Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Adipose-Derived Stem Cell Exosomes Alleviate Psoriasis Serum Exosomes-Induced Inflammation by Regulating Autophagy and Redox Status in Keratinocytes
Authors Kim HR , Lee SY , You GE, Kim HO , Park CW, Chung BY
Received 4 October 2023
Accepted for publication 7 December 2023
Published 23 December 2023 Volume 2023:16 Pages 3699—3711
DOI https://doi.org/10.2147/CCID.S439760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Hye Ran Kim,1 So Yeon Lee,1 Ga Eun You,2 Hye One Kim,1 Chun Wook Park,1 Bo Young Chung1
1Department of Dermatology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, 07441, Korea; 2Research and Development Institute, Biosolution, Seoul Technopark, Seoul, 01811, Korea
Correspondence: Bo Young Chung, Department of Dermatology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, 1 Singil-ro, Yeongdeungpo-gu, Seoul, 07441, Korea, Tel +82-2-829-5221, Fax +82-2-832-3237, Email [email protected]
Introduction: Exosomes play a key role in cell communication and are involved in both pathological and physiological processes. Autophagy dysfunction and oxidative stress are linked to immune-mediated inflammatory diseases such as psoriasis. Stem cell-derived exosomes exhibit immunomodulatory and antioxidant efficacy.
Methods: We aimed to investigate the impact of psoriasis serum-derived exosomes on inflammation, oxidative stress, and autophagy in keratinocytes. Additionally, we explored the therapeutic potential of adipose-derived stem cell (ADSC) exosomes against inflammation induced by psoriasis serum exosomes. To validate psoriasis patient serum-derived exosomes and ADSC exosomes, we used nanoparticle tracking analysis, Western blotting, flow cytometry, and immunofluorescence. qPCR was used to study changes in the gene expression of proinflammatory cytokines and oxidative stress markers in HaCaT cells treated with psoriasis serum-derived exosomes or ADSC exosomes. The effects of these exosomes on autophagy in HaCaT cells were evaluated by Western blotting and immunofluorescence.
Result: The treatment of HaCaT cells with psoriasis serum-derived exosomes increased proinflammatory cytokine production and oxidative stress-related factor (Nox2 and Nox4) expression and decreased Nrf2 expression via P65/NF-κB and P38/MAPK activation. Compared with healthy control serum-derived exosomes, psoriasis serum-derived exosomes decreased ATG5, P62, Beclin1, and LC3 expression and autophagosome production in HaCaT cells. Conversely, ADSC exosomes suppressed proinflammatory cytokine and oxidative stress production, and restored autophagy in HaCaT cells treated with psoriasis serum-derived exosomes.
Discussion: These findings suggest that ADSC exosomes exhibit a suppressive effect on psoriasis serum exosome-induced inflammation and oxidative stress by regulating autophagy in keratinocytes.
Keywords: exosomes, adipose-derived stem cell, psoriasis, autophagy, oxidative stress
Introduction
Psoriasis is a chronic, inflammatory skin disease characterized by keratinocyte activation and immune cell infiltration.1 Beyond its impact on the skin, psoriasis is recognized as a systemic condition associated with comorbidities such as psoriatic arthritis, cardiovascular diseases, and mental disorders.2 Consequently, it profoundly affects patients’ physical and psychological health as well as their quality of life.3 The precise pathogenesis of psoriasis remains unclear, but it is widely accepted to involve complex molecular mechanisms driven by abnormal immune cell activity and signaling with the surrounding tissue microenvironment.1 Various signaling pathways, including redox, NF-κB, and MAPK, play a role in the development of psoriasis.4–6 Autophagy, a crucial process responsible for maintaining tissue homeostasis by degrading and recycling damaged organelles and proteins, has been implicated in psoriasis pathogenesis, with growing evidence suggesting its dysfunction.7,8 Several studies have demonstrated that mesenchymal stem cells (MSCs) derived from the skin of psoriatic patients exhibit over-expression of genes encoding Th1 and TH17 cytokines compared to healthy controls.9–11 Consequently, MSCs in the skin of patients with psoriasis become dysfunctional, losing their anti-inflammatory properties and adopting a pro-inflammatory role, thereby contributing to the pathogenesis of psoriasis through interactions with keratinocytes and immune cells.
