Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Adherence to Treatment Recommendations for Chronic Obstructive Pulmonary Disease - Results from the Swedish National Airway Register
Authors Larsson K, Ekberg-Jansson A, Stridsman C , Hanno M, Vanfleteren LEGW
Received 15 January 2021
Accepted for publication 11 March 2021
Published 6 April 2021 Volume 2021:16 Pages 909—918
DOI https://doi.org/10.2147/COPD.S300299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Kjell Larsson,1 Ann Ekberg-Jansson,2 Caroline Stridsman,3 Malin Hanno,4 Lowie EGW Vanfleteren5,6
1Integrative Toxicology, National Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden; 2Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 3Department of Public Health and Clinical Medicine, Division of Medicine, Umeå University, Umeå, Sweden; 4Boehringer Ingelheim AB, Stockholm, Sweden; 5COPD Center, Department of Respiratory Medicine and Allergology, Sahlgrenska University Hospital, Gothenburg, 413 45, Sweden; 6Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, 413 45, Sweden
Correspondence: Kjell Larsson
Integrative Toxicology, National Institute of Environmental Medicine, IMM, Karolinska Institutet, Stockholm, SE-171 77 Tel +46 70 582 07 63
Email [email protected]
Introduction: Swedish guidelines adhere to the international GOLD document regarding management of chronic obstructive pulmonary disease (COPD). Based on data from the Swedish National Airway Register (SNAR) the aim was to evaluate adherence to guidelines of pharmacological treatment of COPD in Swedish primary and secondary care.
Methods: During a period of 18 months, data on symptoms (CAT, mMRC), lung function, exacerbation history and pharmacological treatment from 15,595 COPD patients from 853 primary care and 125 secondary care clinics were collected from SNAR. Patients with a co-diagnosis of asthma were excluded. Patients were divided into four treatment groups: no pharmacological treatment, short-acting bronchodilators alone, long-acting bronchodilators alone and ICS alone or in combination with bronchodilators.
Results: Of the patients, 29% were in GOLD group A, 58% in group B, 2% in group C and 11% in group D. CAT score was ≥ 10 and mMRC score was below 2 in 30.9% of the patients and mMRC score was ≥ 2 and CAT score < 10 in 4.2% of the patients. In 61.4% of the patients, no exacerbation was registered during the last year. Long-acting bronchodilators were prescribed for 78% and ICS for 46% of all patients. In groups A, B, C and D, respectively, 21%, 11%, 11% and 5% did not receive any inhaler therapy; 67%, 81%, 81% and 90% received long-acting bronchodilators; 33%, 46%, 55% and 71% received any ICS containing therapy and 19%, 34%, 39% and 61% received triple therapy.
Discussion: Data from the SNAR indicate that only a minority of COPD patients were untreated. There was a liberal use of ICS containing drug combinations in subjects who do not have an indication for ICS. A considerable proportion of subjects at high risk of exacerbations did not receive ICS treatment.
Keywords: chronic obstructive pulmonary disease, COPD, glucocorticoids, registry
Introduction
The current Swedish treatment recommendations for chronic obstructive pulmonary disease (COPD) were most recently updated in 2015 1 and are mainly based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) from 2011.2 GOLD recommends classifying patients with COPD in four groups (group A, B, C and D). These four groups were based on a combination of airflow obstruction (FEV1), respiratory symptoms as assessed by the COPD assessment test (CAT) and/or Medical Research Council dyspnoea scale (mMRC) and the risk of exacerbations as assessed by exacerbation rate in the last 12 months. In the GOLD document from 2017 3 lung function was deleted from the A-B-C-D classification which later on was based on symptoms and exacerbation assessed by exacerbation history after lung function having been measured for confirmation of the COPD diagnosis. Group B and D are characterized by a higher degree of symptoms compared to A and C, while group C and D are characterized by a higher risk of exacerbations compared to groups A and B. Today the clinical practice in Sweden is mainly adapted to the GOLD 2017 classification and patient characterisation for treatment decisions is mostly based on the severity of symptoms and risk of exacerbation defined by exacerbation history.
