152–1.91 kg versus 0.87 kg with the placebo.126 Similar number of patients reported adverse events in the liraglutide and placebo groups. However, the overall rates of adverse events and gastrointestinal side effects were higher with liraglutide. Liraglutide was efficacious in improving glycemic status with the liraglutide group having a reduction of hemoglobin A1c by 0.64% and the placebo group having an increase in A1c by 0.42% at week 26. In adults, worst weight response was noted in those with higher baseline weight.127
In children and adolescents with obesity, a meta-analysis has shown that GLP-1 agonists (liraglutide or exenatide) have been associated with modest reduction in BMI [mean difference - 1.24[−1.71, −0.77)] kg/m² and in body weight [mean difference −1.5 (−2.5, −0.5) kg].128 The study included 6 trials with liraglutide and 3 trials with exenatide. Subgroup analysis to compare the efficacy of both drugs did not reveal significant difference between both drugs in lowering BMI (exenatide (MD −1.11 [−1.67, −0.55] kg/m2, I2 0%) and liraglutide (MD −1.58 [−2.42, −0.70] kg/m2, I2 0%)). The glycemic status did not appear to impact the effect of GLP-1 receptor agonists on reduction of weight or BMI. Modest decrease in systolic blood pressure was noted, though there were no improvements in lipid profile. Increased risk of nausea was noted.128 Other side effects of GLP-1 agonists include vomiting, diarrhea, abdominal pain, hypoglycemia, elevated transaminases, and pancreatitis.128
Semaglutide is showing promising weight reducing effect in adults with obesity and T2DM compared to liraglutide.129 In a double-blind randomized, placebo-controlled trial with semaglutide, including a total of 180 adolescents (age from 12 to <18 years), the mean change in BMI from baseline in the treatment group was 16.1% relative to 0.6% in the placebo group.130
Tirzepatide is a dual GLP-1 and glucose dependent insulinotropic peptide (GIP) receptor agonist that has been shown to cause substantial weight loss in adults.131 There is an ongoing clinical trial to demonstrate the metabolic and weight reduction effect of tirzepatide in adolescents (National Library of medicine, NCT number: NCT05260021).
Insulin
Insulin therapy is commonly associated with weight gain.132,133 The weight gain is secondary to catch up in weight after previous weight loss, reduction in glucosuria, increase in appetite and conscious or subconscious overeating due to fear of risk of hypoglycemia.133 Strategies to limit insulin-associated weight gain include limiting the total dose of the insulin by increasing physical activity that promotes insulin sensitivity and using oral antidiabetic medications, such as metformin or GLP-1 analogs that are associated with weight loss.132
Other Pharmacologic Options
Topiramate is a medication that has been used for treatment of epilepsy and migraine prophylaxis in children. It has been shown to lead to weight loss through its central effect on neurotransmitters. In a double-blind randomized control trial in overweight and obese adults with T2DM, topiramate was associated with a reduction in body weight by 6.6 ± −3.3 kg and decrease in body fat in lean body mass.134 There was decrease in daily average energy intake in the topiramate group. Additionally, there was reduction in hemoglobin A1c by 1.1 ± −0.9%.134 Other studies have demonstrated modest reduction in BMI when used as a monotherapy in children and adolescents with severe obesity.135,136 As topiramate is a teratogen and can decrease the efficacy of oral contraceptives, female adolescents that are started on topiramate should be counseled against pregnancy.
Phentermine is a sympathomimetic stimulant medication that potentiates the release of norepinephrine in the hypothalamic region causing satiety.137,138 In a retrospective chart review of adolescents receiving weight management services in a pediatric clinic, greater reduction in BMI was noted at 1 month (−1.5%, 95% CI: −2.6, −0.6%; P=0.001), 3 months (−2.9%, CI −4.5, −1.4%, P<0.001) and 6 months (−4.1%; 95% CI: −7.1, −1%, P=0.009) in comparison with standard of care lifestyle modification therapy in children with obesity.138
A combination of both topiramate and phentermine has been approved by FDA for treatment of children with obesity ≥12 years of age.139 In a double-blind randomized placebo-controlled trial including 233 adolescents with obesity from 12 to 17 years of age, the efficacy of two different phentermine/topiramate dose combinations (high dose 15 mg of phentermine and 92 mg of extended release topiramate and mid-dose of 7.5 mg of phentermine and 46 mg of extended release topiramate) in addition to lifestyle interventions was examined. After 56 weeks of treatment, the high-dose treatment group had mean BMI reduction of 10.44% relative to the placebo group and the mid-dose had a slightly lower reduction of 8.11%. Adverse events were reported almost equally between high dose and placebo group (51.8% vs 52.2%). However, these side effects were less frequently reported in mid-dose group (37%).140
Currently, there is an ongoing Phase I clinical trial to evaluate the efficacy of phentermine and topiramate combination in adolescents with T2DM (National Library of medicine, NCT number: NCT04881799).