Exosomes are 50–150 nm extracellular vesicles that contain nucleic acids, lipids, and proteins. They facilitate cell-to-cell communication and membrane transport. These vesicles can be found in various body fluids (blood, urine, breast milk, and saliva) as well as in cell culture supernatants. The molecular composition of exosomes varies depending on physiological or pathological conditions, cell origin, and tissue type.12
Both immune and nonimmune cells secrete exosomes, which play a role in regulating the immune system. This includes T-cell activation, polarization, immune suppression, and anti-inflammation.13 Previous research has shown that exosomes are critical in cellular signaling and are implicated in the development of disorders, such as autoimmune diseases and tumor growth.14–16
With the development and use of biologics inhibiting TNF-α, IL-17, and IL-23, the therapeutic efficacy in psoriasis treatment has improved in recent years.17,18 However, there are still nonresponders to biologics, indicating treatment resistance and failure.19 Psoriatic arthritis, affecting approximately 30% of psoriasis patients, presents an additional challenge with poorer treatment outcomes compared to psoriasis alone.20 Regenerative medicine based on stem cells offers a promising alternative. Mesenchymal stem cells (MSCs) have demonstrated immunoregulatory or antioxidative properties, contributing to tissue homeostasis.21,22 MSCs have been clinically explored for various diseases, such as graft-versus-host disease, Crohn’s disease, and myocardial infarction.23,24 Preclinical studies have also shown the therapeutic potential of MSC secretome-derived products, including exosomes, in processes related to cell differentiation, inflammation, and oxidative stress.25 In this study, we verified the detrimental effects of psoriasis serum-derived exosomes and the beneficial potential of adipose tissue-derived stem cell (ADSC) exosomes in regulating inflammation, oxidative stress, and autophagy in keratinocytes.
Materials and Methods
Patients and Exosome Isolation
The study was approved by the Institutional Review Board at Hallym University Kangnam Sacred Heart Hospital (IRB no. 2022-03-020). Exosomes were isolated from blood samples from healthy controls and psoriasis patients using an exosome purification kit (Exo-spin™ mini, Cells guidance systems, St. Louis, MO, USA) as manufacturer’s instruction as follows. The serum was centrifuged at 1000 x g for 5 minutes, and the supernatant was transferred to a new microcentrifuge tube, then spun at 16,000 x g for 30 minutes at 4°C. Next, the supernatant was transferred to a new microcentrifuge tube, and Exo-spin™ Buffer was added in a 2:1 ratio. The mixture was mixed by inverting the tube and incubated at 4°C overnight. The mixture was then centrifuged at 16,000 x g for 1 hour at 4°C. The pellet containing exosomes was resuspended in 100 µL of PBS. This resuspended exosome-containing pellet (100 µL) was applied to the top of the Exo-spin™ column and placed into a 1.5 mL microcentrifuge tube. The liquid entered the column matrix under gravity. Then, 180 µL of PBS was added to the top of the column and eluted. The Exo-spin™ column was removed from the sample collection tube. The sample collection tube containing the isolated exosomes was centrifuged at 100 x g for 30 seconds.
ADSC Isolation and Culture
Human adipose tissue was obtained through liposuction surgery with informed consent from a 30-year-old male. Adipose tissue was washed with a phosphate buffer solution to remove red blood cells, followed by incubation with 0.075% collagenase-II (Sigma‒Aldrich, St. Louis, MO, USA) at 37 °C for 40 minutes. After centrifugation and filtration through a 100 µm cell strainer, the resulting cells were cultured in expansion media containing α-MEM (Welgene, Gyeongsan, Gyeongsang, Korea), 10% FBS (Capricorn Scientific GmbH, Ebsdorfergrund, NRW, Germany), and 5 ng/mL recombinant bFGF (R&D system, Minneapolis, MN, USA). The expansion medium was replaced every 2 days, and the cells were subcultured until passage 6.
Preparation of ADSC-Conditioned Media
ADSCs were seeded at 3.7×109 cells/cm2 and cultured in the expansion media for 3 days. Then, to remove FBS and particles, the cells were cultured in α-MEM without FBS. After 24 h, the cells were washed with phosphate buffer solution and cultured in α-MEM containing 10% exosome-depleted SR (XenoFree CTS; Gibco Life Technologies, Carlsbad, CA, USA) for 2 days. The ADSC-conditioned media were harvested and filtered through a 0.22-µm filter.
Separation of ADSC-Derived Exosomes via a TFF System
Exosomes from ADSC-conditioned media were isolated using a TFF system equipped with a 500-kDa cutoff hollow fiber membrane (EMD Millipore Corp., Billerica, MA, USA). The conditioned media (1 L) was processed through the TFF system, maintaining a fluid velocity of 180 r/min and a pressure below 1.5 psi. Subsequently, PBS (2 L) was introduced into the TFF system to enhance exosome purity and buffer exchange. The resulting exosomes were then filtered through a 0.22-µm filter.
