Back to Journals » Infection and Drug Resistance » Volume 16
Acute Pulmonary Actinomycosis Induced by Immunotherapy and Chemotherapy Used for SCLC Treatment: A Case Report and Literature Review
Authors Kuang J, Luo Z , Zhou F
Received 27 August 2023
Accepted for publication 21 November 2023
Published 11 December 2023 Volume 2023:16 Pages 7575—7580
DOI https://doi.org/10.2147/IDR.S429699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ju Kuang, Zhihai Luo, Fu Zhou
Department of Respiratory and Critical Care Medicine, Affiliated Banan Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Fu Zhou, Tel +86-17702395630, Email [email protected]
Abstract: A 66-year-old male patient diagnosed with small-cell lung cancer received chemotherapy and immunotherapy, resulting in successful tumor control. However, the patient subsequently experienced a fever and rapid progression of the pulmonary cavity. Despite sampling bronchoscopic bronchoalveolar lavage fluid for targeted next-generation sequencing (tNGS), the cause remained unidentified. Adding bronchoalveolar lavage fluid to sense metagenomic next-generation sequencing (mNGS) confirmed the infection caused by actinomycetes. The patient’s condition improved after active anti-infection treatment. This case was further analyzed and discussed through a comprehensive literature review, focusing on molecular microbiological diagnosis and treatment processes. The points outlined were as follows: the advancement of molecular microbiology has gradually reduced the challenges associated with diagnosing rare infectious diseases such as pulmonary actinomycosis. Additionally, in immunodeficient individuals, certain infectious diseases with a chronic course may exhibit acute and aggressive characteristics, which is of concern to all colleagues. Currently, tNGS and mNGS are widely employed in clinical settings as practical tools for diagnosing infectious diseases. Notably, these two methods are not substitutes for each other but complement each other.
Keywords: pulmonary actinomycosis, lung cancer, targeted next-generation sequencing, metagenomic next-generation sequencing
Introduction
Actinomycetes are normal parasites of the human oral cavity, dental caries, and tonsil crypts. Actinomycosis confirmed by pathological biopsy and pathogen metagene examination could be frequently observed in patients with compromised immunity. The disease mainly invades the oral cavity and neck; lung actinomycosis, accounting for 15% of actinobacterial infections is rare. Actinomycosis has a low incidence, with clinical and imaging manifestations similar to malignant tumors or chronic infectious diseases, such as lung abscess and tuberculosis; therefore, it is often misdiagnosed. Pulmonary actinomycosis is often considered a chronic infectious disease; however, this case is characterized by an extremely rare clinical acute course.
Clinical Data
A 66-year-old male patient was admitted to the hospital on September 20, 2022, having been diagnosed with lung cancer for half a year and complained of cough and fever for one day. Furthermore, on March 28, 2022, the patient participated in a randomized, double-blind, placebo-controlled, multicenter Phase III clinical study of recombinant whole-human anti-PD-L1 monoclonal antibody injection (ZKAB001) or placebo combined with carboplatin and etoposide for the first-line treatment of extensive-stage small cell lung cancer conducted by Chongqing Medical University.
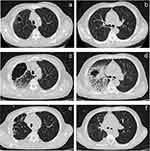
On 2022.4.2, 2022.4.27, 2022.5.18, and 2022.6.29, “ZKAB001 D1+ etoposide D1-D3+ carboplatin D1” was administered in the first five cycles of chemotherapy, and the fourth cycle of immunotherapy was administered alone due to bone marrow suppression. On 2022.7.22, 2022.8.10, and 2022.9.1, he continued the “ZKAB001 D1” single-drug immunotherapy regimen to complete a total of eight cycles of anti-tumor therapy. 2022-9-20 Chest + upper abdomen + lower abdomen + pelvic enhanced computed tomography (CT): 1. Soft tissue shadow of the lower lobe of the right lung, right hilum, mediastinum, and left adrenal mass, consistent with lung cancer with lymph node and adrenal metastases. 2. The anterior cavity of the upper lobe of the right lung, peripheral patches are newer than before, with the possibility of inflammation (Figure 1a and b). On the same day, the patient developed a cough, chest pain, chills, fever, and a temperature of up to 39.4 °C. After admission, the fever was mainly at night and in the early morning, and there was no obvious headache, muscle ache, or other discomfort.
Physical examination on admission revealed a body temperature of 39 °C, pulse of 85 times/min, breathing 20 times/min, blood pressure of 125/70 mmHg, and saturation of hemoglobin with oxygen (SPO2) of 97%. Further observations were as follows: the skin and mucous membranes of the whole body were not yellow, the superficial lymph nodes of the whole body were not enlarged, the lips were red, the oral mucosa was congested, and the tonsils were not swollen; weak breathing sounds, not heard of dry and moist rales; there was no uplift in the precardiac area, no obvious expansion of the cardiac dullness boundary, heart rate 89 beats/min, rhythmical, no murmur. The abdomen was flat and soft, without tenderness, rebound pain, and edema in both lower limbs.
