Back to Journals » International Journal of General Medicine » Volume 15
Admission Levels of Serum P-Selectin and IL-6 Can Predict Development of Deep Venous Thrombosis in Hospitalized Covid-19 Patients
Authors Farouk N , Ashry WMO , EL-Hagrasy HA, Mohamed EF , Eltrawy HH , El-Nasser AM, Shipl W, El Attar S, Kh Sakr L , Abdel Wahab MA , Abdelsalam EM , Sharaf FA, Ahmad IH
Received 5 January 2022
Accepted for publication 20 May 2022
Published 10 June 2022 Volume 2022:15 Pages 5599—5607
DOI https://doi.org/10.2147/IJGM.S357097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nehal Farouk,1 Walaa Mohamed Omar Ashry,2 Hanan A EL-Hagrasy,3 Eman F Mohamed,4 Heba H Eltrawy,5 Asmaa M El-Nasser,6 Walaa Shipl,7 Shahinaz El Attar,7 Lobna Kh Sakr,8 Maisa A Abdel Wahab,1 Eman M Abdelsalam,4 Fawzia A Sharaf,3 Inass Hassan Ahmad9
1Vascular Surgery Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 2Microbiology and Immunology Department, Damietta Faculty of Medicine (Girls), Al-Azhar University, Damietta, Egypt; 3Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 4Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 5Chest Diseases Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 6Medical Microbiology and Immunology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 7Medical Biochemistry and Molecular Biology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 8Radio-Diagnosis Department, faculty of medicine for girls, Al-Azhar University, Cairo, Egypt; 9Endocrinology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
Correspondence: Heba H Eltrawy, Chest Diseases Department, Faculty of medicine for girls, Al-Azhar University, Cairo, Egypt, Tel +20 1006381297, Email [email protected]
Background and Aim: Deep venous thrombosis (DVT) of the lower extremities is common in Covid-19 patients. Interleukin (IL)-6 and P-selectin were found to be elevated in Covid-19 patients. The current study aimed to evaluate P-selectin and IL6 in Covid-19 patients with DVT and to explore its relation to clinical and laboratory parameters in those patients.
Patients and methods: The present retrospective study included 150 hospitalized COVID-19 patients diagnosed on the basis of a positive result of reverse-transcriptase polymerase chain reaction (RT-PCR) test. Laboratory assessments were included for IL-6 and P selectin assessments via enzyme-linked immunosorbent assay. The primary outcome of the present study was the development of DVT detected by Doppler ultrasound (DU) evaluation of the lower extremities during the admission.
Results: The present study included 150 hospitalized Covid-19 patients. DVT was developed in 59 patients (39.3%). DVP patients had significantly higher levels of P selectin [76.0 (63.0– 87.0) versus 63.0 (54.3– 75.0), p < 0.001] and IL-6 [37.0 (27.0– 49.0) versus 18.5 (13.5– 31.5), p < 0.001]. ROC curve analysis revealed good performance of P selectin [AUC (95% CI): 0.72 (0.64– 0.81)] and IL-6 [AUC (95% CI): 0.79 (0.71– 0.86)] in identification of DVT. Logistic regression analysis identified the presence of severe disease [OR (95% CI): 9.016 (3.61– 22.49), p < 0.001], elevated P selectin [OR (95% CI): 1.032 (1.005– 1.059), p = 0.018] and elevated IL-6 [OR (95% CI): 1.062 (1.033– 1.091), p < 0.001] as significant predictors of DVT development in multivariate analysis.
Conclusion: The present study identified a probable role of elevated P-selectin and IL-6 levels in the DVT development in hospitalized Covid-19 patients.
Keywords: Covid-19, deep venous thrombosis, interleukin 6
Introduction
Coronavirus disease 2019 (COVID-19) first emerged in Wuhan, Hubei Province, China, in December 2019.1 Later, COVID-19 first appeared in Egypt in February 2020.2 Thromboembolic incidents are frequent in COVID-19 affected people and are associated with high mortality and morbidity.3–5 Deep venous thrombosis (DVT) of the lower extremities was reported in 14.7–85.4% of hospitalized COVID-19. The use of Doppler ultrasound for the detection of DVT was suggested as a routine assessment in these patients.6,7
The massive inflammation seen in COVID-19 patients is reflected in high levels of inflammatory indicators including C-reactive protein (CRP) and numerous cytokines.8 Interleukin (IL)-6 is regarded as a pro-inflammatory and pleiotropic cytokine that is delivered by a wide range of cell types, such as monocytes, lymphocytes, and fibroblasts. Infection of SARS-COV stimulates IL-6 dose-dependent production of bronchial epithelial cells.9 P-selectin moderately contributes to the activation of platelets and adhesion of specific leukocytes and endothelium-specific platelets.10
P-selectin is an adhesion molecule. In response to inflammatory conditions, it is translocated to the surfaces of platelets and endothelial cells. It is frequently studied as a biomarker of inflammatory conditions.11 Moreover, elevated P-selectin is linked to 1.7-fold elevated venous thrombosis risks.12
The current study aimed to evaluate P-selectin and IL6 in Covid-19 patients with DVT and to explore its relation to clinical and laboratory parameters in those patients.