In both the Swedish guidelines from the Swedish Medical Products Agency in 2009 and 2015 as well as in the GOLD document from 2011 and onwards the position of bronchodilators and inhaled steroids (ICS) in COPD treatment has been clearly defined. Maintenance treatment with bronchodilators, ie, long-acting antimuscarinic drug (LAMA) and/or long-acting β2-agonists (LABA) is recommended as first-line therapy in patients with symptoms, in particular dyspnoea. According to current recommendations, treatment should be commenced with either LAMA or LABA and intensified to combination dual therapy in case of insufficient symptom control. It has been demonstrated that initial treatment with dual bronchodilation yields a more favourable outcome that commencing with either bronchodilator alone.4 Inhaled steroids are recommended for prevention of exacerbations and are thus mainly indicated for COPD patients in GOLD groups C and D. Inhaled steroids must always be prescribed in combination with LABA and are rarely indicated for COPD patients in group A and have not been very frequently recommended for patients in GOLD group B. It has been shown that the adherence to these recommendations is poor and an overprescription of ICS to patients with COPD with infrequent exacerbations has been reported.5
The Swedish National Airway Register (SNAR) held in November 2019 data for more than 80,000 patients with COPD. In the present study, data were collected from the register for a period of 18 months, from June 2018 through November 2019, including 44,512 patients visiting primary care or hospitals. Based on data from the SNAR, the aim of the study was to explore the adherence to national and international treatment recommendations in Swedish primary and secondary care. In particular, the aim was to find out how bronchodilators are used in COPD and to what extent ICS are used in the GOLD A, B, C and D groups.
Patients and Methods
Data Collection
Data were collected from the SNAR which was established in 2013 and includes data from patients with a physician-diagnosed asthma and COPD. In 2019, 1000 clinics in Sweden were linked to SNAR, which includes 853 primary care clinics, 125 secondary care clinics and 22 inpatient wards. Currently, more than 80% of the data are directly transferred from the patients’ medical record files to the register and about 20% are manually entered. Data entered into the register include demography, lung function, exacerbation history, CAT score, mMRC dyspnoea scale score and pharmacological treatment.6
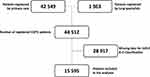
Since 2013, 80,372 patients with COPD have been registered in SNAR. Data from the SNAR were collected between June 1, 2018 and November 30, 2019. After having excluded patients with a concomitant asthma diagnosis data from medical records of 44,512 patients with a COPD diagnosis were obtained whereof 42,549 patients were registered by secondary care and 1963 by specialist care. Data were collected as the latest entry for each patient during the time period from June 1, 2018 to November 30, 2019 (Figure 1).
Patients and Treatments
All patients with a diagnosis of COPD, records of exacerbation history, and CAT score and/or mMRC-score were extracted and, based on that, patients were assigned to group A, B, C or D according to the GOLD classification. To be classified as GOLD group B or D a CAT score ≥10 and/or an mMRC score ≥2 was required. The cut-off score for high exacerbation risk was ≥2 exacerbations or one exacerbation leading to hospitalization during the previous year. One (not hospitalized) or zero exacerbation previous year constituted low risk. An empty record for exacerbations/hospitalisations in the registry implied a null value.
Patients were assigned into four main treatment groups out of which subgroups based on monotherapy or combination of drugs were defined:
No pharmacological treatment
Only short-acting bronchodilators (short-acting beta-agonists, SABA, short-acting antimuscarinics, SAMA or both SABA + SAMA)
Maintenance treatment with long-acting bronchodilators (long-acting beta-agonists, LABA, long-acting antimuscarinics, LAMA or both LABA and LAMA) without ICS
Maintenance treatment with ICS (ICS alone, ICS + LABA, ICS + LAMA, ICS + LABA + LAMA)
Patients with no record of treatment was included in the “No treatment” group. Combinations of drugs indicate both fixed combinations and the different drugs inhaled from separate inhalers. A patient was counted only once for his/her treatment. Hence, a patient treated with, eg, a combination of ICS and LABA was counted once for ICS/LABA and not for ICS or LABA alone and a patient treated with LAMA + LABA was counted once for LAMA/LABA and not for LAMA or LABA alone. Five of the subgroups (“Any SABD”, “Any LA”, “Any LA No ICS”, “Any ICS”, “No ICS” in Table 3) were based on calculations of patient number from different groups.
