Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Add-On Intermittent Theta Burst Stimulation Improves the Efficacy of First-Episode and Recurrent Major Depressive Disorder: Real-World Clinical Practice
Authors Li G , Lei L, Yang C , Liu Z, Zhang KR
Received 13 September 2022
Accepted for publication 6 December 2022
Published 13 January 2023 Volume 2023:19 Pages 109—116
DOI https://doi.org/10.2147/NDT.S388774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Gaizhi Li,1,2 Lei Lei,1 Chunxia Yang,1 Zhifen Liu,1 Ke-Rang Zhang1,2
1Department of Psychiatry, First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2Department of Psychiatry, First Clinical Medical College, Shanxi Medical University, Taiyuan, People’s Republic of China
Correspondence: Zhifen Liu; Ke-Rang Zhang, Department of Psychiatry, First Hospital of Shanxi Medical University, Shanxi Medical University, No. 85 Jiefang Nan Road, Taiyuan, Shanxi, 030001, People’s Republic of China, Tel +86 13703586547 ; +86 18135078183, Email [email protected]; [email protected]
Objective: Repetitive transcranial magnetic stimulation (rTMS) is an effective and evidence-based treatment for major depressive disorder (MDD). This retrospective study aimed to explore the efficacy of add-on iTBS treatment in MDD in real-world clinical practice.
Methods: One hundred and fifty-nine inpatients with MDD in a general hospital were included in this study. These patients were treated with at least 8 sessions of iTBS, in addition to antidepressants and supportive psychotherapy. Symptoms of depression and anxiety were assessed with the Hamilton Depression Rating Scale (HDRS) and the Hamilton Rating Scale for Anxiety (HAMA) at baseline and after 2– 4 weeks of treatment. The improvement degree of depressive and anxious symptoms was compared between the first-episode MDD (n=107) and recurrent MDD (n=52) groups.
Results: Depressive and anxious symptoms were reduced significantly after the add-on iTBS treatment; the response and remission rates in the first-episode MDD group were 55.14% and 28.97%, which were 63.46% and 28.85% for the recurrent MDD group, respectively (P> 0.05). The response rate and remission rate of anxiety in the first-episode MDD group was 64.13% and 57.45% for HAMA, and 66.67% and 62.50% for the recurrent MDD group (P> 0.05).
Conclusion: Our findings indicated that antidepressant and anti-anxiety efficacy of add-on iTBS treatment remains equivocal in real-world clinical practice, regardless of a first-episode depression diagnosis or recurrent depression.
Keywords: depression, anxious, add-on iTBS, real world, efficacy
Introduction
MDD (major depressive disorder) is a major leading cause of disability in the world and is also associated with personal, family and social burden. Antidepressants and cognitive behavioral therapy (CBT) are two primary treatments for MDD; however, only about 50% of patients with MDD benefit from these traditional treatments.1 Although conventional methods of increasing dose and using augmentation strategies will increase overall response rates, these trials require patients to endure prolonged episodes of depression.2 Failure to respond to treatment can increase suicide risk, contribute to worsening of medical co-morbidities, disability, cognitive impairment and death; thus, there is a clinical need for additional antidepressant treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation method increasingly used to treat psychiatric disorders as a new treatment, primarily depression. TMS was approved by Food and Drug Administration in 2007 as a therapy for MDD.3 rTMS is also mrecommended for treatment of MDD and as an add-on treatment of medicine for MDD, which is widely used due to its non-invasive technique.4–6
Previous studies reported the effectiveness and safety of rTMS for treatment of MDD; however, these studies were mostly randomized controlled trials comparing results with those of sham rTMS.7–9 Other studies also included meta‐analyses.10–12 Additionally, a few studies reported the efficacy of rTMS for the treatment of MDD in real-world practice. One such study, Carpenter et al reported TMS as an effective treatment for those unable to benefit from initial antidepressant medication.13 Connolly et al also reported that rTMS is effective and safe for acute and maintenance treatment of patients with MDD after 6 months follow-up.14 Dowling et al found that depressive and anxiety symptoms were significantly improved with 4-week rTMS treatment in an Australian private hospital setting.15 These observational clinical studies examined the effectiveness of rTMS by reporting remission rate and response rate. Few studies explored the mechanism of rTMS. As Zhao et al reported, 20 sessions of one month rTMS treatments for elderly TRD patients led to a significant reduction both in depressive symptoms and molecular levels (ie, plasma IL-1β, and tumor necrosis factor (TNF)-α) compared with the controls.16 However, other molecular studies of rTMS did not report similar findings. Li et al found that TBS works by modulating fronto-cingulate circuit in TRD rather than directly affecting the targeting DLPFC.17 The specific molecular mechanism of rTMS/iTBS is unclear.