Patients and Methods
The present retrospective study was conducted at Al-Zahara University and Al-Azhar Specialist Hospitals. Patients included in the study were admitted during the period from November 2020 to April 2021. Access to patients’ data was approved by the ethical committee of Al-Azhar Faculty of Medicine in accordance with Helsinki Declaration on clinical research involving human subjects. The study included 150 hospitalized COVID-19 patients diagnosed on the basis of a positive result of reverse-transcriptase polymerase chain reaction (RT-PCR) test of a sample collected by a nasopharyngeal swab. Patients were excluded if they had liver cell failure (n = 4), renal failure (n = 4), acute or chronic infections (n = 7), immunological diseases (n = 5) (eg systemic lupus erythematosus and autoimmune thyroiditis) or cancer (n = 3) before admission or if they had a history of DVT (n = 3).
Collected data included patients’ demographics, clinical data, laboratory investigations, radiological findings and therapeutic interventions. Covid-19 severity was assessed using the Infectious Diseases Society of America/American Thoracic Society Criteria. Patients were classified to have severe disease if they had ≥1 major criterion (septic shock with need for vasopressors or invasive mechanical ventilation) or ≥3 minor criteria (respiratory rate ≥30 breaths/min, PaO2/FiO2 ratio ≤250, multilobar infiltrates, confusion/disorientation, uremia (BUN level ≥20 mg/dl), leukopenia as a result in infection alone (WBC count <4000 cells/mL), thrombocytopenia (platelets count <100,000/mL), hypothermia (core temperature <36°C), and hypotension requiring aggressive fluid resuscitation).13
For laboratory assessment, five mL of venous blood were drawn on admission and allowed (within 10−20 minutes) to coagulate at room temperature, centrifuged for 20 minutes at 2000 −3000 rpm to extract the serum that had been aliquoted, and stored at −80°C. A portion of serum was used for assessment of blood urea, serum creatinine, ALT, AST, serum albumin, bilirubin, lipid profile tests and serum ferritin using the automated clinical analyzer BIOLIS 24i (Carolina Chemistries, North Carolina, USA). One other portion was used for CRP and D –Dimer analysis using Immulite 1000 (Siemens, USA). The third portion was stored at −80 to be used for IL-6 and P selectin assessments via enzyme-linked immunosorbent assay (ELISA) technique with kits obtained from (Bio-Techne Ltd, Minneapolis, USA) with catalog numbers D6050 and DPSE00, respectively, according to manufacturer’s instructions.
The primary outcome of the present study was the development of DVT detected by Doppler ultrasound (DU) evaluation of the lower extremities during the admission. The decision to perform Duplex ultrasonography was determined by clinical suspicion. DU examination included grayscale (B-mode), color, as well as spectral Doppler assessment (Figures 1 and 2). Examinations included a deep venous system in the thigh and calf and both saphenous veins.
 |
Figure 1 Normal color Doppler ultrasound of the right long saphenous vein (longitudinal view), (A) B-mode (gray scale) and (B) color flow image. |
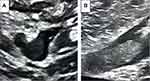 |
Figure 2 Doppler ultrasound of the right common femoral vein in B-mode, (A) transverse and (B) longitudinal views, showing intraluminal thrombus. |
Data obtained from the present study were presented as number and percent for categorical data or median and interquartile range (IQR) for numerical data for numerical data. Categorical data were compared using Fisher’s exact or chi-square tests as appropriate, while numerical data were compared using Mann–Whitney U-test. Correlation analysis was achieved using the Spearman correlation coefficient. Receiver operating characteristic (ROC) curve analysis was used to identify the sensitivity and specificity of the investigated markers. Logistic regression analysis was utilized to identify predictors of DVT development in the studied patients. All the statistical operations were processed using SPSS 25 (IBM, USA) with p value <0.05 considered statistically significant.
