Back to Journals » Pragmatic and Observational Research » Volume 14
Administrative Databases and Diagnostic Therapeutic and Assistance Paths -PDTA- in the Monitoring Treatment of Rheumatoid Arthritis: The Experience of ATS Pavia
Authors Bruno GM , Valentino MC , Brunetti A , Di Matteo S, Begovic I, Croce E, Sakellariou G, Bugatti S, Perotti P, Vecchio S, Migliazza S, Langella R , Colombo GL
Received 26 November 2022
Accepted for publication 3 April 2023
Published 19 April 2023 Volume 2023:14 Pages 29—38
DOI https://doi.org/10.2147/POR.S399221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. David Price
Giacomo M Bruno,1,2 Maria Chiara Valentino,2 Alessandra Brunetti,3 Sergio Di Matteo,2 Ivan Begovic,2 Edoardo Croce,4 Garifallia Sakellariou,5 Serena Bugatti,6 Pietro Perotti,7 Silvia Vecchio,7 Simona Migliazza,7 Roberto Langella,8 Giorgio L Colombo1,2
1Department of Drug Sciences, Center of Pharmaceuticals Economics and Medical Technologies Evaluation, CEFAT.Unipv - University of Pavia at Centro di Ricerca SAVE Studi, Milano, Italy; 2S.A.V.E. Research Center - Studi Analisi Valutazioni Economiche, Milano, Italy; 3Research and Innovation Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 4Scientific Direction - IRCCS istituto Ortopedico Galeazzi, Milan, Italy; 5Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy; 6Department of Internal Medicine and Therapeutics, Division of Rheumatology, University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 7U.O.C. Epidemiological Observatory – Health Protection Agency of Pavia (ATS Pavia), Pavia, Italy; 8SIFO Regional Secretariat - Società Italiana di Farmacia Ospedaliera e dei Servizi Farmaceutici delle Aziende Sanitarie, Milano, Italy
Correspondence: Giacomo M Bruno, Department of Drug Sciences, Center of Pharmaceuticals Economics and Medical Technologies Evaluation, CEFAT.Unipv - University of Pavia at Centro di Ricerca SAVE Studi, Milano, 20149, Italy, Email [email protected]
Background: The current flows of the SSN represent the set of interest whose interconnection alone justifies the current study. These flows can be interconnected with other sources, institutional or otherwise, in order to answer well-defined questions.
Objective: The objective of the study is to verify, through the analysis of administrative databases, any differences in the consumption of health resources between biological off-patent originator drugs and biosimilars in real clinical practice, with particular reference to the rheumatology area.
Methods: Through the use of assisted databases (BDA) of ATS Pavia we evaluated the differences in terms of consumption of health resources related to the different drugs under analysis. Annual and daily costs were calculated by total patient cost, stratified for different treatments, considering the sum of total costs for the prescriptions of drugs subject to the analysis. Another objective was to evaluate the adherence of the drugs of interest, by utilizing specific indicators (MPR).
Results: A total of 145 patients were analyzed. Among enrolled patients, 26.9% of users were treated with a biosimilar drug, while 73.1% with a biologic originator. Adherence is higher if it is considered the population treated with biosimilar drugs (82.1%). Total cost (including drug prescriptions, hospitalizations, outpatient services, tests for any cause) during the observation period of 1 year is 14,274.08. 87.7% of the total is attributable to drugs. Non-hospitalized patients are the least expensive, whether they were treated with biologics or biosimilars.
Conclusion: In our sample, biosimilar drugs tend to be underused: the treatment of a patient with a chronic autoimmune disease is a clinical process that involves many health professionals, and a criticality could also derive from the difficult communication between the various professional figures who get involved with the whole patient treatment.
