Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Adjuvant Lenvatinib Plus PD-1 Antibody for Hepatocellular Carcinoma with High Recurrence Risks After Hepatectomy: A Retrospective Landmark Analysis
Authors Ouyang J , Wang Z, Yuan K , Yang Y , Zhou Y, Li Q , Yang N, Zhao H, Zhao H , Zhou J
Received 6 June 2023
Accepted for publication 29 August 2023
Published 6 September 2023 Volume 2023:10 Pages 1465—1477
DOI https://doi.org/10.2147/JHC.S424616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manal Hassan
Jingzhong Ouyang,1,* Zhengzheng Wang,1,* Kun Yuan,2,* Yi Yang,3,4 Yanzhao Zhou,1 Qingjun Li,1 Nanmu Yang,1 Haitao Zhao,5 Hong Zhao,3,4 Jinxue Zhou1
1Department of Hepatobiliary and Pancreatic Surgery, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan, People’s Republic of China; 2School of Public Health, Capital Medical University, Beijing, People’s Republic of China; 3Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 4Key Laboratory of Gene Editing Screening and Research and Development (R&D) of Digestive System Tumor Drugs, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 5Department of Liver Surgery, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Zhao, Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100020, People’s Republic of China, Email [email protected] Jinxue Zhou, Department of Hepatobiliary and Pancreatic Surgery, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan, 450003, People’s Republic of China, Email [email protected]
Purpose: To evaluate the efficacy and safety of lenvatinib plus programmed death-1 (PD-1) antibody as postoperative adjuvant therapy in patients with hepatocellular carcinoma (HCC) at high risks of recurrence.
Patients and Methods: A series of 137 patients with HCC at high risks of recurrence who underwent hepatectomy at our hospital between October 2019 and January 2022 were retrospectively analyzed. Recurrence-free survival (RFS), overall survival (OS), and treatment-related adverse events (TRAEs) were assessed. Landmark analysis was used to compare short- and long-term RFS. Univariable and multivariable analyses were used to identify prognostic factors, and subgroup analyses were performed according to high risks of recurrence.
Results: A total of 85 patients underwent hepatectomy alone and 52 patients received postoperative adjuvant therapy. Compared with the hepatectomy group (HG), RFS was significantly improved in the adjuvant therapy group (ATG) (P < 0.001), but OS was not (P = 0.098). Landmark analysis revealed that RFS within 6 months of the HG was significantly different from that of the ATG (P < 0.001) but not after 6 months (P = 0.486). Multivariable analysis showed that without adjuvant therapy, high Child-Pugh classification, high alpha-fetoprotein levels, microvascular invasion, and satellite lesions were independent risk factors for recurrence within 6 months after hepatectomy. Subgroup analysis revealed that patients with MVI significantly benefited from adjuvant therapy in RFS. But for OS, adjuvant therapy was only significantly effective in patients with single tumor. The most common treatment-related adverse events during adjuvant therapy were hypertension (36.5%), rash or itching (28.8%), diarrhea (23.1%), and fatigue (21.2%).
Conclusion: Postoperative adjuvant lenvatinib plus PD-1 antibody significantly improved RFS in patients with HCC at high risks of recurrence with acceptable safeties.