Results
The present study included 150 hospitalized Covid-19 patients. DVT was developed in 59 patients (39.3%). Comparison between patients with DVT and patients without revealed that the former subgroup had significantly higher frequency of diabetes (55.9% versus 30.8%, p = 0.004) and severe disease (81.4% versus 26.4%, p < 0.001). In addition, DVT patients had significantly higher LDH levels [median (IQR): 312.0 (244.0–412.0) versus 248.5 (183.3–381.3), p = 0.019], CRP [24.0 (10.0–40.0) versus 19.4 (5.6–32.0), p = 0.016], D-dimer [2.5 (1.6–8.6) versus 0.5 (0.34–1.2), p < 0.001]. Moreover, DVP patients had significantly higher levels of P selectin [76.0 (63.0–87.0) versus 63.0 (54.3–75.0), p < 0.001] and IL-6 [37.0 (27.0–49.0) versus 18.5 (13.5–31.5), p < 0.001]. Among the died 42 patients, there were 36 patients (61.0%) in the DVT group versus 6 patients (6.6%) in the non-DVT group (p < 0.001) (Table 1).
 |
Table 1 Clinical and Laboratory Findings in the Studied Patients (n = 150) |
Correlation analysis identified significant correlation between P selectin and RBS (r = 0.26, p = 0.043), LDH (r = 0.32, p=0.015), and IL-6 and between IL-6 and creatinine (r = 0.4, p = 0.002) and D-dimer (r = 0.62, p < 0.001) in DVT patients. In non-DVT patients, there were significant correlation between P selectin and creatinine (r = 0.39, p < 0.001), albumin (r = 0.42, p < 0.001), ferritin (r = −0.32, p = 0.002), LDH (r = 0.42, p < 0.001), CRP (r = 0.45, p < 0.001), D-dimer (r = 0.27, p = 0.01) and IL-6 (r = 0.66, p < 0.001). Almost similar correlations were recognized between IL-6 and other clinical and laboratory variables (Table 2).
 |
Table 2 Correlation Between P Selectin and IL-6 and Clinical and Laboratory Data |
ROC curve analysis revealed good performance of P selectin [AUC (95% CI): 0.72 (0.64–0.81)] and IL-6 [AUC (95% CI): 0.79 (0.71–0.86)] in identification of DVT (Table 3, Figures 3 and 4). Logistic regression analysis identified the presence of severe disease [OR (95% CI): 9.016 (3.61–22.49), p < 0.001], elevated P selectin [OR (95% CI): 1.032 (1.005–1.059), p = 0.018] and elevated IL-6 [OR (95% CI): 1.062 (1.033–1.091), p < 0.001] as significant predictors of DVT development in multivariate analysis (Table 4).
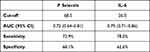 |
Table 3 Value of P Selectin and IL-6 in Prediction of DVT |
 |
Table 4 Predictors of DVT in the Studied Patients |
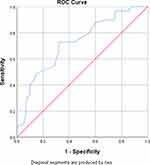 |
Figure 3 ROC curve for P selectin levels and DVT. |
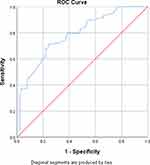 |
Figure 4 ROC curve for IL-6 levels and DVT. |
Discussion
In the present study, DVT was diagnosed in 39.3% of the studied Covid-19 patients. In accordance with these results, Marone et al14 identified DVT in 42 out of 101 Covid-19 patients. Our results revealed that DVT patients had statistically significantly higher LDH, D-Dimer and CRP levels in comparison to other patients in line with Pan et al15 who found that subjects with thrombotic complications had elevated LDH and D-dimer.
The current study showed a significant increase in P-selectin and IL6 levels in DVT patients as compared to non-DVT patients. This novel finding may be supported by the conclusions of Agrati et al.16 In their work, they reported a significant increase in P-selectin plasma levels in COVID-19 patients in comparison to healthy subjects. They demonstrated the essential role of the interaction of platelet-endothelium as a main element of COVID-19 multi-faced pathogenic mechanism resulting in the local activation of the hemostatic system that forms pulmonary thrombi. They recommended examining whether therapies inhibit platelet function or those that inhibit platelet endothelial interaction can reduce the thrombotic and inflammatory complications, thus reducing mortality and morbidity in cases with COVID-19.
In another work, using mass cytometry, Bongiovanni et al17 demonstrated increased P-selectin surface expression in COVID-19 patients in comparison to controls. Also, Comer et al18 revealed a significant increase in plasma P-selectin, thrombopoietin, and platelets factor 4 levels in COVID 19. In contrast to previous studies, Venter et al19 found that the plasma level of soluble P-selectin decreased in cases with COVID-19 than healthy individuals using ELISA technique. They explained their findings by high rate of P-selectin attachment to its receptors on platelets and other cells.