The size and quantity of exosomes were determined using nanoparticle tracking analysis (NTA) (NS300; Malvern Instruments, Malvern, Worcestershire, UK). The protein content of the exosome solution was measured using the BCA assay (iNtRON biotechnology, Seongnam-si, Gyeonggi-do, Korea). Exosomes were stored at −80 °C for future use.
Cell Culture and Treatments
The immortalized human keratinocyte cell line HaCaT (Welgene, Daegu, Korea) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, Walkersville, MD, USA). DMEM was supplemented with 1% penicillin‒streptomycin and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) and incubated in a CO2 incubator at 37 °C (5% CO2).
Based on the efficacy of ADSC exosomes by concentrations on the proliferation of human skin fibroblasts (Biosolution Ltd., Nowon, Seoul, Korea), we determined the concentration of ADSC exosomes (3.7 x 109/mL) for further experiments (Supplementary Figure 1). The concentration of exosomes from serum (2.5 x 108/mL) was determined based on the previous study.26 Further, this concentration of exosomes from psoriasis serum did not affect cell viability in HaCaT cells (data not shown). HaCaT cells were treated with healthy control serum-derived exosomes (2.5 x 108/㎖), psoriasis serum-derived exosomes (2.5 x 108/㎖), ADSC-derived exosomes (3.7x109/㎖), healthy control serum-derived exosomes (2.5 x 108/㎖) + ADSC-derived exosomes (3.7x109/㎖), or psoriasis serum-derived exosomes (2.5 x 108/㎖) + ADSC-derived exosomes (3.7x109/㎖) for 24 h.
Nanoparticle Tracking Analysis
The size and number of extracellular vesicles (EVs) in conditioned medium were analyzed using NanoSight (NS300, Malvern, UK) with a 488 nm laser. The sample was loaded into the chamber using a 1 mL disposable syringe, and a 40-second video was recorded to capture all events. The video was then analyzed using NTA software based on Brownian motion. EVs were diluted with PBS until the appropriate number of EVs was detected, and measurements were taken at the same camera level and detection threshold. The analysis was repeated four times for each sample, providing data on the number and size distribution of exosomes.
Exosome Uptake Assay
HaCaT cells treated with psoriasis serum exosomes or stem cell exosomes were labeled with a green fluorescent dye, Vybrant DiO cell-labeling solution (Sigma‒Aldrich, USA). Cells were washed with PBS, fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.2% Triton X-100 in 1% bovine serum albumin (BSA) for 10 minutes, and blocked with 3% BSA for one hour at room temperature. Cell-labeling solution was added to HaCaT cells treated with psoriasis serum exosomes or stem cell exosomes and incubated at 37 °C for 2 hours. The cells were visualized under fluorescence microscopy using Leica microsystems DFi8 LASX software light microscopy (Leica, Wetzlar, Germany).
Quantitative PCR
Total RNA extraction was performed using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A First Strand cDNA synthesis kit (Roche Applied Science, Mannheim, Germany) was used to synthesize cDNA from 1 µg of total RNA. Quantitative reverse transcriptase-PCR (qRT‒PCR) was performed in triplicate using TaqMan master mix (Applied Biosystems, Foster City, CA, USA) and Real-Time PCR System (Applied Biosystems). The primer details for mRNA detection can be found in Supplementary Table 1. mRNA levels of IL-1β, IL-6, TNF-α, NOX2, NOX4, and Nrf2 were normalized to GAPDH. Fold changes were calculated using the ΔΔCt method, and relative quantification was performed using a Light Cycler 96 instrument (Roche Diagnostics, Mannheim, Germany).
Western Blot Assay
Cells were lysed in pro-prep lysis buffer (Intron, Seoul, Korea) supplemented with a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein concentrations were measured using a bicinchoninic acid solution with copper (II) sulfate (Sigma‒Aldrich, St. Louis, MO, USA). Equal amounts of protein (20 µg) were separated by 10% SDS‒PAGE, transferred to ECL nitrocellulose membranes (GE Healthcare, Buckinghamshire, UK), and blocked for 1 hour with 5% skim milk in TBST. Membranes were incubated overnight at 4 °C with the antibodies listed in Supplementary Table 2. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit, 1:1000; Abcam, Cambridge, UK) and chemiluminescent luminol (LUMINOGRAPH II; Atto, Tokyo, Japan). Immunocomplexes were visualized using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus; Amersham International PLC, Little Chalfont, UK). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control for Western blots.