Orlistat is another medication that is approved for weight management in adolescents with obesity. Orlistat acts by inhibiting gastric and pancreatic lipases preventing the absorption of free fatty acids.141 A randomized, double-blind study including 539 adolescents with obesity demonstrated a mild BMI reducing effect in the orlistat group by 0.55 kg/m2 relative to the control group after 54 weeks of treatment.142 There were no significant differences in glucose levels between the 2 groups. Though the 2-hour insulin levels for those receiving orlistat were lower than at baseline, the decrease was not significantly different from that in the placebo group. Gastrointestinal side effects were common in the orlistat group reaching up to 50%, which limit the tolerability of the medication for many patients.142
Sodium-glucose co-transporter 2 (SGLT2) inhibitors block the renal reabsorption of glucose thereby increasing urinary glucose excretion and lowering plasma glucose level.143,144 SGLT2 inhibitors have been associated with modest weight loss in adults.145 However, pediatric data on SGLT2 inhibitors in adolescents are limited. Dapagliflozin was not associated with any change in BMI z score in a multicenter, placebo-controlled, double-blind randomized Phase 3 study in youth and young adults with T2DM.146
Metabolic and Bariatric Surgery
Metabolic and bariatric surgery (MBS) should be considered as an option for weight loss for adolescents who have T2DM with class II obesity (BMI percentile ≥120% of the 95th percentile to <140% of the 95th percentile or BMI ≥ 35 kg/m2 to <40 kg/m2, whichever is lower) or class III obesity (BMI percentile ≥140% of the 95th percentile or ≥40 kg/m2).147,148 MBS should not be considered in the presence of medically correctable cause of obesity, a medical, cognitive, psychosocial condition affecting the adherence to postoperative recommendations, lack of patient’s or family’s motivation to abide by post-surgical recommendations and current or planned pregnancy within 12 to 18 months of the surgery.147,148
MBS is associated with excellent weight loss and remission of T2DM in adolescents. The two most performed procedures in adolescents are the laparoscopic sleeve gastrectomy and the Roux-en-Y gastric bypass (Figure 2). In the TEEN-LABS consortium study, among 242 adolescents who underwent MBS, mean weight decreased by 27% three years post-surgical intervention.149 There was no significant difference in weight reduction between patients who underwent Roux-en-Y gastric bypass (161 participants) or sleeve gastrectomy (67 participants) (28% vs 26% respectively). Additionally, 95% (95% CI; 85 to 100) of patients who had T2DM at the time of surgery were able to achieve remission. Similarly, high rates of remission were noted for other comorbidities (86% for abnormal kidney function, 76% for prediabetes, 74% for elevated blood pressure and 66% for dyslipidemia).149
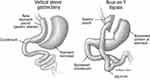 |
Figure 2 Commonly performed metabolic and bariatric procedures performed in adolescents. |
When comparing the data of 30 adolescents in the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) who had T2DM at the time of their MBS (24 underwent Roux-en-Y gastric bypass and 6 underwent vertical sleeve gastrectomy) to 63 adolescents in the TODAY study (medical controls) matched by baseline age (13–18 years), race, sex, ethnicity, and baseline BMI (>35), surgical intervention was associated with a decrease in BMI by 29% relative to a 3.7% increase in the TODAY participants.150 Also, patients in the Teen-Labs group had better glycemic control with a mean reduction of HbA1c by 1.3% in the Teen-LABS group relative to patients in the TODAY group who had increase in their HbA1c by 1.4%.150 Similarly, Teen-LABS group showed better improvement in other comorbidities relative to TODAY group.150
Despite the potential cardiometabolic benefits of MBS, these procedures have been associated with potential risks, including vitamin and mineral deficiencies and need for additional surgery for complications possibly related to surgery.150 Therefore, surgical intervention should be considered for weight loss when medical treatment fails to achieve weight loss.
There are significant barriers to surgery in adolescents that qualify for MBS. These include a stigma around MBS as treatment for obesity, lack of access due to insurance coverage, lack of awareness amongst physicians about the benefits of MBS and family concerns about a surgical procedure during adolescence.151 Additionally, despite higher prevalence of obesity and T2DM in ethnic minorities, white adolescents undergo MBS more frequently than Blacks (2.5 times higher) and Hispanics (2.3 times). Almost two thirds of adolescent bariatric surgeries in the Bariatric Outcomes Longitudinal Database, a national database that collects bariatric surgical care data in the United States were performed in White youth.152 Therefore, there is need to address racial and ethnic disparities in access to MBS.153
Less invasive surgical procedures, such as endoscopic intragastric balloon have been considered as an option when surgical intervention is not feasible. In a retrospective longitudinal study including 27 adolescents with obesity treated with intragastric balloon (age 14–19 years), mean BMI decreased from 37.04 to 31.18 kg/m2 in 6 months period with no serious side effects reported.154 Similar favorable outcome has been shown when a swallowable intragastric balloon was used in 16 children with obesity. Their mean weight decreased from 95.8 ± 18.4 kg to 83.6 ± 27.1 kg.155 Currently, there is an ongoing clinical trial to estimate the efficacy of intragastric balloon in adolescent population (National Library of Medicine, NCT number: NCT03233048).