Keywords: hepatocellular carcinoma, postoperative adjuvant therapy, lenvatinib, PD-1 antibody, efficacy, safety
Introduction
Hepatocellular carcinoma (HCC) is the sixth most widespread malignant tumor and the fourth leading cause of cancer-related death worldwide.1 Hepatectomy has long been the backbone of curative therapies for HCC, with a 5-year survival of 70–80%.1,2 However, the five-year recurrence rate of up to 70% is a major obstacle to survival.3
Postoperative adjuvant therapy is considered a potential treatment for preventing recurrence and improving prognosis. However, there is currently no recognized postoperative adjuvant therapy. Local therapies, such as transcatheter arterial chemoembolization (TACE),4 hepatic arterial infusion chemotherapy (HAIC),5 portal vein infusion chemotherapy,6 and radiation therapy7 have long been widely used as postoperative adjuvant therapies for patients with HCC, but their efficacy in recurrence and survival is uncertain, and the guidelines do not make recommendations.8 And the “STORM” trial confirmed that sorafenib was not an effective intervention for disease recurrence and survival benefit as an adjuvant therapy after hepatectomy.9 However, in several randomized controlled trials, both local and systemic adjuvant therapies have provided survival benefits in patients with high risks of recurrence, such as microvascular invasion (MVI), satellite nodules, multiple tumors, alpha-fetoprotein (AFP) > 400 ng/mL, and large tumors (especially those > 5 cm).10,11
With an increasing variety of anti-angiogenesis targeted drugs (tyrosine kinase inhibitors [TKIs] and anti-vascular endothelial growth factor [VEGF] antibodies) and PD-1 antibodies, targeted therapy plus immunotherapy have significantly improved the prognosis of patients with HCC, gradually leading to a paradigm shift in its clinical treatment of HCC.12,13 Drawing on the experience of traditional adjuvant therapies, TKIs plus PD-1 antibodies may also be an alternative to postoperative adjuvant therapies for patients with high risks of recurrence. However, few studies have reported this.
Here, we conducted a retrospective, real-world study to evaluate the efficacy and safety of lenvatinib plus PD-1 antibody as a postoperative adjuvant therapy in patients with HCC at high risks of recurrence.
Materials and Methods
Study Design and Participants
Consecutive patients who underwent hepatectomy for HCC between October 2019 and January 2022 at Henan Cancer Hospital were reviewed. Baseline data of all patients were retrospectively collected from electronic medical records. The cut-off date for follow-up was October 1, 2022.
The inclusion criteria were as follows: 1) age ≥ 18 years; 2) preoperative treatment-naïve; 3) HCC with R0 surgical resection confirmed by postoperative histopathology; 4) no macrovascular invasion or extrahepatic metastasis; 5) Eastern Cooperative Oncology Group score ≤ 1; 6) Child-Pugh A or B; 7) hepatic resection and adjuvant therapy at our hospital; 8) no evidence of residual or recurrent tumor by postoperative radiology at 4–6 weeks after hepatectomy; and 9) presence of one or more high-risk factors for recurrence: MVI, multiple lesions, AFP > 400 ng/mL, maximum tumor size > 5 cm, and satellite nodules. The exclusion criteria were as follows: 1) severe postoperative complications; 2) concurrent cardiac, pulmonary, cerebral, or renal dysfunction; 3) history of active autoimmune or immunodeficiency diseases; 4) pathological diagnosis of cholangiocarcinoma or other types of cancer; 5) history of other malignancies; 6) loss to follow-up within 6 months; and 7) incomplete clinical or follow-up data.
Adjuvant Lenvatinib Plus PD-1 Antibody Treatment
All patients were re-examined at our hospital 4–6 weeks after the hepatectomy. If no recurrence was found, adjuvant therapy was recommended because of one or more high-risk factors of recurrence, including MVI, multiple lesions, AFP level > 400 ng/mL, maximum tumor size > 5 cm, and satellite nodules. The dose of lenvatinib was dependent on the patient’s weight (≥ 60 kg, 12 mg; < 60 kg, 8 mg, once daily). For PD-1 antibodies, pembrolizumab, camrelizumab, or toripalimab were administered intravenously at a dose of 200 mg every 3 weeks. Adjuvant therapy was started 6–8 weeks after hepatectomy and was continued until tumor progression, intolerable toxicity, patient refusal, or intravenous PD-1 antibody six times, whichever occurred first. Based on whether adjuvant therapy was accepted, all included patients were divided into the following two groups: (1) patients with HCC who received adjuvant therapy with lenvatinib plus PD-1 antibody after hepatectomy (the adjuvant therapy group, the ATG); and (2) patients with HCC who underwent hepatectomy alone (the hepatectomy group, the HG).