Keywords: real world data, biosimilar, rheumatic diseases, PDTA, EULAR recommendations, rheumatoid arthritis
Introduction
The advent of Electronic Medical Records (EMRs) and the enormous enhancement of computing capabilities have made it possible today to collect health data electronically, in large quantities, very quickly and at the very moment in which the service is provided. It is therefore possible to use data relating to large numbers of subjects to describe the impact of care and to predict the results and response to various treatments of future patients.1 This information can be obtained at a relatively low cost, being based on data already routinely collected as part of current practice and representative of real populations and the assistance actually provided. Using real-world data (RWD), we therefore refer to data relating to the state of health of patients or the provision of assistance, collected routinely from a series of sources, such as computerized files or administrative databases relating to hospitalizations, prescriptions or outpatient services.2,3
Rheumatoid arthritis (RA) is a chronic and progressive autoimmune disease characterized by inflammation of the joints resulting in progressive disability. The disease has a higher prevalence among women and about two-thirds of patients are in an age group compatible with full employment. Fatigue and depression can be associated with the disease, with important consequences on the patient’s psychosocial well-being, as well as systemic complications, such as cardiovascular diseases, which can jeopardize their survival.4,5 The mainstay of therapy in RA is represented by disease-modifying anti-rheumatic drugs (DMARDs). The introduction of biological DMARDs (bDMARDs) into therapy has allowed a new therapeutic frontier, in particular for patients who do not respond to traditional therapies or who do not tolerate them.6,7 The entry into the market of biosimilar drugs, despite being seen as a tool capable of expanding access to biological drugs while contributing to the financial sustainability of the health system, has given rise to discussions with the scientific and political-economic-health community, in particular on the safety and efficacy profiles of biosimilars and on the comparability/substitutability with reference to the drugs of origin.8,9 One potential way of investigating RA in primary care is the use of health-care databases.
In order to make this project usable and concrete, we analyze the case of the differences in terms of costs and treatment adherence between biological and biosimilar drugs in real clinical practice with particular reference to patients with RA.
The variables are collected directly in the administrative databases owned by ATS, whose mission is to produce data for governance and fill unmet needs. The researchers provided the ATS operators with the inclusion criteria so that they could proceed with the coring: Pathology exemption (code 006), hospital admissions recorded in the previous 3 years, all hospital admissions in which the main or secondary diagnosis is main and/or secondary 714.0, 714.1, 714.2, 714.30, 714.32, excluding patients affected by HIV or drug addicts or with psychiatric pathologies from the selection, finally including those with a biosimilar or biological prescription.
All the data required for the execution of this study are included in the administrative databases of ATS Pavia: retrospective data are “secondary nature” data, ie already collected by ATS Pavia and the flow of information that is generated is useful for incidence studies to predict how local resources are used.
The tracer drugs for the analysis have been Infliximab and Etanercept, considering that at the time of data extraction there was a prescription volume of biosimilar drugs not less than 10% of the total in the therapeutic area considered.
Furthermore, it should be noted that the analysis was conducted exclusively with the use of databases owned by ATS (Health Protection Company) Pavia (approximately 530,000 patients) and, according to the privacy law, the analysis was carried out entirely on aggregated data provided by ATS following the requested outputs.
All subjects who manage the data of the study guarantee to keep the confidentiality of personal data in the database.
Methods
Data Sources
The analyses were carried out starting from the assisted databases (BDA) owned by ATS of Pavia - U.O.C. Epidemiological Observatory. The administrative databases used are Flow of Territorial Pharmaceutical Products, Flow of the Outpatient Specialist and Instrumental Diagnostics, Flow of Hospital Discharge Cards (SDO) and Mortality Cards. In accordance with the privacy law, the analysis is conducted entirely within ATS, which provides aggregated data following the required outputs.