Aspiration therapy is an innovative modality for weight management. A percutaneous gastrostomy tube allows patients to remove a portion of their ingested meals with subsequent lower caloric consumption.156 Aspiration therapy has been approved for weight management in adults. There is an ongoing clinical trial to evaluate the weight reduction effect of aspiration therapy in adolescents (National Library of Medicine, NCT number: NCT03598920).
A family-centered approach is crucial when considering surgical intervention to ensure that adolescents and parents/caregivers understand and are willing and able to follow post-surgical requirements. Comprehensive psychosocial evaluation, is important for assessing factors that could influence adherence and post-surgical outcomes, determining psychosocial readiness for surgery, and recommendations to mitigate contra-indications (eg, treatment for poorly managed psychiatric conditions, eating disorders, or substance abuse).147
Conclusion
The rising prevalence of T2DM among adolescents is posing a major challenge to the healthcare system. Weight reduction via lifestyle interventions is an essential component for management of adolescents with T2DM. Adolescents with T2DM have various biological, socioeconomic, cultural, psychological, and financial barriers to weight management. A multidisciplinary approach that is family focused and is delivered in a culturally appropriate manner is recommended to achieve weight management in youth with T2DM. Lifestyle interventions and pharmacotherapy have shown modest efficacy in achieving weight reduction in adolescents with T2DM. Bariatric surgery is associated with excellent weight reduction and remission of T2DM in youth. Emerging therapies for weight reduction in youth include digital technologies, newer GLP-1 agonists and endoscopic procedures.
Disclosure
Dr Bridget K Biggs reports donation to Mayo Clinic aimed at improving care of adolescents with obesity. Funds have supported research and program development for lifestyle interventions from Delaney Family and The Vincent Dowling Family Foundation, outside the submitted work. None of the authors have any conflicts of interest relevant to this manuscript.
References
1. Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326(8):717–727. doi:10.1001/jama.2021.11165
2. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3). doi:10.1542/peds.2017-3459
3. Stierman BA, Carroll J, Margaret D. National health and nutrition examination survey 2017–March 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes. Available from: https://stacks.cdc.gov/view/cdc/106273.
4. Magge SN, Wolf RM, Pyle L, et al. The coronavirus disease 2019 pandemic is associated with a substantial rise in frequency and severity of presentation of youth-onset type 2 diabetes. J Pediatr. 2022;251:51–59.e2. doi:10.1016/j.jpeds.2022.08.010
5. Wu HJ, Patterson CC, Zhang XE, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022;185:109785. doi:10.1016/j.diabres.2022.109785
6. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi:10.1016/S0140-6736(05)61032-X
7. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi:10.1046/j.1365-2362.32.s3.3.x
8. Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16(2):81–90. doi:10.1038/s41574-019-0286-3
9. Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem. 2020;126(1):67–73. doi:10.1080/13813455.2018.1493606
10. Klingensmith GJ, Connor CG, Ruedy KJ, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 2016;17(4):266–273. doi:10.1111/pedi.12281
11. Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4–11. doi:10.1111/j.1399-5448.2009.00519.x
12. Twig G, Zucker I, Afek A, et al. Adolescent obesity and early-onset type 2 diabetes. Diabetes Care. 2020;43(7):1487–1495. doi:10.2337/dc19-1988
13. Awa WL, Fach E, Krakow D, et al. Type 2 diabetes from pediatric to geriatric age: analysis of gender and obesity among 120,183 patients from the German/Austrian DPV database. Eur J Endocrinol. 2012;167(2):245–254. doi:10.1530/EJE-12-0143
14. Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981;20(2):87–93. doi:10.1007/BF00262007
15. Saleh M, Kim JY, March C, Gebara N, Arslanian S. Youth prediabetes and type 2 diabetes: risk factors and prevalence of dysglycaemia. Pediatr Obes. 2022;17(1):e12841. doi:10.1111/ijpo.12841
16. Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4(4):114–123. doi:10.4239/wjd.v4.i4.114
17. Srinivasan S, Chen L, Todd J, et al. The first genome-wide association study for type 2 diabetes in youth: the progress in diabetes genetics in youth (ProDiGY) consortium. Diabetes. 2021;70(4):996–1005. doi:10.2337/db20-0443
18. Pena AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med. 2020;18(1):72. doi:10.1186/s12916-020-01516-x
19. Brazo-Sayavera J, Aubert S, Barnes JD, Gonzalez SA, Tremblay MS. Gender differences in physical activity and sedentary behavior: results from over 200,000 Latin-American children and adolescents. PLoS One. 2021;16(8):e0255353. doi:10.1371/journal.pone.0255353
20. Meeks KA, Freitas-Da-Silva D, Adeyemo A, et al. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med. 2016;11(3):327–340. doi:10.1007/s11739-015-1302-9
21. Pham TM, Carpenter JR, Morris TP, Sharma M, Petersen I. Ethnic differences in the prevalence of type 2 diabetes diagnoses in the UK: cross-sectional analysis of the health improvement network primary care database. Clin Epidemiol. 2019;11:1081–1088. doi:10.2147/CLEP.S227621
22. Titmuss A, Davis EA, Brown A, Maple-Brown LJ. Emerging diabetes and metabolic conditions among Aboriginal and Torres Strait Islander young people. Med J Aust. 2019;210(3):111–113 e1. doi:10.5694/mja2.13002
23. Kriska A, Delahanty L, Edelstein S, et al. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131(3):e850–6. doi:10.1542/peds.2012-0620
24. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–167. doi:10.1210/jc.2010-1642
25. Dong YH, Zou ZY, Yang ZP, et al. Association between high birth weight and hypertension in children and adolescents: a cross-sectional study in China. J Hum Hypertens. 2017;31(11):737–743. doi:10.1038/jhh.2017.22
26. Franks PW, Looker HC, Kobes S, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes. 2006;55(2):460–465. doi:10.2337/diabetes.55.02.06.db05-0823
27. Jefferies C, Carter P, Reed PW, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. 2012;13(4):294–300. doi:10.1111/j.1399-5448.2012.00851.x
28. Chao LC, Vidmar AP, Georgia S. Spike in diabetic ketoacidosis rates in pediatric type 2 diabetes during the COVID-19 pandemic. Diabetes Care. 2021;44(6):1451–1453. doi:10.2337/dc20-2733
29. American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl1):S180–S199. doi:10.2337/dc21-S013
30. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(12):2648–2668. doi:10.2337/dci18-0052
31. Shah AS, Zeitler PS, Wong J, et al. ISPAD clinical practice consensus guidelines 2022: type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23(7):872–902. doi:10.1111/pedi.13409
32. Sunil B, Ashraf AP. Dyslipidemia in pediatric type 2 diabetes mellitus. Curr Diab Rep. 2020;20(10):53. doi:10.1007/s11892-020-01336-6
33. Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16(11):678–689. doi:10.1038/nrm4074
34. Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735–1741. doi:10.2337/dc12-2420
35. Group TS, Bjornstad P, Drews KL, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416–426. doi:10.1056/NEJMoa2100165
36. Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–835. doi:10.1001/jama.2017.0686
37. Songer TJ, Haymond MW, Glazner JE, et al. Healthcare and associated costs related to type 2 diabetes in youth and adolescence: the TODAY clinical trial experience. Pediatr Diabetes. 2019;20(6):702–711. doi:10.1111/pedi.12869
38. Katz LL, Anderson BJ, McKay SV, et al. Correlates of medication adherence in the TODAY cohort of youth with type 2 diabetes. Diabetes Care. 2016;39(11):1956–1962. doi:10.2337/dc15-2296
39. Saydah S, Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep. 2010;125(3):377–388. doi:10.1177/003335491012500306
40. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258–279. doi:10.2337/dci20-0053
41. Gulley LD, Shomaker LB. Depression in youth-onset type 2 diabetes. Curr Diab Rep. 2020;20(10):51. doi:10.1007/s11892-020-01334-8
42. Wang J, Wu X, Lai W, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ Open. 2017;7(8):e017173. doi:10.1136/bmjopen-2017-017173