Follow-Up and Clinical Outcomes
The first follow-up occurred 4–6 weeks after hepatectomy and then once every 2–3 months for two years. Thereafter, follow-up was performed every 6 months. Each follow-up included laboratory tests and abdominal radiological examination. RFS was regarded as the primary outcome, while the secondary outcomes were OS and treatment-related adverse events (TRAEs). RFS was defined as the duration between the date of hepatectomy and the date of tumor progression, death, or the end of follow-up. OS was measured from the time of hepatectomy to patient death or the end of follow-up. TRAEs were evaluated according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
For all patients, once recurrence or metastasis was detected, re-hepatectomy, radiofrequency ablation, TACE, or systemic therapy was administered after a multidisciplinary discussion. The multidisciplinary team included medical oncologists, surgical oncologists, and radiologists to ensure that reasonable decisions were made to maximize the survival benefits.
Statistical Analysis
Data on baseline characteristics were presented using descriptive statistics. Categorical variables were expressed as frequencies (percentages) and compared using Fisher’s exact test or χ2-test, Continuous variables were expressed as median (interquartile range, IQR) and compared using the Mann–Whitney test. Survival curves were estimated using the Kaplan-Meier method and compared using the Log rank test. Landmark analysis was used to compare the RFS within 6 months and after 6 months between the two groups.14,15 Univariable and multivariable logistic regression models were used to identify the variables related to recurrence within 6 months, and all variables in the univariable analysis were entered into multivariable logistic regression analysis through the stepwise forward selection method. The variables in the model with the best predictive effect were identified as independent risk factors. Subgroup analysis were stratified according to age, AFP level, BCLC stage, number, maximum size, MVI, Edmonson tumor grade, and satellite nodules. Data were analyzed using the R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-tailed P-value < 0.05.
Results
Patient Characteristics
137 patients were enrolled in this study, including 85 patients in the HG (median follow-up time 28.5 months, IQR: 25.2–31.7 months) and 52 patients in the ATG (median follow-up time 14.8 months, IQR: 12.8–17.6 months; mean duration of adjuvant therapy 3.1 months) (Figure 1). The baseline characteristics were acceptably balanced between the two groups, except for sex (P = 0.020, Table 1).
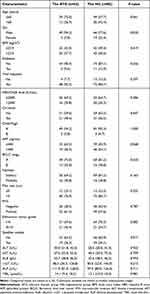 |
Table 1 Baseline Characteristics of Patients (n=137) |
Among the 21 (38.9%) patients with recurrence in the ATG, there were 17 (81.0%) cases of intrahepatic recurrence, 2 (9.5%) cases of extrahepatic metastasis, and 2 (9.5%) cases of intrahepatic recurrence with extrahepatic metastasis, including 19 (90.5%) cases of recurrence within 12 months and 2 (9.5%) cases of recurrence after 12 months; and in the HG, there were a total of 68 (80.0%) patients with recurrence, 55 (80.9%) cases of intrahepatic recurrence, 4 (5.9%) cases of extrahepatic metastasis, and 9 (13.2%) cases of intrahepatic recurrence with extrahepatic metastasis, including 55 (80.9%) cases of recurrence within 12 months and 13 (19.1%) cases of recurrence after 12 months. There was no significant difference in the recurrence pattern between the two groups (P for recurrence site = 0.792; P for recurrence time = 0.488). And treatments for recurrence were not significantly different between the two groups (P = 0.968, Table 2).