Data accessed from SNAR is not freely available. For data extraction, researchers need a permission by a national ethical board and by the Centre of Registers in Västra Götaland, Sweden, which supports the development of national quality registers. Thus, the current study was approved by the Swedish Ethical Review Agency, Dnr 2019–04915, and data extraction and statistical processing was conducted by Centre of Registers Västra Götaland.
Statistical Analyses
Data were analysed and presented using descriptive statistics. Data are presented as mean values and standard deviation (SD). Association between ICS use and different outcomes in GOLD groups A and B was analysed by means of logistic regression and odds ratios (OR) and 95% confidence interval are given. Data extraction and statistical processing were conducted by Centre of Registers Västra Götaland, Sweden, which supports the development of national quality registers. SAS software 9.4 (SAS Institute Inc, Cary, NC, USA) was used for statistical analyses.
Results
Patients
Among the 44,512 registered patients with COPD during the study period, data on symptoms and/or exacerbation classification according to GOLD A–D groups were missing in 28,917 patients leaving 15,595 patients, 8380 patients directly transferred, and 7215 patients manually transferred, for further analyses.
Patient characteristics are given in Table 1. Of the 15,595 included COPD patients, 4481 (29%) was classified as GOLD group A, 9095 (58%) as GOLD group B, 314 (2%) as GOLD group C and 1705 (11%) as GOLD group D. Mean age (SD) was 72 (8.6) years in the total population and was similar in the four groups. Also, gender distribution, body mass index (BMI) and smoking habits were similar in the GOLD A, B, C and D groups. Lung function assessed by FEV1 in percent of predicted value was 59% in the whole population, 64% in group A, 57% in group B, 62% in group C and 47% of predicted value in group D.
 |
Table 1 Patient Characteristics |
In GOLD group A, 75.4% of the patients and in GOLD group B, 67.7% of the patients had no exacerbations registered during the previous year. In groups C and D, less than 10% of the patients had <2 exacerbations registered during the year prior to inclusion. Mean (SD) CAT score of the whole study population was 13 (7.0); 7 (2.5) in group A, 15 (6.0) in group B, 7 (2.4) in group C, and 19 (7.0) in group D (Table 1).
Of the 15,595 patients CAT scores (but not mMRC) were available in 4744 patients and mMRC (but not CAT scores) were available in 492 patients leaving 10,359 patients with both CAT and mMRC scores. CAT ≥10 and mMRC ≥2 was observed in 4237 (40.9%) patients and CAT ≥10 and mMRC <2 was observed in 3202 (30.9%) of the patients. In 435 (4.2%) patients CAT score was below 10 and mMRC ≥2 and in 2485 (24.0%) of the patients CAT score was below 10 and mMRC below 2 (Table 2). Hence, 435 (14%) of all the 2920 patients with a CAT score below 10 had high mMRC while 3252 (56%) of all the 5687 patients with a CAT-score ≥f 10 had low mMRC (<2) scores (Table 2).
 |
Table 2 Outcomes in 10,539 COPD Patients in Whom Both CAT Scores and mMRC Scores Were Available |
Pharmacological Treatment
Results of the pharmacological treatment in the different groups according to GOLD (A-D) are given in Table 3 and Figure 2.
 |
Table 3 Treatment of 15,595 COPD Patients According to the GOLD A-B-C-D Classification |
GOLD Group A
In GOLD group A, 21% of the patients did not receive any pharmacological treatment and 10% were treated with short-acting bronchodilator (SABD) alone, mostly a beta-agonist. Two-thirds (67%) of the patients in GOLD group A were treated with long-acting bronchodilators (LABA and LAMA alone or in combination) with or without combination with ICS and 36.2% had LAMA and/or LABA without ICS as maintenance therapy. One-third (33%) was treated with ICS alone or in combination with bronchodilators. The proportion of patients with ICS alone was less than 3%. One out of five patients (19.4%) in group A was on triple therapy (LABA + LAMA + ICS).