TBS (theta-burst stimulation) is a novel rTMS pattern which induces more rapid and long-lasting effects on synaptic plasticity.18 Several randomized trials that targeted the left DLPFC with iTBS (intermittent TBS) and biTBS (left iTBS and right cTBS) have shown that their efficacy is superior to that of sham stimulation in patients with depression.19–21 The real-world study of iTBS showed superior results to rTMS.22 However, the effectiveness of add-on iTBS for first-episode and recurrent MDD in the real world is not clear.
We hypothesized that the add-on rTMS (iTBS) is equivocal regardless of MDD episodes. In this current study, we aimed to compare the efficacy of add-on rTMS (iTBS) for MDD, in addition to antidepressants and supportive psychotherapy for first-episode and recurrent MDD in real-world practice.
Methods
Participants
This is a retrospective study of patients hospitalized in inpatient facilities, diagnosed with MDD between Oct-1-2015 and July-1-2021. Participants diagnosed with MDD (first-episode and recurrent) using the ICD-10 were included within the department of psychiatry in a 3A grade hospital in Taiyuan city, Shanxi Province, China. The inclusion criteria consisted of: (1) age: between 18 and 65 years old; (2) Hamilton Depression Rating Scale (HDRS) score >14. The exclusion criteria consisted of: (1) any history of neurological diseases, other physical diseases and presence of comorbidities of other disorders; (2) any other mental disorders, eg, schizophrenia, schizoaffective disorder, substance use disorder, OCD, panic disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, Axis II personality disorders, or mental retardation; (3) pregnancy or breastfeeding. Axis II personality disorders were excluded by using Structured Clinical Interview for DSM-IV and an Axis II Personality Disorders (SCID-II) Interview.
Only patients judged to be appropriate for iTBS treatment were included in the study. Thirteen hundred and ninety-one patients diagnosed with MDD were included in this study; 671 of them were excluded due to coexisting treatment (including computerized cognitive remediation therapy, biofeedback therapy, modified electroconvulsive therapy); 473 patients were excluded due to the number of iTBS between 1 to 7; 79 patients were excluded due to lack of HDRS score; 9 participants were excluded due to hospital stay (less than 1 week or more than 4 weeks). Finally, 159 patients were treated with add-on iTBS, antidepressants and supportive psychotherapy. The flow diagram of the sample included in this study is shown Figure 1.
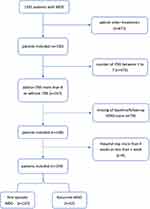 |
Figure 1 Participant Flow. |
This study was approved by the First Hospital of Shanxi Medical University Institutional Review Board and informed consent was obtained from all the participants prior to commencement of this study. This study complies with the Declaration of Helsinki.
iTBS Treatment
The iTBS was delivered by a trained medical technician utilizing a magnetic stimulator (MT-10, Beijing, China; Magstim-Rapid2, England; OSF-3/T, Wuhan, China) with a figure-eight coil. The patients were seated in a comfortable chair and received iTBS stimulation targeting the left DLPFC with 1200 pulses. The DLPFC was determined 5 cm anterior to the motor threshold location along a superior oblique plane, with a rotation point about the tip of the patient’s nose. The iTBS repeating burst consists of three repeating pulses (stimuli) delivered every 20 ms (50 Hz); bursts are repeating every 200 ms (5 Hz).23 On the first day, the resting motion threshold of patients was measured to determine the stimulation intensity (80–120% motor threshold, MT). Each week consisted of 5 sessions; every patient was treated with at least 8 sessions.