43. Sellers EAC, McLeod L, Prior HJ, Dragan R, Wicklow BA, Ruth C. Mental health comorbidity is common in children with type 2 diabetes. Pediatr Diabetes. 2022;23(7):991–998. doi:10.1111/pedi.13389
44. Silverstein J, Cheng P, Ruedy KJ, et al. Depressive symptoms in youth with type 1 or type 2 diabetes: results of the pediatric diabetes consortium screening assessment of depression in diabetes study. Diabetes Care. 2015;38(12):2341–2343. doi:10.2337/dc15-0982
45. Palmer DL, Berg CA, Wiebe DJ, et al. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. J Pediatr Psychol. 2004;29(1):35–46. doi:10.1093/jpepsy/jsh005
46. Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration Type 1 diabetes. Diabet Med. 2002;19(8):635–642. doi:10.1046/j.1464-5491.2002.00752.x
47. Walders-Abramson N, Venditti EM, Ievers-Landis CE, et al. Relationships among stressful life events and physiological markers, treatment adherence, and psychosocial functioning among youth with type 2 diabetes. J Pediatr. 2014;165(3):504–508e1. doi:10.1016/j.jpeds.2014.05.020
48. Bonnet F, Irving K, Terra JL, Nony P, Berthezene F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178(2):339–344. doi:10.1016/j.atherosclerosis.2004.08.035
49. Walter KN, Wagner JA, Cengiz E, Tamborlane WV, Petry NM. Substance use disorders among patients with type 2 diabetes: a dangerous but understudied combination. Curr Diab Rep. 2017;17(1):2. doi:10.1007/s11892-017-0832-0
50. Underwood JM, Brener N, Thornton J, et al. Overview and methods for the youth risk behavior surveillance system - United States, 2019. MMWR Suppl. 2020;69(1):1–10. doi:10.15585/mmwr.su6901a1
51. Jones CM, Clayton HB, Deputy NP, et al. Prescription opioid misuse and use of alcohol and other substances among high school students - youth risk behavior survey, United States, 2019. MMWR Suppl. 2020;69(1):38–46. doi:10.15585/mmwr.su6901a5
52. Carlsson S, Hammar N, Grill V, Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care. 2003;26(10):2785–2790. doi:10.2337/diacare.26.10.2785
53. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):958–967. doi:10.1016/S2213-8587(15)00316-2
54. Group TS, Wilfley D, Berkowitz R, et al. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care. 2011;34(4):858–860. doi:10.2337/dc10-1704
55. Nip ASY, Reboussin BA, Dabelea D, et al. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for diabetes in youth study. Diabetes Care. 2019;42(5):859–866. doi:10.2337/dc18-2420
56. Pinhas-Hamiel O, Levy-Shraga Y. Eating disorders in adolescents with type 2 and type 1 diabetes. Curr Diab Rep. 2013;13(2):289–297. doi:10.1007/s11892-012-0355-7
57. Winston AP. Eating Disorders and Diabetes. Curr Diab Rep. 2020;20(8):32. doi:10.1007/s11892-020-01320-0
58. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl1):S15–S33. doi:10.2337/dc21-S002
59. Khadilkar A, Oza C. Glycaemic control in youth and young adults: challenges and solutions. Diabetes Metab Syndr Obes. 2022;15:121–129. doi:10.2147/DMSO.S304347
60. Marcus MD, Wilfley DE, El Ghormli L, et al. Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr Obes. 2017;12(4):337–345. doi:10.1111/ijpo.12148
61. Draznin B, Aroda VR, Bakris G, et al. 14. Children and adolescents: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl1):S208–S231. doi:10.2337/dc22-S014
62. Hallal PC, Victora CG, Azevedo MR, Wells JC. Adolescent physical activity and health: a systematic review. Sports Med. 2006;36(12):1019–1030. doi:10.2165/00007256-200636120-00003
63. Hay J, Maximova K, Durksen A, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. 2012;166(11):1022–1029. doi:10.1001/archpediatrics.2012.1028
64. Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi:10.1186/1479-5868-7-40
65. Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19(2):81–87. doi:10.1097/MED.0b013e3283514e13
66. Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21(1):1–12. doi:10.1055/s-2000-8847
67. Slaght JL, Wicklow BA, Dart AB, et al. Physical activity and cardiometabolic health in adolescents with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2021;9(1):e002134. doi:10.1136/bmjdrc-2021-002134
68. Springer SC, Silverstein J, Copeland K, et al. Management of type 2 diabetes mellitus in children and adolescents. Pediatrics. 2013;131(2):e648–64. doi:10.1542/peds.2012-3496