The study is monocentric and the evaluation will follow up the potential involvement of other centers through protocol amendments. The target population of this observational retrospective study includes ATS Pavia patients diagnosed with RA and at least one biologic or biosimilar drug prescription between January 2017 and December 2019 (recruitment period). In BDA cases were identified by setting as extraction criteria hospitalized patients: this study includes a sample of individuals of legal age of both genders, with diagnosis (main and/or secondary, code 7140) and/or pathology exemption (code 006) from 2017 to 2019 and prescription of Biological – originator (and possible concomitant therapies) and prescription of Biological – biosimilar (and possible concomitant therapies). The prescription date of the first drug during the inclusion period represented the “index date” for each patient. From the index date onward, each patient was observed for 1 year (follow-up period) also recording the time elapsed in days between the date of diagnosis and the first prescription, or the date of death for the deceased within the year of observation. For each individual patient, this database records information regarding daily clinical practice, such as demographic and clinical data (diagnostic and therapeutic) but also consumption and costs of the health-care resources administered. Each patient was uniquely identified through a specific internal study number. The correspondence between the internal number assigned and the patient’s identity is kept exclusively at the Center. The study was conducted according to the guidelines of the Declaration of Helsinki, and according to the Italian law, approved by local Ethic Committee (Comitato Etico Pavia - Fondazione I.R.C.C.S. Policlinico “San Matteo” - P-20210018856, Pavia, 6/11/21).
The patient cohort was selected relying on a brief descriptive analysis of the Personal Data dataset, in particular of the demographic characteristics of the patients and the distribution of age of diagnosis, gender and year of diagnosis. The interrogation of data relating to diagnoses, interventions and procedures was carried out in accordance with the ICD-9-CM classification system (International Classification of Diseases, 9th revision - Clinical Modification).
The registration of the pharmaceutical prescription takes place by means of a list of products coded by a commercial name and relative pharmaceutical form, ATC (Anatomical Therapeutic Chemical classification system - WHO) classification, A.I.C. (Marketing Authorization), and active ingredient with the drugs are registered.
The outcomes between off-patent originating biologic drugs and biosimilars in real clinical practice are analyzed considering that at the time of data extraction (2016–2019) a prescription volume was present in the therapeutic area considered of biosimilar drugs not less than 10% of the total.
Subjects were treated with Etanercept (both biologic and biosimilar), Infliximab (both biologic and biosimilars) Abatacept, Adalimumab Tofacitinib, Tocilizumab, Golimumab, Baricitinib, Sarilumab (biologics only).
Data Preparation
A dataset was generated to evaluate the consumption of each drug of interest. We considered only the consumption of biological drugs and biosimilars that occurred during the observation period, identifying the first and last prescriptions for each therapy within the observation year. Once the data of interest had been extracted, it was possible to proceed with the creation of new useful variables (in terms of costs and adherence). The costs for each patient, determined by drugs, hospitalizations, and specialist visits, were identified 1 year after the start of the observation. After identifying consumption, it was possible to calculate adherence using the Defined Daily Dose (DDD) defined as the average dose of a drug taken daily by an adult patient, with reference to the main therapeutic indication of the drug itself: the database was then enriched with days of therapeutic coverage, obtained from the correct posology and the DDD.
Adherence was determined for each therapeutic category using the medication–possession ratio (MPR). MPR reflects the proportion of days during which patients possessed a supply medication. The levels of adherence have been categorized as follows: less than 40%: non-adherent, between 40% and 80%: on average adherents, greater than 80%: strongly adherent.
Data Processing and Statistical Analysis
Statistical processing was possible using the RStudio software, on which different datasets were prepared according to the analyses to be performed.
The following indicators are used to assess the differences:
- Persistence is defined as the period of days of therapy between the first dispensation and the interruption of therapy, it will be calculated as a continuous variable, such as the number of days of therapy for which therapy is available, without interruptions. The total number of days in therapy of the patient will be analyzed through the use of the “Defined Daily Dose” (DDD).
- Treatment adherence will be calculated using the Medical Possession Rate (MPR) technique, which is defined as the ratio between the number of packs dispensed during the persistence period multiplied by the number of DDD per pack, divided by the total number of days until therapy is interrupted.
The costs for each patient are identified 1 year after the start of the observation. Drugs (for patients who have received therapy), hospitalizations and specialist visits contribute to the costs.