GOLD Group B
In group B, 11% of the patients were not prescribed any pharmacological treatment for COPD. More than four out of five patients (81%) in GOLD group B were treated with long-acting bronchodilators as maintenance therapy and 36.7% were prescribed long-acting bronchodilator therapy without concurrent ICS. Almost half of the patients (46%) were treated with ICS. ICS-monotherapy was less than 2%. More than one-third (34%) of the patients in group B were prescribed triple therapy (LABA + LAMA + ICS).
GOLD Group C
Group C was small (2% of the whole group) and 11% did not receive any pharmacological treatment. Long-acting bronchodilators were prescribed as maintenance therapy to 81% of the C group patients, in 29% of the patients without combination with ICS. More than half (55%) used ICS but only 1% as monotherapy. In the C group, 39% of the patients were prescribed triple therapy (LABA + LAMA + ICS).
GOLD Group D
In the D group, 5% were not prescribed any pharmacological treatment and 4% had only short-acting bronchodilators. In 90% of the patients, long-acting bronchodilators were prescribed, and in 19.9% without combination with ICS. More than two-thirds of the patients (71%) in group D were treated with ICS and 61% were prescribed triple therapy (LAMA + LABA + ICS).
Prescriptions of Inhaled Steroids in GOLD Groups A and B
To find out what defines ICS treatment in GOLD groups A and B (in which ICS is not indicated according to Swedish recommendations) a multiple logistic regression including gender, age, FEV1, CAT score, and exacerbations rate was conducted. The age distribution of ICS prescription was similar in GOLD groups A and B, whereas females were overrepresented in both groups A and B. Age or gender did not have an influence on prescription of ICS, neither in GOLD group A nor in GOLD group B (Table 4).
Lung function as assessed by FEV1 had a slight and similar influence on ICS prescription in GOLD group A [OR (95% CI) 1.02 (1.01–1.02); p<0.001] and GOLD group B [OR (95% CI) 1.02 (1.01–1.02); p<0.001]. Also, CAT score had a slight influence on ICS prescription, OR (95% CI) 0.96 (0.93–0.99); p=0.01 in group A and OR (95% CI) 0.97 (0.95–0.98); p<0.0001 in group B (Table 4).
In GOLD group A 79.5% and in GOLD group B 73.7% of the patients had no exacerbations during the last year. History of exacerbation had a strong influence on prescription of ICS in GOLD groups A and B; OR (95% CI) 0.61 (0.51–0.73); p<0.0001 and OR (95% CI) 0.63 (0.56–0.71); p<0.0001, respectively (Table 4).
Discussion
Based on medical records of almost 16,000 patients with COPD, registered in The Swedish National Airway Register (SNAR) during 18 months in 2018–2019 it was found that almost 9 out of 10 COPD patients belonged to GOLD group A or B. From the present data, it seems clear that the recommendations of pharmacological COPD treatment are not fully implemented according to current international and Swedish guidelines. There was an overprescription of inhaled steroids as approximately one-third of the patients in GOLD group A and almost half of the patients in group B were prescribed ICS. In addition, one out of five patients in group A and one out of three patients in group B were prescribed triple therapy, ie, a combination of LABA, LAMA and ICS. There were also indications of under prescription as 1 patient out of 10 in group D was prescribed only short-acting bronchodilators or no pharmacological treatment at all. Long-acting bronchodilators were commonly used as maintenance therapy and were prescribed to four out of five patients.
One of the aims of the study was to explore prescription patterns of ICS in COPD. Therefore, patients with a reported concomitant asthma diagnosis in SNAR were excluded as COPD patients who also have asthma most likely are treated with ICS because of their asthma. In early studies, it could not be demonstrated that ICS alone had a preventive effect on exacerbations in COPD7,8 but in more recent studies, it has been demonstrated that ICS in combinations with LABA do prevent exacerbations.9–12 Guidelines recommend ICS in COPD for the prevention of future exacerbations and this exacerbation risk is defined by the history of number and severity of exacerbations in the last year. It is recommended not to treat subjects with ICS if they are classified as having a low risk of exacerbation and thus belong to group A or B.