69. Yoo H, Suneja U. Pediatric Obesity Nutritional Guidelines. StatPearls; 2022.
70. Gidding SS, Dennison BA, Birch LL, et al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–559. doi:10.1542/peds.2005-2374
71. Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114(6):1569–1573. doi:10.1542/peds.2003-0649-F
72. Gummesson A, Nyman E, Knutsson M, Karpefors M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1295–1305. doi:10.1111/dom.12971
73. Catalan V, Aviles-Olmos I, Rodriguez A, et al. Time to consider the “exposome hypothesis” in the development of the obesity pandemic. Nutrients. 2022;14(8):1597. doi:10.3390/nu14081597
74. Aller E, Mariman ECM, Bouwman FG, van Baak MA. Genetic predictors of >/=5% weight loss by multidisciplinary advice to severely obese subjects. J Nutrigenet Nutrigenomics. 2017;10(1–2):32–42. doi:10.1159/000469662
75. Lamiquiz-Moneo I, Mateo-Gallego R, Bea AM, et al. Genetic predictors of weight loss in overweight and obese subjects. Sci Rep. 2019;9(1):10770. doi:10.1038/s41598-019-47283-5
76. O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US preventive services task force. JAMA. 2017;317(23):2427–2444. doi:10.1001/jama.2017.0332
77. Janicke DM, Steele RG, Gayes LA, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J Pediatr Psychol. 2014;39(8):809–825. doi:10.1093/jpepsy/jsu023
78. Reinehr T, Widhalm K, l’Allemand D, et al. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity. 2009;17(6):1196–1199. doi:10.1038/oby.2009.17
79. Kumar S, King EC, Christison AL. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57–65 e4. doi:10.1016/j.jpeds.2018.12.049
80. Tucker JM, Stratbucker W, King EC, et al. Characteristics of paediatric weight management in the United States: associations with program retention and BMI outcomes in the paediatric obesity weight evaluation registry (POWER). Pediatr Obes. 2022;17(2):e12848. doi:10.1111/ijpo.12848
81. Berkowitz RI, Marcus MD, Anderson BJ, et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes. 2018;19(2):191–198. doi:10.1111/pedi.12555
82. Smith SM, Sumar B, Dixon KA. Musculoskeletal pain in overweight and obese children. Int J Obes. 2014;38(1):11–15. doi:10.1038/ijo.2013.187
83. Fainardi V, Passadore L, Labate M, Pisi G, Esposito S. An overview of the obese-asthma phenotype in children. Int J Environ Res Public Health. 2022;19(2):636. doi:10.3390/ijerph19020636
84. Cediel G, Pacheco-Acosta J, CastiUo-Durdn C. Vitamin D deficiency in pediatric clinical practice. Deficiencia de vitamina D en la practica clinica pediatrica. Arch Argent Pediatr. 2018;116(1):e75–e81. doi:10.5546/aap.2018.eng.e75
85. Sluggett L, Wagner SL, Harris RL. Sleep duration and obesity in children and adolescents. Can J Diabetes. 2019;43(2):146–152. doi:10.1016/j.jcjd.2018.06.006
86. Andersen IG, Holm JC, Homoe P. Obstructive sleep apnea in obese children and adolescents, treatment methods and outcome of treatment - A systematic review. Int J Pediatr Otorhinolaryngol. 2016;87:190–197. doi:10.1016/j.ijporl.2016.06.017
87. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi:10.1378/chest.09-0360
88. Pinhas-Hamiel O, Zeitler P. Barriers to the treatment of adolescent type 2 diabetes--a survey of provider perceptions. Pediatr Diabetes. 2003;4(1):24–28. doi:10.1034/j.1399-5448.2003.00027.x
89. Lee V. The experiences and views of children with type 2 diabetes and their families. Diabetes Care Child Young People. 2020;10:165.
90. Gately PJ, Cooke CB, Barth JH, Bewick BM, Radley D, Hill AJ. Children’s residential weight-loss programs can work: a prospective cohort study of short-term outcomes for overweight and obese children. Pediatrics. 2005;116(1):73–77. doi:10.1542/peds.2004-0397
91. Fonseca H, Palmeira AL, Martins S, Ferreira PD. Short- and medium-term impact of a residential weight-loss camp for overweight adolescents. Int J Adolesc Med Health. 2014;26(1):33–38. doi:10.1515/ijamh-2012-0107
92. Vlaev I, Taylor MJ, Taylor D, et al. Testing a multicomponent lifestyle intervention for combating childhood obesity. BMC Public Health. 2021;21(1):824. doi:10.1186/s12889-021-10838-1
93. Evans EW, Wing RR, Pierre DF, Howie WC, Brinker M, Jelalian E. Testing the effect of summer camp on excess summer weight gain in youth from low-income households: a randomized controlled trial. BMC Public Health. 2020;20(1):1732. doi:10.1186/s12889-020-09806-y
94. Kouvari M, Karipidou M, Tsiampalis T, et al. Digital health interventions for weight management in children and adolescents: systematic review and meta-analysis. J Med Internet Res. 2022;24(2):e30675. doi:10.2196/30675
95. Likhitweerawong N, Boonchooduang N, Kittisakmontri K, Chonchaiya W, Louthrenoo O. Effectiveness of mobile application on changing weight, healthy eating habits, and quality of life in children and adolescents with obesity: a randomized controlled trial. BMC Pediatr. 2021;21(1):499. doi:10.1186/s12887-021-02980-x
96. Lei S, Inojosa JRM, Kumar S, et al. Effectiveness of a weight loss program using digital health in adolescents and preadolescents. Child Obes. 2021;17(5):311–321. doi:10.1089/chi.2020.0317
97. Alotaibi M, Alnajjar F, Cappuccio M, Khalid S, Alhmiedat T, Mubin O. Efficacy of emerging technologies to manage childhood obesity. Diabetes Metab Syndr Obes. 2022;15:1227–1244. doi:10.2147/DMSO.S357176
98. Yang HJ, Kang JH, Kim OH, et al. Interventions for preventing childhood obesity with smartphones and wearable device: a protocol for a non-randomized controlled trial. Int J Environ Res Public Health. 2017;14(2):184. doi:10.3390/ijerph14020184
99. Mohammed Nawi A, Che Jamaludin FI. Effect of internet-based intervention on obesity among adolescents in Kuala Lumpur: a school-based cluster randomised trial. Malays J Med Sci. 2015;22(4):47–56.