 |
Table 2 Comparison of Recurrence Patterns and Treatments After Recurrence Between Adjuvant Therapy and Hepatectomy Groups |
Efficacy
The HG had a median RFS of 5.5 months (95% confidence interval (CI): 4.5–10.2 months) and 6-, 12-, 18-, and 24-month RFS rates of 47.1%, 35.0%, 26.9% and 24.2%, respectively. The median RFS for the ATG was not reached, and 6-, 12-, 18-, and 24-month RFS rates of 80.8%, 63.2%, 56.5% and 56.5%, respectively. The ATG was associated with better RFS than HG (hazard ratio (HR): 0.441, 95% CI: 0.289–0.673; P < 0.001) (Figure 2A). Median OS was 26.6 months (95% CI: 20.1 months-not evaluated (NE)) in the HG, with 6-, 12-, 18-, and 24-month OS rates of 88.2%, 73.5%, 61.6% and 56.2%, respectively. The median OS was 26.4 months (95% CI: 19.9 months-NE) in the ATG, with 6-, 12-, 18-, and 24-month OS rates of 98.1%, 88.2%, 72.7% and 62.3%, respectively. There was no significant difference in the OS between the two groups (HR: 0.595, 95% CI: 0.336–1.053; P = 0.097) (Figure 2B).
 |
Figure 2 Kaplan‒Meier survival curves based on recurrence-free survival (A) and overall survival (B) of the adjuvant therapy group (ATG) and the hepatectomy group (HG). |
Landmark Analysis
We further performed a landmark analysis of the RFS by marking the time of 6 months. The RFS of the ATG within 6 months after hepatectomy was more beneficial than that of the HG (P < 0.001); however, there was no significant difference after 6 months (P = 0.486, Figure 3).
 |
Figure 3 Landmark analysis based on recurrence-free survival of the adjuvant therapy group (ATG) and the hepatectomy group (HG). |
Prognostic Factor Analysis
According to univariable analysis, adjuvant therapy, AFP levels, MVI, and satellite nodules had significant effect on recurrence within 6 months after hepatectomy. Multivariable analysis showed that without adjuvant therapy (OR: 6.144, 95% CI: 2.441–15.465; P < 0.001), high Child-Pugh classification (OR: 7.447, 95% CI: 1.046–53.002; P = 0.045), high AFP levels (OR: 2.343, 95% CI: 1.029–5.335; P = 0.043), MVI (OR: 2.896, 95% CI: 1.257–6.672; P = 0.013), and satellite nodules (OR: 3.791, 95% CI: 1.579–9.102; P = 0.003) were independent risk factors for recurrence within 6 months after hepatectomy (Table 3).
 |
Table 3 Logistic Regression Analysis to Predict Recurrence Within 6 Months in Patients with HCC After Hepatectomy |
Subgroup Analysis
A subgroup analysis was performed to further explore the impact of adjuvant therapy on postoperative outcomes in patients with HCC with a high risk of recurrence. The ATG had similar RFS compared with the HG in subgroups of patients with AFP > 400 ng/mL, BCLC B stage, multiple lesions, MVI-negative, Edmondson tumor grade III–IV, and satellite nodules. The ATG had better RFS than the HG in subgroups of patients with AFP ≤ 400 ng/mL, BCLC A stage, solitary lesion, MVI-positive, Edmondson tumor grade I–II and no satellite lesion, and subgroups of age or maximum tumor size (Figure 4A). In the subgroup analysis of OS, only patients with BCLC A stage or solitary tumor showed significant differences between the ATG and HG (Figure 4B).
Safety
TRAEs occurred in most of the patients (90.4%) treated with adjuvant therapy. The most common TRAEs were hypertension (36.5%), rash or itching (28.8%), diarrhea (23.1%), and fatigue (21.2%). There were 9 (17.3%) cases of grade 3 TRAEs, including 3 (5.8%) cases of hypertension, 2 (3.8%) cases of diarrhea, 2 (3.8%) cases of rash or itching, 1 (1.9%) case of increased ALT/AST, and 1 (1.9%) case of thrombocytopenia (Table 4). Most TRAEs are reversible following symptomatic treatment. However, 2 patients with severe rash or itching (grade 3) discontinued lenvatinib and resumed its use after the symptoms were relieved. One patient with increased ALT/AST (grade 3) delayed PD-1 antibody therapy because of hepatoprotective therapy during the fourth immunotherapy treatment. No grade 4 or fatal TRAEs was observed.