Cell Immunofluorescence
HaCaT cells were seeded on coverslips and allowed to adhere overnight. Cells were washed with PBS, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 in 1% BSA for 10 min, blocked with 3% BSA for one hour at room temperature and incubated overnight at 4 °C with anti-LC3B (Abcam, Cambridge, UK). Cells were then incubated in goat anti-Rb HRP (Abcam, Cambridge, UK) and the secondary antibody conjugated with FITC (Abcam, Cambridge, UK) at 1:200 for 1 h. Stained HPDFs were captured and visualized using a microscope (Leica Micro systems, Germany). For nuclear counterstaining, Vectashield mounting medium was used along with DAPI (Vector Laboratories, Burlingame, CA).
Flow Cytometry
Passage 4 and 7 ADSCs were detached with 0.05% trypsin/EDTA (Welgene, Gyeongsan, Gyeongsang, Korea; LS015-01) at 37 °C for 3 min and washed using FACS buffer (1% BSA in PBS). The cells were blocked using FcR blocking reagent (Miltenyi Biotec, Auburn, CA, USA; 130-059-901) and stained with FITC-labeled antibodies against CD34 (BD Biosciences, San Diego, CA, USA; 555821) and CD29 (Dako, Glostrup, Copenhagen, Denmark; F7068) for 1 hour at RT. Fluorescence was analyzed using a BD FACSCalibur Flow Cytometer (BD bioscience, Heidelberg, Baden-Württemberg, Germany; 342975), and the data were analyzed by BD Cell Quest Pro software.
Statistical Analyses
Statistical analyses were conducted with GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA). Data were analyzed using the Student’s t-test and one-way analysis of variance with Tukey’s post-hoc test. P values < 0.05 were considered statistically significant.
Results
Characterization of Exosomes Isolated from the Serum of Psoriasis Patients and Adipose-Derived Stem Cells
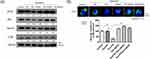
The ADSCs were positive for the MSC marker CD29 and negative for the endothelial marker CD34, as assessed by flow cytometry (Figure 1A). These ADSCs had a typical fibroblastic-like morphology (Figure 1B).
We isolated exosomes from the serum of patients with psoriasis (n = 6), healthy controls (n = 6) and ADSCs. The existence of exosomes was verified by nanoparticle tracking analysis, which revealed a peak at 126.6 nm with 4.62×107 particles/mL in the serum of healthy controls, a peak at 147.7 nm with 8.46×107 particles/mL in the serum of psoriasis patients, and a peak at 123.7 nm with 2.64×108 particles/mL in the exosomes of ADSCs (Figure 2A). Western blot analysis revealed that the particles expressed molecular markers recognized for exosomes, such as CD63 and CD9 (Figure 2B). Therefore, the particles isolated from the serum of patients with psoriasis, normal controls, and ADSCs were confirmed to be exosomes.
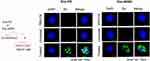
Next, to confirm the uptake of exosomes by recipient HaCaT cells, we labeled exosomes derived from psoriasis serum or ADSCs with DiI and added them to recipient HaCaT cells for 2 h. Immunofluorescence assays showed that HaCaT cells exhibited efficient uptake of exosomes (Figure 3).
The Effects of Psoriasis Serum-Derived Exosomes and ADSC-Derived Exosomes on Proinflammatory Cytokine Production in HaCaT Cells
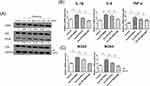
To explore the effects of psoriasis serum-derived exosomes and ADSC-derived exosomes on proinflammatory cytokine production in HaCaT cells, we treated HaCaT cells with healthy control serum-derived exosomes, psoriasis serum-derived exosomes, ADSC-derived exosomes, healthy control serum-derived exosomes + ADSC-derived exosomes or psoriasis serum-derived exosomes + ADSC-derived exosomes for 24 h. The mRNA production of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, in HaCaT cells treated with psoriasis serum-derived exosomes was elevated compared to that in untreated controls and healthy control serum-derived exosome-treated HaCaT cells. ADSC exosome treatment significantly decreased IL-1β, IL-6 and TNF-α expression in psoriatic serum-derived exosome-stimulated HaCaT cells compared with psoriatic serum-derived exosome-stimulated HaCaT cells (Figure 4A).