The combination of ICS and LABA is today recommended as first-line therapy in Sweden when considering ICS treatment in COPD. According to Swedish guidelines, the use of ICS alone in COPD must be avoided.1 The adherence to the recommendations on this point was found to be high in Sweden as only 2% of the patients who took ICS were prescribed ICS alone. In a British study, 63% of the COPD patients in GOLD groups A and B were treated with ICS.10 In that study, a concurrent asthma diagnosis and exacerbations were found to be strong predictors of ICS treatment. In a recent Swedish study, ICS was commonly prescribed to patients with mild or moderate COPD.5 In that study, in which concurrent asthma was not excluded, 46% of the patients in GOLD stage 1 or 2, ie, patients with an FEV1 above 50% of predicted value, were prescribed ICS alone or on combination with other drugs. These findings are likely similar to those of the present study in which lung function measurements indicated that most of the patients had a mild or moderate disease, mean FEV1 was 59% of predicted value.
Despite a low exacerbation rate in the present study, ICS was prescribed to almost half (45.5%) of all patients. In a study focused on withdrawal of ICS in COPD patients the International Primary Care Respiratory Group (IPCRG) algorithm for ICS withdrawal was used and it was found that there was a possible indication for stopping steroid treatment in 55% of the patients who were on steroid treatment. They also found that ICS was indicated in 18% of the patients who were not treated with ICS.13 In a Swedish study, it was demonstrated that approximately 30% of the GOLD A patients and 50% of the GOLD B patients were treated with ICS in combination with a bronchodilator.14 In another study from the UK, it was reported that 75% of the COPD patients with FEV1 above 50% of predicted value were treated with ICS.15 Similar results were found in an Italian study where 45% of the patients in GOLD groups A and B were treated with ICS16 and in a Swiss study showing that between 30% and 40% of the patients in GOLD groups A and B were treated with ICS.17 Hence, the present results seem to corroborate previous results demonstrating an overprescription of ICS in COPD.
In Sweden prescription of triple therapy has been increased substantially during recent years and was, in 2014, the most commonly prescribed treatment for COPD patients in all GOLD groups except for GOLD group A.14 In an observational study from the UK it was shown that one out of 4 COPD patients were prescribed triple therapy within 1 year from the COPD diagnosis irrespective of group classification.18 It thus seems that COPD patients rather fast end up in triple therapy which often may be indicated but, in many cases, reflects an overprescription of ICS. To explore factors that may be of importance for ICS prescription in GOLD groups A and B, ie, in the groups in which ICS is not indicated according to current guidelines, specific analyses (multiple logistic regression) were conducted. Age and gender did not influence ICS prescription whereas lower lung function and higher CAT score had a minor, but significant, influence on the ICS prescription. Exacerbation history, however, had a stronger influence on ICS prescriptions. From these findings, it may be concluded that, when ICS are prescribed outside general recommendations, this may be based on clinical outcomes such as lung function, symptoms and exacerbations history.
According to current guidelines, long-acting bronchodilators are the first choice of pharmacological treatment in COPD.1,3 There are also several studies supporting treatment with a combination of LAMA and LABA in favour of either LAMA or LABA alone4,19–21. In the present study long-acting bronchodilators were prescribed to almost 80% of the patients, 2 out of 3 in GOLD group A and 9 out of 10 in GOLD group D. Long-acting bronchodilators thus seem to be frequently used as basic therapy for COPD in Sweden. The benefit of dual bronchodilatation also seems to have been received well and half of the patients in GOLD groups B, C and D, who were not on ICS were treated with both LAMA and LABA. In group A, two out of five patients who were not treated with ICS were prescribed dual bronchodilatation. It should, however, be noted that a limited number of patients in GOLD group C (11.5%) and D (5.4%) did not receive any pharmacological treatment. The reason for this is not clear but the finding indicates that there may still be physicians who do not follow current guidelines for COPD treatment.