 |
Table 4 Number of Patients with TRAEs During the Adjuvant Treatment Period |
Discussion
At present, hepatectomy is the main radical therapy for HCC. However, the 5-year recurrence rate is as high as 70%, and approximately 30% even in patients who meet the Milan criteria.3,16 Reducing recurrence is critical for enhancing the overall prognosis of patients with HCC. Postoperative adjuvant therapy is considered as an effective way to reduce recurrence, but in addition to antiviral therapy, adjuvant therapy continues to be controversial and is currently not recommended by guidelines.2,8 Our study showed that adjuvant lenvatinib plus PD-1 antibody after hepatectomy is beneficial for RFS and has manageable safety.
Randomized controlled trial revealed that adjuvant TACE significantly improved RFS (P = 0.01, HR: 0.68, 95% CI: 0.49–0.93), but increased the 1-year RFS rate by only 15.8%.11 Another multicenter randomized controlled trial indicated that adjuvant HAIC only increased the 1- and 2-year RFS rates by 15% and 16.7% in patients with MVI.17 As for immunotherapy, multicenter randomized controlled trial demonstrated that adjuvant immune checkpoint inhibitors increased the 1- and 2-year RFS rates by 9% and 12.3%.18 Chen et al demonstrated that adjuvant PD-1 antibody increased the 1- and 2-year RFS rates by 24.4% and 22.8% in patients with high risks of recurrence.19 However, our study showed that adjuvant lenvatinib plus PD-1 antibody increased the 1- and 2-year RFS rates by 28.2% and 32.3%. Similar results were found by Li et al in patients with HCC receiving adjuvant TKIs plus PD-1 antibody (increased 1-year RFS rate by 27.9%).20 Adjuvant targeted therapy plus immunotherapy provided additional advantages in improving RFS compared with adjuvant TACE, HAIC, or PD-1 antibody monotherapy. This may be related to the synergetic anticancer effect of the combination therapy.21 Different from the above studies,11,18–20 the ATG in our study did not significantly improve OS (HR: 0.595, 95% CI: 0.336–1.053; P = 0.097). In our study, the 1-year OS rate of the ATG improved by 14.7%, superior to adjuvant TACE,11 HAIC,17 or immune monotherapy.19 However, the 2-year OS rate of the ATG in our study improved by only 6.1%. Comparing the survival curves and cumulative risk tables for OS, we believe that this may be due to the short follow-up time of the ATG (median follow-up time: 14.8 vs 28.5 months).
Although the Log rank test showed a significant difference in RFS, landmark analysis revealed that RFS of the ATG was significantly beneficial within 6 months (P < 0.001) and not after 6 months (P = 0.486). This result indicated that adjuvant lenvatinib plus PD-1 antibody is an appropriate therapy for patients with high risks of recurrence who are more tend to recur in the short term. Interestingly, 6 months is the approximate time when the 7th cycle of PD-1 antibodies should have been received (hepatectomy to discharge 15 days + discharge to the first follow-up 30 days + the 1st-6th immunotherapy doses 21×5 days + 30 days = 6 months). This raises the question of whether prolonged the duration of adjuvant therapy result in benefit for RFS.