100. Ercelik ZE, Caglar S. Effectiveness of active video games in overweight and obese adolescents: a systematic review and meta-analysis of randomized controlled trials. Ann Pediatr Endocrinol Metab. 2022;27(2):98–104. doi:10.6065/apem.2244036.018
101. Cummings C, Crochiere R, Lansing AH, Patel R, Stanger C, Digital Health A. Program targeting physical activity among adolescents with overweight or obesity: open trial. JMIR Pediatr Parent. 2022;5(1):e32420. doi:10.2196/32420
102. Kelly AS. Enhancing weight loss with financial incentives in teens with severe obesity. NIH. Available from: https://grantome.com/grant/NIH/R01-DK113631-04.
103. Jebeile H, Gow ML, Lister NB, et al. Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity. J Nutr. 2019;149(7):1189–1197. doi:10.1093/jn/nxz049
104. Liese AD, Ma X, Reid L, et al. Health care access and glycemic control in youth and young adults with type 1 and type 2 diabetes in South Carolina. Pediatr Diabetes. 2019;20(3):321–329. doi:10.1111/pedi.12822
105. Lu H, Holt JB, Cheng YJ, Zhang X, Onufrak S, Croft JB. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Serv Res. 2015;15:541. doi:10.1186/s12913-015-1185-5
106. Lee JM, Davis MM, Menon RK, Freed GL. Geographic distribution of childhood diabetes and obesity relative to the supply of pediatric endocrinologists in the United States. J Pediatr. 2008;152(3):331–336. doi:10.1016/j.jpeds.2007.08.037
107. Skelton JA, Goff DC, Ip E, Beech BM. Attrition in a multidisciplinary pediatric weight management clinic. Child Obes. 2011;7(3):185–193. doi:10.1089/chi.2011.0010
108. DeSilva S, Vaidya SS. The application of telemedicine to pediatric obesity: lessons from the past decade. Telemed J E Health. 2021;27(2):159–166. doi:10.1089/tmj.2019.0314
109. Whitley A, Yahia N. Efficacy of clinic-based telehealth vs. face-to-face interventions for obesity treatment in children and adolescents in the United States and Canada: a systematic review. Child Obes. 2021;17(5):299–310. doi:10.1089/chi.2020.0347
110. Yin W, Liu Y, Hu H, Sun J, Liu Y, Wang Z. Telemedicine management of type 2 diabetes mellitus in obese and overweight young and middle-aged patients during COVID-19 outbreak: a single-center, prospective, randomized control study. PLoS One. 2022;17(9):e0275251. doi:10.1371/journal.pone.0275251
111. Hood KK, Beavers DP, Yi-Frazier J, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. 2014;55(4):498–504. doi:10.1016/j.jadohealth.2014.03.011
112. Van Buren DJ, Wilfley DE, Marcus MD, et al. Depressive symptoms and glycemic control in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Res Clin Pract. 2018;135:85–87. doi:10.1016/j.diabres.2017.11.008
113. Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75(6):577–587. doi:10.1007/s40265-015-0347-4
114. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. doi:10.4088/JCP.09r05346blu
115. Whalen DJ, Belden AC, Tillman R, Barch DM, Luby JL. Early adversity, psychopathology, and latent class profiles of global physical health from preschool through early adolescence. Psychosom Med. 2016;78(9):1008–1018. doi:10.1097/PSY.0000000000000398
116. Hilliard ME, Powell PW, Anderson BJ. Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. Am Psychol. 2016;71(7):590–601. doi:10.1037/a0040359
117. Jaser SS. Family interaction in pediatric diabetes. Curr Diab Rep. 2011;11(6):480–485. doi:10.1007/s11892-011-0222-y
118. Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872. doi:10.1002/14651858.CD001872.pub2
119. Bennich BB, Roder ME, Overgaard D, et al. Supportive and non-supportive interactions in families with a type 2 diabetes patient: an integrative review. Diabetol Metab Syndr. 2017;9:57. doi:10.1186/s13098-017-0256-7
120. McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178–184. doi:10.1001/jamapediatrics.2013.4200
121. Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–2128. doi:10.1056/NEJMoa1916038
122. Group TS, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi:10.1056/NEJMoa1109333
123. Borzutzky C, King E, Fox CK, et al. Trends in prescribing anti-obesity pharmacotherapy for paediatric weight management: data from the POWER Work Group. Pediatr Obes. 2021;16(1):e12701. doi:10.1111/ijpo.12701
124. Ard J, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821–2839. doi:10.1007/s12325-021-01710-0
125. Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. Obes Rev. 2021;22(6):e13177. doi:10.1111/obr.13177