All the participants were given supportive psychotherapy routinely; the number of supportive psychotherapy sessions for all patients were recorded by the psychotherapist.
Clinical Assessment
HDRS
The 24-item Hamilton Depression Rating Scale (HDRS) was used to assess the severity of depressive symptoms, as assessed by the psychologist in our hospital. The HDRS scale is divided into seven structural parts: anxiety/somatization (items 10–12, 15, 17), cognitive disturbance (items 2, 3, 9, 19–21), psychomotor retardation (items 1, 7, 8, 14), sleep disturbance (items 4–6), weight loss (item 16), diurnal variation (item 18), and hopelessness (items 22–24).24
HAMA
The Hamilton Anxiety Rating Scale (HAMA) is a widely used 14-item clinician-administered rating tool, which was used to assess the severity of anxious symptoms. The HAMA scale is divided into two structural parts: psychic anxiety (items 1–6, 14) and somatic anxiety (items 7–13).25
Data Analysis
All the data were analyzed with SPSS Statistics for Windows (version 21.0; IBM, Armonk, NY). For the characteristics of the study sample, measurement data were described as means ± standard deviations (SD) and count data were described in number of cases (%).
The primary outcomes for the patients in this study were the response and remission rates at end point (two to four weeks) via HDRS and HAMA. The response was calculated as reduction of HDRS (or HAMA) ≥50% change and remission was less than 7.
Socio-demographics, clinical characteristics, HDRS and HAMA scores were compared using two independent sample T-tests and the chi-squared test. Changes in HDRS and HAMA scores at different time-points were analyzed by a paired T-test.
Results
Effectiveness of Add-On iTBS in All Participants
The demographic data of the participants is shown in Table 1. After 2 to 4 weeks of treatment, the HDRS remission rate of the add-on iTBS group was 28.93% and the response rate was 57.86%. In addition, the HAMA remission and response rates were 59.15% and 65%, respectively. See Table 1.
 |
Table 1 Demographic Data and Clinical Characteristics of MDD Group |
Socio-Demographic and Clinical Characteristics of First-Episode and Recurrent MDD Groups
Socio-demographic and clinical characteristics were compared between the two groups. Age, gender, educational years, age of onset, number of supportive psychotherapy sessions, dose of antidepressants (all antidepressants were converted to equivalent dose of fluoxetine) were not statistically significant between the two groups (P>0.05). The illness duration of the recurrent MDD group was significantly longer than the first-episode MDD group (P<0.001). More than half of the patients in this study had a HAMA score higher than 14 (65.04% in the first-episode MDD group and 70.58% in the recurrent MDD group; P=0.492). The HDRS total score and other structural factors, the HAMA total score and structural factors were not of great significance between the two groups (P>0.05). See Table 2.
 |
Table 2 Demographic Data and Clinical Characteristics of First-Episode and Recurrent MDD Groups |
Comparison of Add-On iTBS Efficacy in First-Episode and Recurrent MDD Groups
The HDRS response and remission rates for the first-episode MDD group were 55.14% and 28.97%, respectively, and 63.46% and 28.85%, respectively, for the recurrent MDD group (P>0.05). The response and remission rates of the first-episode MDD group were 64.13% and 57.45% for HAMA and 66.67% and 62.50%, respectively, for the recurrent MDD group (P>0.05). See Figure 2 and Table 3.
 |
Table 3 Comparison of Add-On iTBS Treatment After 2 to 4 Weeks of Treatment (First-Episode vs Recurrent MDD Groups) |
Discussion
The aim of the current study was to explore the acute treatment efficacy of add-on iTBS treatment for depressive and anxious symptoms of inpatients with MDD. The add-on iTBS group was given antidepressants, supportive psychotherapy and add-on iTBS, and compared between the first-episode and recurrent MDD patients. The illness duration of the recurrent MDD group was longer than the first-episode group, which is in accordance with previous studies. Both groups showed significant reduction in HDRS/HAMA scores. However, the difference in improvement of depressive and anxious symptoms between the two groups is not statistically significant (P>0.05).