Multivariable logistic regression results showed that without adjuvant therapy, high Child-Pugh classification, high AFP levels, MVI, and satellite nodules were independent risk factors for recurrence within 6 months after hepatectomy. Previous studies have reported that tumor pathological characteristics are more likely to be independent risk factors for early recurrence, such as multiple tumors, AFP levels, MVI and satellite nodules.22–24 It is widely considered that intrahepatic tumor metastasis and the aggressiveness of primary cancers are the main causes of early recurrence.25 It has been shown that number is a limited prognostic factor for early recurrence of HCC, and compared with number, biological characteristics better reflect the aggressiveness of tumor, such as AFP levels, MVI, and satellite nodules.26 Therefore, we may make a better selection for postoperative adjuvant therapy after considering tumor biological risk factors. Child-Pugh classification is an independent risk factor for recurrence within 6 months after hepatectomy in our study, possibly because of the different effect of combined systemic therapy on liver function compared with conventional adjuvant therapy. Impairment of liver function is one of the most common TRAEs in patients with HCC treated with lenvatinib plus PD-1 antibody.12 Child-Pugh classification was also proven to be an independent risk factor for prognosis of patients with unresectable HCC treated with lenvatinib plus PD-1 antibody.27 However, there were only 7 (5.1%) patients with Child-Pugh B grade in our study, and Child-Pugh classification did not have a significant effect on recurrence within 6 months in univariable logistic regression (P = 0.097; HR: 4.000, 95% CI: 0.747–21.408). Therefore, we believe that whether Child-Pugh classification is an independent predictor of recurrence in the short term among patients with HCC treated with adjuvant lenvatinib plus PD-1 antibody still needs to be further validated with large sample data.
There is continued controversy about the subgroups that are recognized to significantly benefit from postoperative adjuvant therapy. In our subgroup analysis, RFS of subgroup with AFP ≤ 400 ng/mL, single tumor, well-differentiated tumor and no satellite nodules significantly benefited from adjuvant lenvatinib plus PD-1 antibody. This finding that only the subgroups with low tumor aggressiveness can benefit from adjuvant therapy is not an individual case. For example, RFS significantly benefited from adjuvant TACE in the well-differentiated subgroup,11 from adjuvant HAIC in the single tumor subgroup,17 and from adjuvant immune checkpoint inhibitors in the number of tumors ≤ 3 subgroup, the negative hepatic venous tumor thrombus subgroup, the negative satellite nodule subgroup, and the AFP ≤ 400ng/mL subgroup.18,19 Hepatologists and oncologists have long tended to believe that HCC patients with MVI are more likely to benefit from adjuvant therapy. Adjuvant TACE,28 adjuvant HAIC,17 adjuvant stereotactic body radiotherapy,29 and adjuvant sorafenib30 have all been proven to significantly improve RFS in HCC patients with MVI. In our subgroup analysis, postoperative adjuvant lenvatinib plus PD-1 antibody significantly improved RFS of HCC patients with MVI (P < 0.001; HR: 0.323, 95% CI: 0.172–0.606). Similarly, in a multicenter Phase II trial, Zhao et al demonstrated that HCC patients with MVI benefited more from postoperative adjuvant camrelizumab plus apatinib compared to other subgroups.31 Therefore, we should establish practical models based on MVI to identify the subgroups that may benefit from postoperative adjuvant targeted therapy plus immunotherapy. In addition, in our subgroup analysis, single tumor was the subgroup whose OS benefited from adjuvant therapy significantly. Although similar results have been reported in other adjuvant therapies,20,32 OS is influenced by treatment after recurrence. Thus, the effect of adjuvant therapy on OS remains controversial. Although no significant difference existed in treatment after recurrence between the two groups (P = 0.968), our study is limited by the shortage of follow-up time for the ATG, and further validation is needed regarding the role of adjuvant therapy on OS in each subgroup.
Consistent with the safety of TKIs plus PD-1 antibody during the perioperative period in resectable HCC, we found that hypertension is one of the most common TRAEs of lenvatinib plus PD-1 antibody.20,33,34 TKIs damage tumor vascular epithelial cells, causing an increase in nitric oxide synthase and a decrease in nitric oxide, which explains why hypertension occurs.35 Among all patients who received adjuvant therapy, three patients who did not complete 6 cycles of anti-PD-1 antibodies were excluded due to severe immune checkpoint inhibitor (ICIs)-related pneumonia, ICI-related hepatitis, ICI-related myocarditis with myositis (Figure 1), and 9 (17.3%) patients with grade 3 TRAEs were relieved after the intervention. The remaining TRAEs were considered grade 1–2 (82.7%).