The Effects of Psoriasis Serum-Derived Exosomes and ADSC-Derived Exosomes on the Expression of Oxidative Stress in HaCaT Cells
The expression of the oxidative stress-related factors NOX2 and NOX4 was increased in psoriasis serum-derived exosome-treated HaCaT cells compared to negative controls and healthy control serum-derived exosome-treated HaCaT cells. In contrast, the expression of Nrf2, an antioxidant signaling-related factor, was decreased in psoriasis serum-derived exosome-treated HaCaT cells compared to negative controls and healthy control serum-derived exosome-treated HaCaT cells.
ADSC exosome treatment significantly decreased NOX2 and NOX4 mRNA expression in psoriasis serum-derived exosome-stimulated HaCaT cells compared to psoriasis serum-derived exosome-only treatment in HaCaT cells. Furthermore, Nrf2 mRNA expression was increased in ADSC exosome-treated HaCaT cells in psoriasis serum-derived exosome-stimulated HaCaT cells compared with psoriasis serum-derived exosome-treated HaCaT cells (Figure 4B).
The Relevance of the p65NF-κB and p38MAPK Signaling Pathways to Proinflammatory Cytokine and Oxidative Stress Production Induced by Psoriasis Serum-Derived Exosomes in HaCaT Cells
Next, we determined the possible signaling pathway associated with the activation of psoriasis serum-derived exosomes in HaCaT cells. Treatment of HaCaT cells with exosomes derived from the serum of psoriasis patients increased the phosphorylation of P65/NF-κB and P38/MAPK compared to the control and healthy control serum-derived exosome treatment groups. However, ADSC exosomes showed no apparent changes in the phosphorylation of P65/NF-κB and P38/MAPK compared to the control and healthy control serum-derived exosome treatment groups (Figure 5A).
To confirm the involvement of P65/NF-κB and P38/MAPK signaling in psoriasis serum-derived exosome-induced oxidative stress and proinflammatory cytokine production changes, HaCaT cells were pretreated with inhibitors of P65/NF-κB (PDTC) and P38/MAPK (SB203580) for 1 h and then stimulated with exosomes isolated from psoriasis patient serum for 24 h. The upregulation of IL-1β, IL-6, TNF-α, NOX2, and NOX4 mRNA expression was significantly blocked by PDTC and SB203580 in psoriasis patient serum-derived exosome-treated HaCaT cells. Taken together, these data showed that the increase in IL-1β, IL-6, TNF-α, NOX2, and NOX4 production by psoriasis serum-derived exosomes was dependent on P65/NF-κB and P38/MAPK signaling (Figure 5B and C).
The Effects of Psoriasis Serum-Derived Exosomes or ADSC-Derived Exosomes on Autophagy in HaCaT Cells
To evaluate the effects of psoriasis serum exosomes or ADSC exosomes on autophagy in HaCaT cells, we treated HaCaT cells with psoriasis serum exosomes or ADSC-derived exosomes for 24 h. Then, we checked the change in the expression of autophagy-related factors using Western blot analysis. Psoriasis serum-derived exosome treatment decreased the expression of autophagy-related factors, including ATG5, P62, Beclin1, and LC3B, in HaCaT cells compared to negative controls and healthy controls’ serum-derived exosome-treated group (Figure 6A). The formation of autophagosomes displaying LC3B punctae per cell was reduced in psoriasis serum-derived exosome-stimulated HaCaT cells compared to negative controls and healthy controls’ serum-derived exosome-treated group. ADSC-derived exosome treatment increased the expression of ATG5, P62, Beclin1, and LC3B in psoriasis serum-derived exosome-stimulated HaCaT cells compared with psoriasis serum-derived exosome-stimulated HaCaT cells. Furthermore, ADSC-derived exosome treatment induced an increase in LC3B puncta per cell in psoriasis serum-derived exosome-stimulated HaCaT cells compared to psoriasis serum-derived exosome-stimulated HaCaT cells (Figure 6B and C).
Discussion
This study found that psoriatic patient-derived circulating exosomes can promote inflammation and oxidative stress in recipient keratinocytes by inhibiting autophagy and activating P65/NF-κB and P38/MAPK signaling. Moreover, it demonstrated the therapeutic potential of ADSC-derived exosomes in reversing the upregulation of pro-inflammatory cytokines, oxidative stress, and autophagy dysfunction induced by psoriasis serum-derived exosomes in HaCaT cells.