Both CAT and mMRC was available in 10,539 patients. In two-thirds of the patients there was a conformity between CAT and mMRC, ie, CAT ≥10 and mMRC was ≥2 or CAT <10 and mMRC <2. However, in one-third of the patients, there was a discrepancy between CAT and mMRC where CAT was the more sensitive measure to reveal symptoms. Hence, a minority of the patients (4%) had a high mMRC score while CAT score was low whereas, on the other hand, a considerable number of patients (31%) had a high CAT score while mMRC score was low. The mMRC scale only measures dyspnoea whereas CAT also reveals other symptoms such as cough, phlegm, sleep disturbance, limited activities and energy. Thus, CAT gives a more holistic picture than does mMRC, and patients who do not experience dyspnoea but other symptoms as their major problem will be captured by using CAT and missed by mMRC. This is in agreement with a recent study in which it was demonstrated that a substantial number of COPD patients report other than respiratory symptoms as their main problems.22
During recent years, the position of ICS in the treatment of COPD has been debated. There are data indicating that a combination of bronchodilators (LAMA + LABA) may be more favourable in preventing exacerbations than the combination of ICS and LABA23 and there are studies showing that triple therapy prevents exacerbations more effectively than do bronchodilators.24,25 It is important to consider a concomitant asthma diagnosis or a history of asthma when considering ICS in COPD as asthma increases the indication for ICS treatment. It has also become clear that the levels of circulating eosinophils predict ICS response in COPD patient with exacerbations. Therefore, the recommendation to use ICS in COPD patients suffering from (infrequent) exacerbations should also be based on a possible concurrent asthma and circulating eosinophils exceeding 0.3 × 109 cells/L.26–28 As we do not have data on blood eosinophil count, we do not know whether or not the decision to prescribe ICS could have been guided by blood eosinophils count. This is, however, not likely as the association between blood eosinophilia and response to steroids was not (and is still not) generally recognized in primary care at the time of the study. It is likely that assessment of blood eosinophilia will be included in the SNAR within a not far distant future.
There are limitations to this study. Data required for group classification were missing in a high number of patients which may have influenced the results. The patients had a mild disease and the exacerbations rate was low with only 13% of the patients in groups C and D. One reason for this may be that the coverage of the SNAR is more complete in primary care, where the patients with mild and moderate disease are found. A major strength, however, is the high level of patients with COPD included from all counties in Sweden in 2018–2019, which gave an up-to-date information about adherence to treatment recommendations in COPD.
In conclusion, data from the SNAR indicate that only a minority of registered subjects were untreated or inappropriately treated with ICS monotherapy. However, there is a liberal use of ICS containing drug combinations and a drift towards the use of triple therapy even in subjects who do not have a clear indication of ICS treatment according to recommendations. On the other hand, also a considerable proportion of subjects at high risk of exacerbations did not receive ICS treatment. Quality registries like SNAR with continuous feedback to the prescribers might help to improve adherence to guidelines.
Acknowledgments
The authors would like to thank Caddie Zhou, Registercentrum, Västra Götaland, Sweden for performing statistical calculations.
Support Statement
This study was funded by Boehringer Ingelheim AB, Stockholm, Sweden.
Disclosure
Kjell Larsson has, during the last 5 years, on one or more occasion served in an advisory board, served as a speaker and/or participated in education activities arranged by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Sanofi, Novartis, Orion and Teva. Ann Ekberg-Jansson has during the last 5 years, participated in education activities arranged by Boehringer Ingelheim. Caroline Stridsman has during the last 5 years, served in an advisory board or as a speaker in activities arranged by AstraZeneca, Boehringer Ingelheim, and Novartis. Malin Hanno is employed by Boehringer Ingelheim AB, Sweden. Lowie EGW Vanfleteren has during the last 5 years received grants and personal fees from AstraZeneca and personal fees from GSK, Novartis, Boehringer Ingelheim, Menarini, Resmed, Chiesi, AGA Linde, Verona, and Pulmonx. The authors report no other conflicts of interest in this work.