Previous studies reported efficacy and safety of rTMS/iTBS for MDD.18,26 Few studies reported the safety and efficacy of iTBS for MDD in real-world clinical practice. Li et al reported that the response rate was 40% after 2 weeks of iTBS treatment for refractory MDD; Plewnia et al reported a higher response rate (80%) but only 5 patients with MDD received TBS treatment.17,20 The multi-center real-world study of iTBS by Blumberger et al22 showed 49% response rate and 32% remission rate. Connolly et al reported a remission rate of 35.3% and response rate of 41.2%. Dowling et al showed a remission rate of 28% and a response rate of 54% for rTMS treatment in the real world.14,15 Sackeim et al reported that response (58–83%) and remission (28–62%) rates were notably high across self-report and clinician-administered assessments.27 The remission rate of 28.93% and response rate of 57.86% of HDRS score in this real-world study appears to be similar with previous reports. Previous studies reported remission of 37.1% after 6 and more weeks of rTMS.13 The remission and response rates of depression in the two groups is comparable, which may be due to the short time follow-up.
Our findings observed that anxiety symptoms are also greatly improved after treatment. The HAMA response rate of 65% and remission rate of 59.15% is also in consistent with previous studies. The study of Clarke et al showed improvement in depression and no significant difference in remission rates between the depression group and depression comorbid anxiety disorders group after rTMS treatment.28 Zhang et al reported overall improvement of somatic anxiety of 52.29% at 2 weeks and 71.64% at 4 weeks; and overall improvement of psychic anxiety of 44.90% at 2 weeks and 65.55% at 4 weeks.29 Previous studies generally compare improvements with baseline or sham rTMS.
Consistent with previous reports about the efficacy of rTMS/iTBS in treatment-resistant MDD, this study observed improvements in depressive and anxious symptoms regardless of the episodes.
Limitations
There are several limitations in this naturalistic study that should be considered in the interpretation of the results. Firstly, the follow-up times in the current study vary from two to four weeks, and increasing the follow-up time may create more robust evidence regarding the long-term benefit of add-on iTBS. Secondly, the specific medication used for the patients in this study varied, but the dosage of the antidepressants was converted to equivalent of fluoxetine. Thirdly, all patients were accepting supportive psychotherapy; however, the number of supportive psychotherapy sessions was comparable between the two groups. All participants being recruited from one treatment center is also a limitation in terms of generalizing the results.
Conclusion
The potential for an adjunctive benefit of iTBS when used in combination with pharmacotherapy can be partly answered by the present report. In conclusion, our findings suggest the therapeutic equivalence of add-on iTBS for first-episode and recurrent MDD (two to four weeks) in real-world clinical practice. Further research into the long-term efficacy and mechanisms of iTBS may provide clues on how this technique may be harnessed for greater therapeutic potential.
Acknowledgments
We thank all the volunteers who participated in this study. This study is supported by National Natural Science Foundation of China (82001802), the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20200039).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Katon W, Unützer J, Russo J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety. 2010;27:19–26. doi:10.1002/da.20613
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi:10.1176/appi.ajp.163.1.28
3. Horvath JC, Mathews J, Demitrack MA, Pascual-Leone A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. J Vis Exp. 2010;45. doi:10.3791/2345
4. Hutton TM. The clinical application of transcranial magnetic stimulation. Psychiatr Ann. 2014;446:305–309. doi:10.3928/0485713-20140609-09
5. McClintock SM, Reti IM, Carpenter LL, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation rTMS in the treatment of depression. J Clin Psychiatry. 2018:79. doi:10.4088/JCP.16cs10905
6. Chinese Society of ECT & Neurostimulation. Chinese experts consensus on repetitive transcranial tagnetic stimulation. J Transl Med. 2018;71:4–9. Chinese.