The interplay between keratinocytes and immune cells plays a crucial role in the pathogenesis of psoriasis, which is characterized by dysregulated keratinocyte proliferation and differentiation.1 Our study demonstrated that psoriasis serum-derived exosomes increased the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in recipient HaCaT cells through the P65/NF-κB and P38/MAPK signaling pathways. A previous study also highlighted the involvement of exosomes in psoriasis pathogenesis. For instance, exosomes were found to facilitate communication between keratinocytes and neutrophils, promoting psoriasis progression in cell and animal models.26,27 Additionally, the secretion of heat shock protein 90 (HSP90) was increased in IFN-γ-induced exosomes from HaCaT cells, and plasma exosomes from a psoriatic mouse model showed elevated expression of Hsp90α, a potent immunomodulatory.28 Cheung et al demonstrated that exosomes can act as carriers for nonpeptide antigens, triggering the production of IL-17A and IL-22 by CD1a-autoreactive T cells in psoriasis patients.29 Collectively, these findings, including ours, suggest that exosomes play a significant role in propagating psoriatic inflammation as cell-to-cell messengers.
Oxidative stress is recognized as a critical contributor to the development and persistence of psoriasis.4,30,31 Markers of oxidative stress are elevated in serum of psoriasis and are associated with disease severity.32,33 Genetic polymorphisms in redox-modulating genes have also been found to be upregulated in psoriasis.30,31 In the psoriasis pathomechanism, oxidative stress activates various signaling pathways, including NF-κB and MAPK, leading to the activation of Th1/Th17 cells, production of proinflammatory cytokines, keratinocyte proliferation, and immune cell infiltration in the skin.4 We found that psoriasis serum exosomes triggered an increase in oxidative stress-related factors, such as NOX2 and NOX4. Conversely, psoriasis serum exosomes suppressed the expression of Nrf2, an essential regulator of antioxidant signaling, in recipient keratinocytes.
Autophagy is a key intracellular degradation pathway that degrades or recycles damaged cellular components via lysosomal machinery. Autophagy plays a critical role in maintaining cellular homeostasis, including metabolism, cell survival, and the immune response, and impaired autophagy is associated with various diseases.34,35 Autophagy dysfunction has been implicated in psoriasis pathogenesis. The ATG16L1 autophagy-related gene polymorphism is linked to psoriasis development.36 Defective autophagy enhanced p62 activity and triggered proinflammatory cytokines in keratinocytes, contributing to psoriasis.37 Klapan et al demonstrated that TNF-α, a central regulator of psoriasis pathogenesis, induced autophagy induction in early stages but inhibited autophagy after prolonged treatment in primary human epidermal keratinocytes.8 Interestingly, we found that psoriasis serum exosomes inhibited autophagy in recipient keratinocytes.
A recent study reported that injecting human umbilical cord MSC exosomes reduced psoriasis-like skin inflammation, including epidermal proliferation and psoriasis area and severity index scores, in imiquimod-induced mice by regulating IL-23 and IL-17 expression.38 In a mouse model of imiquimod-induced psoriasis, topical application of E1-MYC 16.3 human embryonic stem cell-derived MSC exosomes led to reduced C5b-9 and IL-17 levels.39 However, the impact of ADSC-derived exosomes on psoriatic inflammation remains unclear. Our study proved that ADSC exosomes ameliorated the upregulation of IL-1β, IL-6, and TNF-α induced by exosomes from psoriasis serum in human keratinocytes. Previous research has shown that exosomes isolated from MSCs resist oxidative damage.40,41 Our study showed the antioxidant efficacy of ADSC exosomes in recipient keratinocytes, consistent with previous findings. The antioxidant effects of MSC-derived exosomes are thought to involve the transfer of antioxidative enzyme mRNA, proteins, or miRNAs into recipient cells.42
MSC-derived exosome therapy offers several advantages over stem cell transplantation itself.43 MSC-derived exosomes are less harmful by avoiding problems related to transplantation of proliferative and live cells. Furthermore, MSC-derived exosomes can be stored without toxic cryopreservatives for prolonged periods while maintaining their potency, and they can be economically mass-produced under controlled laboratory conditions.43 Specifically, ADSC exosomes have the merit of being one of the most affordable and abundant stem cell sources owing to their less painful collection approach.44 In the current study, we found that ADSC exosomes suppressed the inflammatory reaction induced by psoriasis serum exosomes by regulating oxidative stress and autophagy in recipient keratinocytes. Therefore, our findings suggest that ADSC exosomes hold promising therapeutic potential for the treatment of psoriasis.