The Fisher test on the coefficients of a linear regression model (in the case of the variables relating to costs) suitably estimated as a test for the verification of hypotheses to evaluate the significance with respect to the subdivisions made and the chi-square test on the model of logistic regression estimated for mortality, as a dichotomous variable. To assess significance, the p-value was considered, using 0.05 as the cut-off value.
The hospital admission rate, ie, the number of hospital discharges, is analyzed considering patients persisting for a period of at least 6 months.
The average number of specialist visits is calculated keeping into account persistent patients during the therapy period and specialist visits for any cause. Patient mortality data are estimated focusing on the number of persistent patients in the same period considered for hospital admissions and, once again, we considered all-cause mortality. Costs are estimated based on total patient cost, stratified for patients treated with biologics and treated with biosimilar drugs, considering the sum of the total costs of all prescriptions, hospital admissions for any cause, outpatient services for any cause and examinations for any cause during the one-year persistence period.
Another cost analysis will be performed on a scenario that includes only disease-related prescriptions and only disease-related hospitalizations, with all-cause data from outpatient services and examinations. All costs will be assessed from the point of view of the Italian National Health System.
Results
Overall, 145 patients met the inclusion criteria and were considered in the analysis. The age at diagnosis, meant as the first prescription, is mainly concentrated between 50 and 70 years (Figure 1).
 |
Figure 1 Age classes distribution. |
bDMARDs object of this study were used as analysis drivers in order to “track”, in the database, the subjects they were administered to. Observing the use of the different therapies, 26.9% (n=39) of users were treated with a biosimilar drug, while 73.1% (n=106) were treated with a biologic originator (Figure 2).
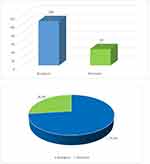 |
Figure 2 Patient distribution by biological and biosimilar drugs. |
Considering the cost’s composition (Figure 3), it is shown that 87.7% (€ 12,511.73) of the total cost (included drug prescriptions, hospitalizations, outpatient services, tests for any cause during the observation period of 1 year) is due to drugs, 6.8% (€ 966.84) to hospitalizations and 5.6% (€ 795.51) to services (specialist visits).
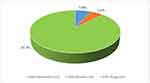 |
Figure 3 Costs composition. |
Average composition of costs can be appreciated using a bar graph that highlights low variability, which shows how many non-hospitalised patients correspond to lower levels of expenditure, whereas only seven patients hospitalized are linked to costs of up to €16,521 euro and the majority (91 patients) had costs connected to medical services. The same tendency can be appreciated both for biologics and biosimilars drugs: even in this case almost the totality did not need for hospitalization and costs concentrated in the lower class (Figure 4A and B).
 |
Figure 4 Cost of recoveries (A) biologics, (B) biosimilars. |
Adherence was calculated using medication possession ratio (MPR) according to the ranges recognized by AIFA. Of a total of 106 patients treated with biologic drugs 60 patients (56,6%) were strongly adherent, 34 average adherents (32,1%) and 12 nonadherent (11,3%) (Figure 5A).
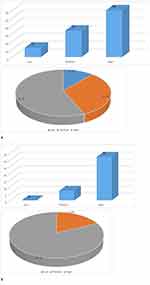 |
Figure 5 Adherence distribution (A) biologics, (B) biosimilars. |
Adherence is higher if it is considered the population treated with a biosimilar drug: among patients treated with a biosimilar almost all patients (32) are high adherent (82.1%), 7 (17.9%), while medium adherent and none are low adherent (Figure 5B).
As a final analysis, the possible differences between the different groups for the items listed (Number of hospitalizations, Cost of hospitalizations, Number of medical services, Cost of medical services, Number of Drugs prescriptions, Cost of Drugs prescriptions, Total costs) was compared using the student test and the significance calculation.
The comparison of biologics and biosimilars shows that most patients take biologics (106 compared to 39 biosimilar users): they represent a slightly lower cost in case the focus is based on hospitalizations cost, in respect to a total cost mostly comparable.