Our study had several limitations. First, owing to the retrospective nature of our analysis, there were unavoidable selection biases and confounders. We did not perform propensity score matching due to the small sample of the ATG. Second, patients infected hepatitis virus accounted for the majority of our cohort. Therefore, the applicability of our results to HCC caused by other etiologies needs to be validated. Third, symptomatic TRAEs such as anorexia, fatigue, and itching mainly relied on the patient’s subjective descriptions. Patients with long follow-up periods may have underreported or exaggerated symptoms, and some patients consulted local hospitals regarding grade 3–4 TRAEs. These factors may have led to bias in the TRAE follow-up results. Fourth, the follow-up time for the ATG was insufficient.
Conclusion
Lenvatinib plus PD-1 antibody has shown promising efficacy and manageable toxicity in patients with HCC at high risks of recurrence after hepatectomy, but the best duration needs to be defined further. We look forward to further randomized controlled trials to support our results and determine the role of lenvatinib plus PD-1 antibody in postoperative adjuvant therapy.
Data Sharing Statement
All data will be available from the corresponding author Jinxue Zhou ([email protected]) with the agreement of institutional ethics committee.
Ethics Approval and Informed Consent
Research was conducted in accordance with the Declaration of Helsinki (1975) and its amendments. This study was approved by the ethics committees of the Affiliated Cancer Hospital of Zhengzhou University and the Henan Cancer Hospital. The requirement for patient consent was waived because the study was retrospective and all data was anonymized or maintained with confidentiality of patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was sponsored by the Henan Provincial Medical Science and Technology Research Project (LLRGJ20220191), Key Scientific Research Project of Colleges and Universities in Henan Province (23A320033), Henan Provincial Science and Technology Project (232102311080), CAMS Innovation Fund for Medical Sciences (CIFMS) (2022-12M-C&T-B-081), Special Research Fund for Central Universities, Peking Union Medical College (No. 3332022026), and Youth Project of Beijing Hope Marothon Special Fund (No. LC2021B17), the National Natural Science Foundation of China (No. 81972311, No. 82141127), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-066), and the Non-profit Central Research Institution Fund of the Chinese Academy of Medical Sciences (No. 2019PT310026).
Disclosure
All authors have no conflicts of interest to declare in this work.
References
1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
2. European Association For The Study Of The Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57(4):1426–1435. doi:10.1002/hep.25832
4. Jiang JH, Guo Z, Lu HF, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol. 2015;21(15):4627–4634. doi:10.3748/wjg.v21.i15.4627
5. Moran A, Ramos LF, Picado O, et al. Hepatocellular carcinoma: resection with adjuvant hepatic artery infusion therapy vs resection alone. A systematic review and meta-analysis. J Surg Oncol. 2019;119(4):455–463. doi:10.1002/jso.25338
6. Li S, Mei J, Wang Q, et al. Postoperative adjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of hepatocellular carcinoma patients with microvascular invasion: a preliminary report of a phase III, randomized controlled clinical trial. Ann Surg Oncol. 2020;27(13):5183–5190. doi:10.1245/s10434-020-08601-8
7. Rong W, Yu W, Wang L, et al. Adjuvant radiotherapy in central hepatocellular carcinoma after narrow-margin hepatectomy: a 10-year real-world evidence. Chin J Cancer Res. 2020;32(5):645–653. doi:10.21147/j.issn.1000-9604.2020.05.09
8. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308
9. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a Phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi:10.1016/S1470-2045(15)00198-9
10. Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res. 2014;44(5):523–531. doi:10.1111/hepr.12159
11. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. doi:10.1158/1078-0432.CCR-17-2899
12. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
13. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
14. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi:10.1200/JCO.1983.1.11.710
15. Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. doi:10.1161/CIRCOUTCOMES.110.957951
16. Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver resection versus local ablation therapies for hepatocellular carcinoma within the Milan criteria: a systematic review and meta-analysis. Ann Surg. 2021;273(4):656–666. doi:10.1097/SLA.0000000000004350
17. Li SH, Mei J, Cheng Y, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III, randomized study. J Clin Oncol. 2023;41(10):1898–1908. doi:10.1200/JCO.22.01142
18. Li L, Wu PS, Liang XM, et al. Adjuvant immune checkpoint inhibitors associated with higher recurrence-free survival in postoperative hepatocellular carcinoma (PREVENT): a prospective, multicentric cohort study. J Gastroenterol. 2023. doi:10.1007/s00535-023-02018-2
19. Chen W, Hu S, Liu Z, et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int. 2023;17(2):406–416. doi:10.1007/s12072-022-10478-6
20. Li J, Wang WQ, Zhu RH, et al. Postoperative adjuvant tyrosine kinase inhibitors combined with anti-PD-1 antibodies improves surgical outcomes for hepatocellular carcinoma with high-risk recurrent factors. Front Immunol. 2023;14:1202039. doi:10.3389/fimmu.2023.1202039
21. Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7(1):333. doi:10.1186/s40425-019-0824-5
22. Chan A, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi:10.1016/j.jhep.2018.08.027
23. Zhang YM, Zhou ZT, Liu GM. Factors predicting early recurrence after surgical resection of hepatocellular carcinoma. J Hepatol. 2019;70(3):571–572. doi:10.1016/j.jhep.2018.10.038
24. Li C, Ouyang W, Yang T. The association of microvascular invasion with satellite nodule, tumor multiplicity, tumor encapsulation and resection margin of hepatocellular carcinoma. J Hepatol. 2022;77(3):890–891. doi:10.1016/j.jhep.2022.03.036
25. Kim HI, An J, Kim JY, et al. Postresection period-specific hazard of recurrence as a framework for surveillance strategy in patients with hepatocellular carcinoma: a multicenter outcome study. Liver Cancer. 2022;11(2):141–151. doi:10.1159/000518837
26. Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62(5):1131–1140. doi:10.1016/j.jhep.2014.12.018
27. Yang X, Chen B, Wang Y, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;17(3):709–719. doi:10.1007/s12072-022-10480-y
28. Wang XH, Zhou QF, Wang CM, et al. Adjuvant transarterial chemoembolization for intermediate-stage hepatocellular carcinoma with microvascular invasion. Br J Surg. 2023;110(8):913–916. doi:10.1093/bjs/znac376
29. Shi C, Li Y, Geng L, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: a randomised controlled trial. Eur J Cancer. 2022;166:176–184. doi:10.1016/j.ejca.2022.02.012
30. Zhang XP, Chai ZT, Gao YZ, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB. 2019;21(12):1687–1696. doi:10.1016/j.hpb.2019.04.014
31. Zhao H, Du S, Zhu Z, et al. 724P Anti-PD-1 antibody SHR-1210 combined with apatinib as adjuvant treatment in patients with hepatocellular carcinoma at high risk of recurrence after radical resection: preliminary results from a multicenter, randomized, controlled phase II trial. Ann Oncol. 2022;33:S873. doi:10.1016/j.annonc.2022.07.848
32. Liu S, Li H, Guo L, et al. Tumor size affects efficacy of adjuvant transarterial chemoembolization in patients with hepatocellular carcinoma and microvascular invasion. Oncologist. 2019;24(4):513–520. doi:10.1634/theoncologist.2018-0305
33. Wu JY, Wu JY, Li YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: a multicenter retrospective study. Front Oncol. 2022;12:985380. doi:10.3389/fonc.2022.985380
34. Xia Y, Tang W, Qian X, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022;10(4):e004656. doi:10.1136/jitc-2022-004656
35. Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22(6):1385–1394. doi:10.1158/1078-0432.CCR-15-1354
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