In previous studies on psoriasis treatment, human umbilical cord mesenchymal stem cell (MSC) - derived exosomes were administered through subcutaneous injection or as an oil-in-water emulsion of MSC exosomes in a psoriasis mice model induced by imiquimod. Building on this prior research, the application of exosomes through subcutaneous injection or topical administration could be explored for treating the psoriatic skin lesions. Topical application of exosomes may offer advantages over oral or intravenous administration, such as bypassing issues associated with first-pass metabolism, resulting in higher bioavailability at the target site. Nevertheless, further investigations through in vivo studies or clinical trials are needed to determine the most effective application method for exosomes.
This study had the limitation of in vitro studies using HaCaT cells. To gain a more comprehensive understanding of the impact of exosomes on the pathogenesis of psoriasis, additional mechanistic analyses are necessary in future studies.
Conclusion
This study showed that psoriasis serum-derived exosomes modified keratinocytes by upregulating proinflammatory cytokines, inducing oxidative stress, and impairing autophagy. ADSC exosomes reversed the effects of psoriasis serum-derived exosomes on keratinocytes, restoring normal levels of proinflammatory cytokines, redox status, and autophagy. These findings contribute to our understanding of the intricate mechanism by which exosomes impact psoriasis pathogenesis and suggest the potential use of ADSC exosomes as a treatment option for psoriasis.
Abbreviations
ADSC, adipose tissue-derived stem cell; BSA, Bovine serum albumin; DMEM, Dulbecco’s Modified Eagle’s medium; EV, Extracellular vesicles; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSP90, Heat shock protein 90; MSC, Mesenchymal stem cell; NTA, Nanoparticle tracking analysis; qRT‒PCR, Quantitative reverse transcriptase-PCR; TFF, Tangential flow filtration.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
This research fulfills the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the Institutional Review Board at Hallym University Kangnam Sacred Heart Hospital (IRB no. 2022-03-020). Patients were fully informed about the study and asked for their consent before participating in the study.
Consent for Publication
The authors confirm that all individuals involved in this publication have provided their consent for the publication of any images, videos, recordings, or other materials, and have been presented with the contents of the article to be published.
Acknowledgments
This study was supported by the 2020 Dr. G grant from Korean dermatologic association.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chhabra S, Dogra S, Sharma K, Raychaudhuri SK, Raychaudhuri SP. Recent update on immunopathogenesis of psoriasis. Indian J Dermatol. 2022;67(4):360–373. doi:10.4103/ijd.ijd_569_22
2. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi:10.1016/j.jaad.2016.07.064
3. Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–708. doi:10.1016/j.jaad.2004.04.014
4. Plenkowska J, Gabig-Ciminska M, Mozolewski P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(17):6206.
5. Arthur JS, Darragh J. Signaling downstream of p38 in psoriasis. J Invest Dermatol. 2006;126(8):1689–1691. doi:10.1038/sj.jid.5700280
6. Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-kappaB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69(2):89–94. doi:10.1016/j.jdermsci.2012.11.002
7. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. doi:10.15252/embj.2021108863
8. Klapan K, Frangez Z, Markov N, Yousefi S, Simon D, Simon HU. Evidence for lysosomal dysfunction within the epidermis in psoriasis and atopic dermatitis. J Invest Dermatol. 2021;141(12):2838–2848 e4. doi:10.1016/j.jid.2021.05.016
9. Orciani M, Campanati A, Salvolini E, et al. The mesenchymal stem cell profile in psoriasis. Br J Dermatol. 2011;165(3):585–592. doi:10.1111/j.1365-2133.2011.10438.x
10. Campanati A, Orciani M, Consales V, et al. Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1-Th17/Th2 imbalance of psoriasis. Arch Dermatol Res. 2014;306(10):915–920. doi:10.1007/s00403-014-1493-3
11. Campanati A, Orciani M, Lazzarini R, et al. TNF-α inhibitors reduce the pathological Th1 -Th17 /Th2 imbalance in cutaneous mesenchymal stem cells of psoriasis patients. Exp Dermatol. 2017;26(4):319–324. doi:10.1111/exd.13139
12. Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi:10.1016/j.semcancer.2014.04.009
13. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi:10.1038/nri3622