References
1. Läkemedelsverket Medical Products Agency (Sweden). Läkemedelverkets expert panel. [Farmakologisk behandling av kroniskt obstruktiv lungsjukdom (KOL) - behandlingsrekommendationer]; 2015. Available from: https://www.lakemedelsverket.se/sv/behandling-och-forskrivning/behandlingsrekommendationer/sok-behandlingsrekommendationer/lakemedel-vid-kroniskt-obstruktiv-lungsjukdom-kol--behandlingsrekommendation. Accessed March 29, 2021
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2011. Available from: https://goldcopd.org/gold-reports/. Accessed March 29, 2021
3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2017. Available from: https://goldcopd.org/gold-reports/. Accessed March 29, 2021
4. Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. doi:10.1186/s12931-019-1193-9
5. Sulku J, Janson C, Melhus H, et al. A cross-sectional study assessing appropriateness of inhaled corticosteroid treatment in primary and secondary care patients with COPD in Sweden. Int J Chron Obstruct Pulmon Dis. 2019;14:2451–2460. doi:10.2147/COPD.S218747
6. Stridsman C, Konradsen J, Vanfleteren L, et al. The Swedish National Airway Register (SNAR) – development, design and utility to date. Eur Clin Respir J. 2020;7(1):1833412. doi:10.1080/20018525.2020.1833412.
7. Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340(25):1948–1953. doi:10.1056/NEJM199906243402503
8. Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353(9167):1819–1823. doi:10.1016/S0140-6736(98)10019-3
9. Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–919. doi:10.1183/09031936.03.00027003
10. Chalmers JD, Tebboth A, Gayle A, Ternouth A, Ramscar N. Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice. NPJ Prim Care Respir Med. 2017;27(1):43. doi:10.1038/s41533-017-0040-z
11. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7
12. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi:10.1183/09031936.03.00031402
13. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535–2548. doi:10.2147/COPD.S93321
14. Sundh J, Aberg J, Hasselgren M, et al. Factors influencing pharmacological treatment in COPD: a comparison of 2005 and 2014. Eur Clin Respir J. 2017;4(1):1409060. doi:10.1080/20018525.2017.1409060
15. Thomas M, Radwan A, Stonham C, Marshall S. COPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary care. COPD. 2014;11(3):300–309.
16. Maniscalco M, Martucci M, Fuschillo S, de Felice A, D’Anna SE, Cazzola M. A case scenario study on adherence to COPD GOLD recommendations by general practitioners in a rural area of southern Italy: the “progetto PADRE”. Respir Med. 2020;170:105985. doi:10.1016/j.rmed.2020.105985
17. Marmy JL, Diedrich JP, Cadus C, et al. Adherence to GOLD recommendations among Swiss pulmonologists and general practitioners. COPD. 2020;1–10.
18. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi:10.2147/COPD.S91694
19. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi:10.1016/S2213-2600(14)70065-7
20. Singh D, D’Urzo AD, Donohue JF, et al. An evaluation of single and dual long-acting bronchodilator therapy as effective interventions in maintenance therapy-naive patients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:2835–2848. doi:10.2147/COPD.S217710
21. Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi:10.1016/j.rmed.2015.08.002
22. Houben-Wilke S, Janssen DJA, Franssen FME, Vanfleteren L, Wouters EFM, Spruit MA. Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores. Health Qual Life Outcomes. 2018;16(1):205. doi:10.1186/s12955-018-1034-4
23. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
24. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
25. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
26. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi:10.1016/S2213-2600(18)30006-7
27. Harlander M, Barrecheguren M, Turel M, Miravitlles M. Should patients switched from D to B in the GOLD 2017 classification be discontinued from Inhaled Corticosteroids? COPD. 2017;14(5):465–468. doi:10.1080/15412555.2017.1342233
28. Vestbo J, Vogelmeier CF, Small M, Siddall J, Fogel R, Kostikas K. Inhaled corticosteroid use by exacerbations and eosinophils: a real-world COPD population. Int J Chron Obstruct Pulmon Dis. 2019;14:853–861. doi:10.2147/COPD.S189585
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.