Concerning considered parameters (number and cost of hospitalizations and therapies), there are no appreciable statistically significant differences between the two samples.
The t-test shows that there is no statistical significance in the comparison between the two groups (biologics vs biosimilars) considering number and cost of (1) hospitalizations, (2) medical services, (3) drug prescriptions, as well as total costs.
Discussion
The innovation of this project is the use of real data from Pavia ATS and not simple literature data. Starting from the information extracted from the assisted database of ATS, we tried to understand the management of patients with RA, but the extrapolations that were made, starting from the extractive algorithm, were inconsistent. Pharmaceutical consumptions, obtained from prescription archives, have long been considered as reliable descriptors of patient exposure to drugs as pathology tracers. However, in order to be able to conduct real pharmacoepidemiological studies, it is important that these archives are integrated both with population personal data and with other health performance descriptors (eg hospital admissions and specialist services). This approach makes it possible to change from a purely descriptive view to an epidemiological one, in which the patient is at the center of the study with the possibility of identifying reference populations. Pathology exemption is not a valid tool in order to identify all the diagnoses of RA, certainly not considering part of it such as patients with comorbidities, exemption for oncological diseases, which include the other, older patients, people who do not apply for the exemption because it is not useful or because it is below a certain income threshold. In addition, information concerning the discontinuation of treatment indicated by doctor is not available. Both biologics and biosimilars need to be suspended in some kind of situations (for example, infections and surgical interventions), and the interruption does not constitute a non-adherent case.10,11 Moreover, there is no possibility to measure it. The possible incompleteness of the information represents a further limitation of this study: it is unknown what kind of investigations the patient conducted outside the public context: in fact, the administrative database from which the information was extracted does not actually include the private regime.
The results of the 2021 OsMed reports of the Italian Medicines Agency (AIFA) on consumption and expenditure related to biosimilar medicines show a high heterogeneity at the regional level.12 In this context, examining the three-year period considered with regard to rheumatological area, the prescription of biologicals overcomes the one of biosimilars: this could be due to a lower knowledge of the availability and operations of biosimilar drugs. The problem is targeting therapy, especially for inflammatory autoimmune diseases, for which it is not so easy to identify the correct biologic that ensures an effective cure, while allowing, at the same time, not to waste resources – which, once again, must be reallocated in the correct way (for example, by finding monitoring systems).13 The complexity of this issue is further increased by the current availability of several different modes of action for the treatment of RA, implying the need to find predictive features before treatment initiation.14
The standard Diagnostic Therapeutic Path used in Lombardy Region is outdated and has proven to be unsuccessful in the management of the onset of RA: for example, in the early stages of the disease the patient relied on the general practitioner, and only later he was accessing specialist care.15
A 2015 validation study of a classification algorithm to identify RA using administrative health databases16 provides a complete validation of classification algorithms for the identification of patients with rheumatoid arthritis (RA) at the population level through health-care administrative databases. This study carried out in the Lombardy region showed that AHDs are valuable tools for the identification of RA cases at the population level and allow estimation of disease prevalence and to select retrospective cohorts: in a randomly selected sample of 827 patients drawn from a tertiary rheumatology centre (training set), clinically validated diagnoses were linked to administrative data including diagnostic codes and drug prescriptions.
Biologic drugs have represented an important turning point for the treatment of serious and life-threatening diseases in fields like oncology, neurodegenerative, dermatological, gastroenterological, and rheumatological.17 But, in this regard, the high costs these drugs have entered the market with raised a problem of sustainability about pharmaceutical expenditure as well as (and above all) limiting access to a small number of patients.18 In this context, the expiry of patent coverage of biologic drugs represents a significant opportunity for patients and for the health system as a whole. In addition to allowing access to new therapies to an increasing number of patients, it would in fact cause savings, in terms of financial resources, which could be re-invested in pharmaceutical innovation. Despite the reasonableness of the preference for the use of biosimilar formulations (or at a lower cost) for biotechnological molecules with expired patents, that is also confirmed by the latest update of the Aifa position paper,9 and the evidence of the obtainable economic benefits, the share of use of biosimilar formulations, out of the total number of molecules with a biosimilar on the market, remains low and with a great variability among the Italian regions.