The present study demonstrated that a significant increase in IL6 in cases with DVT compared with non-DVT patients with COVID-19. In support of our findings, meta-analyses by Udomsinprasert et al,20 Zhang et al21 and Han. et al22 reported that IL6 is elevated in severe COVID 19 cases than non-severe COVID-19 subjects. Likewise, El-Shabrawy et al23 found increased IL 6 and CRP in severe COVID-19 cases in Egypt compared with the mild cases.
In conclusion, the present study identified a probable predictive role of elevated P-selectin and IL-6 levels in DVT development in hospitalized Covid-19 patients. These findings may have significant clinical and therapeutic implications. However, the conclusions of our study may be limited by its retrospective design.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable requests.
Ethics Approval and Consent to Participate
This article was approved by the ethics committee at the Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt (AFMG IRB), ID: 654, reference number: 202102645. A written informed consent was obtained from all patients.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
The authors declare that they have not received any funding.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Mohan B, Nambiar V. COVID-19: an insight into SARS-CoV-2 pandemic originated at Wuhan City in Hubei Province of China. J Infect Dis Epidemiol. 2020;6(4):146. doi:10.23937/2474-3658/1510146
2. Medhat MA, El Kassas M. COVID-19 in Egypt: uncovered figures or a different situation? J Glob Health. 2020;10(1). doi:10.7189/jogh.10.010368
3. Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J, Ogeng’o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–281. doi:10.1016/j.avsg.2020.08.087
4. Ribes A, Vardon-Bounes F, Mémier V, et al. Thromboembolic events and Covid-19. Adv Biol Regul. 2020;77:100735. doi:10.1016/j.jbior.2020.100735
5. Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2):E70–E80. doi:10.1148/radiol.2020203557
6. Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi:10.1016/j.thromres.2020.05.018
7. Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142(2):181–183. doi:10.1161/CIRCULATIONAHA.120.047407
8. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi:10.1126/science.abb8925
9. Yoshikawa T, Hill T, Li K, Peters CJ, Tseng C-TK. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83(7):3039–3048. doi:10.1128/JVI.01792-08
10. AL-aasam MRM, AL-bdairi AJ, ALbdairy JJ. The studying the adhesion molecule p-selectin in patients with ischemic heart disease. Medico Legal Update. 2020;20(2):807–813.
11. Perkins LA, Anderson CJ, Novelli EM. Targeting P-selectin adhesion molecule in molecular imaging: p-selectin expression as a valuable imaging biomarker of inflammation in cardiovascular disease. J Nucl Med. 2019;60(12):1691–1697. doi:10.2967/jnumed.118.225169
12. Shi D, Xu X, Xu Z, et al. P-selectin: an unpredicted factor for deep vein thrombosis after total Hip arthroplasty. Biomed Res Int. 2014;2014:1–6. doi:10.1155/2014/783967
13. Metlay JP, Waterer GW. Update in adult community-acquired pneumonia: key points from the new American Thoracic Society/Infectious Diseases Society of America 2019 guideline. Curr Opin Pulm Med. 2020;26(3):203–207. doi:10.1097/MCP.0000000000000671
14. Marone EM, Bonalumi G, Curci R, et al. Characteristics of venous thromboembolism in COVID-19 patients: a multicenter experience from northern Italy. Ann Vasc Surg. 2020;68:83–87. doi:10.1016/j.avsg.2020.07.007
15. Pan F, Yang L, Li Y, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17(9):1281. doi:10.7150/ijms.46614
16. Agrati C, Bordoni V, Sacchi A, et al. Elevated P-Selectin in severe Covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis. 2021;13(1):e2021016. doi:10.4084/mjhid.2021.016
17. Bongiovanni D, Klug M, Lazareva O, et al. SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis. 2021;12(1):50. doi:10.1038/s41419-020-03333-9
18. Comer SP, Cullivan S, Szklanna PB, et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2):e3001109. doi:10.1371/journal.pbio.3001109
19. Venter C, Bezuidenhout JA, Laubscher GJ, et al. Erythrocyte, platelet, serum ferritin, and p-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci. 2020;21(21):8234. doi:10.3390/ijms21218234
20. Udomsinprasert W, Jittikoon D, Sangroongruangsri D, Chaikledkaew D. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential predictors of severity and mortality for covid-19: systematic review with meta-analysis. J Clin Immunol. 2021;41(1):11–22. doi:10.1007/s10875-020-00899-z
21. Zhang J, Hao Y, Ou W, et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med. 2020;18(1):1–8. doi:10.1186/s12967-020-02571-x
22. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi:10.1080/22221751.2020.1770129
23. El-Shabrawy M, Alsadik ME, El-Shafei M, et al. Interleukin-6 and C-reactive protein/albumin ratio as predictors of COVID-19 severity and mortality. Egypt J Bronchol. 2021;15(1):1–7. doi:10.1186/s43168-021-00054-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