7. Fitzgerald PB, Brown TL, Marston NA, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–1008. doi:10.1001/archpsyc.60.9.1002
8. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi:10.1001/archgenpsychiatry.2010.46
9. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi:10.1016/j.biopsych.2007.01.018
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation rTMS for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi:10.1017/S0033291713000512
11. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65–75. doi:10.1017/S0033291708003462
12. De Risio L, Borgi M, Pettorruso M, et al. Recovering from depression with repetitive transcranial magnetic stimulation rTMS: a systematic review and meta-analysis of preclinical studies. Transl Psychiatry. 2020;10(1):393. doi:10.1038/s41398-020-01055-2
13. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation TMS for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–596. doi:10.1002/da.21969
14. Connolly KR, Helmer A, Cristancho MA, Cristancho PO, Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73:e567–e573. doi:10.4088/JCP.11m07413
15. Dowling NL, Bonwick R, Dharwadkar NP, Ng CH. Repetitive transcranial magnetic stimulation for major depression: a naturalistic observational study in an Australian private hospital. Psychiatry Res. 2020;291:113275. doi:10.1016/j.psychres.2020.113275
16. Zhao X, Li Y, Tian Q, Zhu B, Zhao Z. Repetitive transcranial magnetic stimulation increases serum brain-derived neurotrophic factor and decreases interleukin-1β and tumor necrosis factor-α in elderly patients with refractory depression. J Int Med Res. 2019;47(5):1848–1855. doi:10.1177/0300060518817417
17. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(7):2088–2098. doi:10.1093/brain/awu109
18. Chou PH, Lu MK, Tsai CH, et al. Antidepressant efficacy and immune effects of bilateral theta burst stimulation monotherapy in major depression: a randomized, double-blind, sham-controlled study. Brain Behav Immun. 2020;88:144–150. doi:10.1016/j.bbi.2020.06.024
19. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16:57–65. doi:10.3109/15622975.2014.964768
20. Plewnia C, Pasqualetti P, Grosse S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219–223. doi:10.1016/j.jad.2013.12.025
21. Duprat R, Desmyter S, de Rudi R, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission? J Affect Disord. 2016;200:6–14. doi:10.1016/j.jad.2016.04.015
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–1692. doi:10.1016/S0140-6736(18)30295-2
23. Cárdenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schönfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22(4):294–306. doi:10.1007/s10548-009-0084-7
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi:10.1136/jnnp.23.1.56
25. Hamilton M. The assessment of anxiety states by rating. Br J Psychiatry. 1959;32:50–55. doi:10.1111/j.2044-8341.1959.tb00467.x
26. Avery DH, Isenberg KE, Sampson SM, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008;693:441–451. doi:10.4088/JCP.v69n0315
27. Clarke E, Clarke P, Gill S, et al. Efficacy of repetitive transcranial magnetic stimulation in the treatment of depression with comorbid anxiety disorders. J Affect Disord. 2019;252:435–439. doi:10.1016/j.jad.2019.03.085
28. Sackeim HA, Aaronson ST, Carpenter LL, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with transcranial magnetic stimulation. J Affect Disord. 2020;277:65–74. doi:10.1016/j.jad.2020.08.005
29. Zhang L, Zhu J, Zhang T, et al. Comparative efficacy of add-on rTMS in treating the somatic and psychic anxiety symptoms of depression comorbid with anxiety in adolescents, adults, and elderly patients-A real-world clinical application. J Affect Disord. 2020;276:305–311. doi:10.1016/j.jad.2020.05.151
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