The trend in the use of biosimilars is extremely variable among different areas in Italy. The problem of inadequate adherence and persistence in drug treatment is long-standing and far from a full understanding of the causes and possible solutions. When drugs are taken with a different degree of deviation from the prescribed regimen, situation-specific alterations in the risk/benefit ratio can be created, both for reduced benefit and increased risk and for both conditions.
The EULAR RA management recommendations represent a step forward and the real “game changer” will be intercepting what happens before the diagnosis, finding indicators that allow information to be extracted from the same administrative databases: Diagnostic Algorithm for RA patient of ATS Pavia can be improved, by following the EULAR recommendations for the management of RA.19,20
Our project could solve the problem of diagnosis reconsidering the use of the Diagnostic Algorithm for RA patient of ATS Pavia: this could help doctors quickly identifying the patient that needs treatment, consequentially improving quality of life (given the highly disabling pathology). In front of the availability of expensive new therapies, as well as the growing demand for health by the population, health expenditure faces an obstacle in the limited scope of available resources. If the diagnosis comes late, the treatment is inappropriate: early diagnosis is a determining factor and it would be able to produce, with a timely therapeutic intervention, a human, social and economic advantage for rheumatology patients.
Some specific indicators could be using glucocorticoids in the period (months) before diagnosis (incorrect practice); increasing use of NSAIDs; time that elapsed between starting therapy with MTX or hydroxychloroquine and switch to biological disease-modifying antirheumatic drugs (bDMARDs); determine treatment modification times and people who stop being in treatment because they decided to leave; the execution of some instrumental tests prior to the diagnosis. Then, starting from the diagnosis, an optimization of the course of treatment could include using of b-DMARDs (beyond the use of conventional synthetic DMARDs), for which biosimilars are available, other biologics are available on the market, with different therapeutic targets: it is therefore important to understand which drug to initiate the patient and what are the indicators that make it possible to identify whether a specific biologic drug strategy is the most appropriate for the specific case); adherence to b-DMARDs; monitoring by performing laboratory tests.19,20 The issue is therefore both organizational and cultural: this is because it is necessary to know the evolution of the disease, the existing therapeutic options and, finally, the access opportunities to a biosimilar drug.
Conclusions
Primary analysis was based on the differential in the health resource consumption, in the rheumatology patient treatment, in case he was given an off-patent originator biological drug as well as he was treated with a biosimilar, and on the analysis of the respective adherence: this was for the dual purpose of having a full view, capable to identify the costs of therapy, and in order to extract more specific information in the treated population. Despite limitations of the exclusive use of health administrative data, the result of the analysis is that a lot of patients who may be using biosimilar drugs cannot actually access it.
Disclosure
Professor Serena Bugatti reports personal fees from AbbVie, Bristol Myers Squibb, Eli Lilly, and Galapagos; grants, personal fees from Pfizer, outside the submitted work. Professor Giorgio L Colombo reports grants from Pfizer, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320(9):867–868. doi:10.1001/jama.2018.10136
2. Casula M, Tragni E, Catapano AL. I database amministrativi come fonti di dati per la ricerca farmaco epidemiologica [Administrative databases as data sources for drug-epidemiological research]. CARE. 2011;1:33–36. Italian.
3. Colombo GC, Pastorelli L. Dall’impiego dei Database amministrativi in Sanità ai Bigdata: definizione, utilizzo, opportunità e criticità. [From administrative databases in Healthcare to Big Data: definition, use, opportunities and critical issues]. ClinicoEconomics Italian Article Outcomes Res. 2015;2015:37–48 Italian.