14. Fruhbeis C, Frohlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi:10.1371/journal.pbio.1001604
15. Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. doi:10.4161/cc.8.13.8988
16. Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. doi:10.3389/fphys.2012.00124
17. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi:10.1016/j.jaci.2017.07.004
18. Bellinato F, Gisondi P, Girolomoni G. Latest advances for the treatment of chronic plaque psoriasis with biologics and oral small molecules. Biologics. 2021;15:247–253. doi:10.2147/BTT.S290309
19. Wu JJ, Kearns DG, Lin TC, et al. Characterization of non-responders to interleukin-17 inhibitors in moderate to severe psoriasis patients enrolled in the Corrona((R)) Psoriasis Registry. J Eur Acad Dermatol Venereol. 2021;35(8):e531–e533. doi:10.1111/jdv.17270
20. FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):59. doi:10.1038/s41572-021-00293-y
21. Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19(12):1885–1893. doi:10.1089/scd.2010.0093
22. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi:10.1182/blood-2007-02-069716
23. Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi:10.1016/j.stem.2018.05.004
24. Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129–137. doi:10.1016/j.scr.2008.02.002
25. Witwer KW, Van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. doi:10.1080/20013078.2019.1609206
26. Shao S, Fang H, Zhang J, et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J. 2019;33(6):6813–6828. doi:10.1096/fj.201802090RR
27. Jiang M, Fang H, Shao S, et al. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019;33(12):13241–13253. doi:10.1096/fj.201900642R
28. Lv J, Zhou D, Wang Y, et al. Effects of luteolin on treatment of psoriasis by repressing HSP90. Int Immunopharmacol. 2020;79:106070. doi:10.1016/j.intimp.2019.106070
29. Cheung KL, Jarrett R, Subramaniam S, et al. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213(11):2399–2412. doi:10.1084/jem.20160258
30. Asefi M, Vaisi-Raygani A, Khodarahmi R, et al. Methylentetrahydrofolatereductase (rs1801133) polymorphism and psoriasis: contribution to oxidative stress, lipid peroxidation and correlation with vascular adhesion protein 1, preliminary report. J Eur Acad Dermatol Venereol. 2014;28(9):1192–1198. doi:10.1111/jdv.12262
31. Guarneri F, Sapienza D, Papaianni V, et al. Association between genetic polymorphisms of glutathione S-transferase M1/T1 and psoriasis in a population from the area of the strict of messina (Southern Italy). Free Radic Res. 2020;54(1):57–63. doi:10.1080/10715762.2019.1698738
32. Husni ME, Wilson Tang WH, Lucke M, Chandrasekharan UM, Brennan DM, Hazen SL. Correlation of high-density lipoprotein-associated paraoxonase 1 activity with systemic inflammation, disease activity, and cardiovascular risk factors in psoriatic disease. Arthritis Rheumatol. 2018;70(8):1240–1250. doi:10.1002/art.40499
33. El-Rifaie AA, Sabry D, Doss RW, Kamal MA, Abd El Hassib DM. Heme oxygenase and iron status in exosomes of psoriasis patients. Arch Dermatol Res. 2018;310(8):651–656. doi:10.1007/s00403-018-1852-6
34. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi:10.1038/nature06639
35. Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(7):651–662. doi:10.1056/NEJMra1205406
36. Douroudis K, Kingo K, Traks T, et al. Polymorphisms in the ATG16L1 gene are associated with psoriasis vulgaris. Acta Derm Venereol. 2012;92(1):85–87. doi:10.2340/00015555-1183
37. Lee HM, Shin DM, Yuk JM, et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186(2):1248–1258. doi:10.4049/jimmunol.1001954
38. Zhang Y, Yan J, Li Z, Zheng J, Sun Q. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate psoriasis-like skin inflammation. J Interferon Cytokine Res. 2022;42(1):8–18. doi:10.1089/jir.2021.0146
39. Zhang B, Lai RC, Sim WK, Choo ABH, Lane EB, Lim SK. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int J Mol Sci. 2021;22(2). doi:10.3390/ijms22020720
40. Shen K, Jia Y, Wang X, et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021;165:54–66. doi:10.1016/j.freeradbiomed.2021.01.023
41. Xu P, Xin Y, Zhang Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11(1):264. doi:10.1186/s13287-020-01777-6
42. Zhang W, Liu R, Chen Y, Wang M, Du J. Crosstalk between oxidative stress and exosomes. Oxid Med Cell Longev. 2022;2022:3553617. doi:10.1155/2022/3553617
43. Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi:10.1038/s41409-019-0616-z
44. Mazini L, Rochette L, Amine M, Malka G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci. 2019;20(10). doi:10.3390/ijms20102523
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.