126. Tamborlane WV, Barrientos-Perez M, Fainberg U, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381(7):637–646. doi:10.1056/NEJMoa1903822
127. Kyriakidou A, Kyriazou AV, Koufakis T, et al. Clinical and genetic predictors of glycemic control and weight loss response to liraglutide in patients with type 2 diabetes. J Pers Med. 2022;12(3):424. doi:10.3390/jpm12030424
128. Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: a meta-analysis. J Pediatr. 2021;236:137–147 e13. doi:10.1016/j.jpeds.2021.05.009
129. Iqbal J, Wu HX, Hu N, et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23(6):e13435. doi:10.1111/obr.13435
130. Weghuber D, Barrett T, Barrientos-Perez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387:2245–2257. doi:10.1056/NEJMoa2208601
131. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi:10.1056/NEJMoa2206038
132. Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799–812. doi:10.1111/j.1463-1326.2006.00686.x
133. Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19(12):1655–1668. doi:10.1111/dom.13009
134. Eliasson B, Gudbjornsdottir S, Cederholm J, Liang Y, Vercruysse F, Smith U. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes. 2007;31(7):1140–1147. doi:10.1038/sj.ijo.0803548
135. Berman C, Naguib M, Hegedus E, Vidmar AP. Topiramate for weight management in children with severe obesity. Child Obes. 2022. doi:10.1089/chi.2022.0062
136. Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clin Pediatr. 2015;54(1):19–24. doi:10.1177/0009922814542481
137. Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother. 2013;47(3):340–349. doi:10.1345/aph.1R501
138. Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes. 2017;41(1):90–93. doi:10.1038/ijo.2016.185
139. Dhillon S. Phentermine/topiramate: pediatric first approval. Paediatr Drugs. 2022. doi:10.1007/s40272-022-00532-z
140. Kelly AS, Bensignor MO, Hsia DS, et al. Phentermine/topiramate for the treatment of adolescent obesity. NEJM Evid. 2022;1(6):EVIDoa2200014. doi:10.1056/EVIDoa2200014
141. Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord. 1997;21(Suppl 3):S12–23.
142. Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi:10.1001/jama.293.23.2873
143. Wilding J, Fernando K, Milne N, et al. SGLT2 inhibitors in type 2 diabetes management: key evidence and implications for clinical practice. Diabetes Ther. 2018;9(5):1757–1773. doi:10.1007/s13300-018-0471-8
144. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi:10.1210/jc.2011-2260
145. Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29(8):1295–1303. doi:10.1016/j.jdiacomp.2015.07.011
146. Tamborlane WV, Laffel LM, Shehadeh N, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10(5):341–350. doi:10.1016/S2213-8587(22)00052-3
147. Pratt JSA, Browne A, Browne NT, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14(7):882–901. doi:10.1016/j.soard.2018.03.019
148. Armstrong SC, Bolling CF, Michalsky MP, Reichard KW; Section on Obesity SOS. Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. 2019;144(6). doi:10.1542/peds.2019-3223
149. Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123. doi:10.1056/NEJMoa1506699
150. Inge TH, Laffel LM, Jenkins TM, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018;172(5):452–460. doi:10.1001/jamapediatrics.2017.5763
151. Campbell EG, Alasmar A, Lawrence R, et al. Barriers to metabolic bariatric surgery in adolescents: results of a qualitative study. Surg Obes Relat Dis. 2022;18(6):794–802. doi:10.1016/j.soard.2022.03.010
152. Nunez Lopez O, Jupiter DC, Bohanon FJ, Radhakrishnan RS, Bowen-Jallow KA. Health disparities in adolescent bariatric surgery: nationwide outcomes and utilization. J Adolesc Health. 2017;61(5):649–656. doi:10.1016/j.jadohealth.2017.05.028
153. Steinberger AE, Nickel KB, Keller M, et al. National trends in pediatric metabolic and bariatric surgery: 2010–2017. Pediatrics. 2022;150. doi:10.1542/peds.2022-057316
154. Fittipaldi-Fernandez RJ, Guedes MR, Galvao Neto MP, Klein M, Diestel CF. Efficacy of intragastric balloon treatment for adolescent obesity. Obes Surg. 2017;27(10):2546–2551. doi:10.1007/s11695-017-2699-1
155. De Peppo F, Caccamo R, Adorisio O, et al. The Obalon swallowable intragastric balloon in pediatric and adolescent morbid obesity. Endosc Int Open. 2017;5(1):E59–E63. doi:10.1055/s-0042-120413
156. Swei EC, Sullivan SA. Aspiration therapy. Tech Vasc Interv Radiol. 2020;23(1):100659. doi:10.1016/j.tvir.2020.100659
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