4. AIFA. Agenzia Italiana del Farmaco – Position Paper sui Farmaci Biosimilari - Versione definitiva 13.05.2013. Available from: https://www.aifa.gov.it/sites/default/files/AIFA_POSITION_PAPER_FARMACI_BIOSIMILARI.pdf.
5. Barnes T, Wong E, Thakrar K, et al. The resource cost of switching stable rheumatology patients from an originator biologic to a biosimilar in the UK. Value Health. 2017;20(9):A543. doi:10.1016/j.jval.2017.08.821
6. Rezk MF, Pieper B. Effetto nocebo e aderenza terapeutica treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4:209–218. doi:10.1007/s40744-017-0085-z
7. Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25:102–112. doi:10.18553/jmcp.2019.25.1.102
8. Pani L. Farmaci Biotecnologici e Biosimilari: innovazione e sostenibilità del sistema pubblico, AIFA[Biotechnological and biosimilar drugs: innovation and sustainability of the public system, Italian Medicines Agency]. Available from: https://www.senato.it/documenti/repository/commissioni/comm12/documenti_acquisiti/Prof.%20PANI%20aifa.pdf.
9. Agenzia Italiana del Farmaco. Secondo Position Paper AIFA sui Farmaci Biosimilari [Second Position Paper on Biosimilar Medicines - Italian Medicines Agency]. Available from: https://www.aifa.gov.it/-/secondo-position-paper-aifa-sui-farmaci-biosimilari.
10. Holloway K, van Dijk L. The world medicines situation 2011. Rational use of medicines. Geneva: World Health Organization; 2011. Available from: http://narst.dmsc.moph.go.th/manuals/The%20World%20Medicines%20Situation%202011%20-%20Rational%20Use%20of%20Medicines.pdf.
11. European Medicines Agency and European Commission. Agenzia Europea dei Medicinali e Commissione Europea. Medicinali biosimilari nell’UE - Guida informativa per gli operatori sanitari.[Biosimilar medicines in the EU - Information guide for healthcare professionals]. Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_it.pdf.
12. National Observatory on the use of Medicines. L’uso dei farmaci in Italia. Rapporto Nazionale 2021. Roma: Agenzia Italiana del Farmaco.[The use of drugs in Italy. National Report 2021. Rome: Italian Medicines Agency]. Roma: Agenzia Italiana del Farmaco. Available from: https://www.aifa.gov.it/-/presentato-il-rapporto-nazionale-2021-l-uso-dei-farmaci-in-italia-.
13. Linares A, Fernandez C, Asensi D, et al. Budgetary impact of biosimilar prescription in the treatment of rheumatic diseases. Eur J Hosp Pharm. 2020;27(Suppl 1):A133–A134.
14. Stoffer MA, Smolen JS, Woolf A, et al. Development of patient-centred standards of care for rheumatoid arthritis in Europe: the eumusc.net project. Ann Rheum Dis. 2014;73:902–905. doi:10.1136/annrheumdis-2013-203743
15. Pasina L, Casadei G, Nobili A. A survey among hospital specialists and pharmacists about biosimilars. Eur J Intern Med. 2016;35:e31–e33. doi:10.1016/j.ejim.2016.07.010
16. Carrara G, Scirè CA, Zambon A, et al. A validation study of a new classification algorithm to identify rheumatoid arthritis using administrative health databases: case–control and cohort diagnostic accuracy studies. Results from the RECord linkage on rheumatic diseases study of the Italian society for rheumatology. BMJ Open. 2015;5(1):e006029. doi:10.1136/bmjopen-2014-006029
17. Kay J, Schoels MM, Dörner T, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77:165–174. doi:10.1136/annrheumdis-2017-211937
18. Putrik P, Ramiro S, Kvien TK, et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. are differences related to country’s wealth? Ann Rheum Dis. 2014;73:2010–2021. doi:10.1136/annrheumdis-2013-203819
19. Van der Heijde D, Aletaha D, Carmona L, et al. 2014 update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis. 2015;74:8–13. doi:10.1136/annrheumdis-2014-206350
20. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