Important auxiliary tests after admission: C-reactive protein 59.67 mg/L, blood routine, white blood cells 9.0*10^9/L, hemoglobin 112 g/L, platelets 179*10^9/L, neutrophil percentage 85.2%. Procalcitonin 1.20 ng/mL. Sputum smear: Gram staining, white blood cells >25; epithelial cells 10–25; positive Bacillus 1+ was found and no phagocytosis was observed. Positive cocci 1+ were detected without phagocytosis. No phagocytosis was observed in the negative Bacillus 1+. There were no obvious abnormalities in the blood, sputum, or sputum fungus cultures. Cytokeratin 19 fragment (CRF-211) 2.73 ng/mL, neuronal specific enolase (NSE) 28.07 ng/mL, gastrin-releasing peptide precursor (ProGRP) 1800 pg/mL, squamous cell-cell antigen (SCC) 0.59 ng/mL. D-dimer 1.05 mg/L. Carcinoma embryonic antigen (CEA), prostate-specific antigen (PSA), alpha-fetoprotein (AFP), markers of myocardial injury, blood glucose, renal function, coagulation, brain natriuretic peptide (BNP), liver function, renal function, anti-nuclear antibody spectrum, rheumatoid, and anti-neutrophil cytoplasmic antibodies (ANCA) were not significantly abnormal.
After admission, the patient developed blood in sputum, grayish-yellow pus sputum, and right-sided chest pain. Piperacillin and tazobactam were given 4.5 g q8h anti-infection, followed by phenolsulfoethylamine to stop bleeding and bromhexine to expectorate phlegm. Four days later, the patient’s self-perception improved, but the intermittent fever persisted. 2022.9.24 Chest and abdominal CT: 1. Increased soft tissue density in the right hilum, multiple enlarged lymph nodes in the mediastinum, and thick-walled cavities in the upper lobe of the right lung were observed, suggesting enhanced scanning. 2. Right lung infection. 3. Aortic and coronary artery calcification, bilateral pleural effusion. 4. Left adrenal mass was considered for metastasis based on medical history. 5. Cholecystitis: liver cyst (Figure 1c and d). The patient demonstrated rapid imaging progress and an uncontrolled fever. On 2022.9.25, the antibiotics were adjusted to 1 g q6h+ levofloxacin 0.5 g qd+ oral voriconazole 200 mg qd combined with anti-infection treatment, and bronchoscopy was performed. There was obvious hyperemia in the right middle lobe bronchus, and a little white phlegm was observed in the right upper lobe bronchus. tNGS: human herpes virus (sequence number 20). As tNGS did not provide strong evidence, mNGS was immediately supplemented: Dental actinomyces (sequence number 22153), Oersenia gum (sequence number 3012), Bacillus difficile stage flight (sequence number 3349), Micromonas minuta (sequence number 2286), Pseudopropionibacterium propionate (sequence number 1261), Streptococcus constellations (sequence number 545), Propionibacterium acidogenes (sequence number 1588), Treponema lecithin (sequence number 111), Porphyromonas gingivalis (sequence number 111), and Human herpesvirus type 4 (sequence number 15). The tissue mass of the lavage fluid was shed with respiratory epithelial cells and inflammatory cells, and no tumor cells were found. Due to the positive penicillin skin test of the patient, the antibiotic regimen was ceftriaxone 2 g qd+ clarithromycin 600 mg q6h. The patient did not have a fever the following day, and the clinical symptoms continued to improve. After the regimen lasted for one month, the patient was discharged with oral administration of clindamycin and minocycline. 2022.10.14 follow-up chest CT: Compared with the previous film 2022.9.29: The volume of the sclerenchyma cavity in the upper lobe of the right lung was smaller than that before, and the surrounding inflammation was significantly reduced (Figure 1e and f).
Discussion
Characteristics of this case: The patient had a basis for lung cancer and had completed multiple courses of chemotherapy combined with immunotherapy, making him a typical immunosuppressive population. After onset, he presented with an acute course of disease and persistent high fever, and the lung lesions were extremely aggressive and tissue-destructive. This refreshes our previous understanding of actinomycosis as a chronic granulomatous inflammation. Actinomycetes are common symbiotic flora of the oropharynx, gastrointestinal tract, and urogenital tract and generally do not cause infection.1 Pulmonary actinomycosis may occur when there are risk factors, such as poor oropharyngeal hygiene, risk of aspiration, underlying lung disease, immunosuppression, and pre-existing local or distant actinomycotic infection.2 Actinomycetes can invade almost all organs of the body, including the intracranial, eyes, mouth, nose, pharynx, lungs, digestive tract, reproductive system, and bone joints, and pulmonary actinomycosis accounts for 15%. At present, the vast majority of lung actinomycosis is reported to have a chronic course, and the acute course of the disease is seldom reported. Although immunosuppression is a risk factor for developing pulmonary actinomycosis, it is seldom reported. Cell wall lipoproteins and peptidoglycans can cause pathological changes in the body, while actinobacteria can produce proteolytic enzymes leading to anatomical structural destruction, invading local and distal tissues, and producing the sinus tract and fistula.1 This is what caused the large hole in this case.
Clinical manifestations of pulmonary actinomycosis are usually non-specific, difficult to distinguish from other chronic or infectious lung diseases, and often misdiagnosed, especially lung cancer, with an initial correct diagnosis as low as 4%.3 Even in specialist clinics, the time between symptom onset and diagnosis is often more than six months.1 The main findings of chest CT scans include consolidation, mediastinal or hilar lymph node enlargement, cavity, ground-glass changes, pleural thickening, and effusion in 15–50% of patients, and empyema in 9–15% of pulmonary actinomycosis cases.4
Before the development of molecular etiology, pulmonary actinomycosis was diagnosed mainly by histological and microbiological examination of tissue samples, and the best clinical specimens for microbiological diagnosis were fine needle aspiration and tissue biopsy. The typical manifestation of actinomycetes culture is the appearance of “molar” or “breadcrumb-like” colonies in the broth medium after 3–7 days of culture. Specific staining with fluorescently coupled monoclonal antibodies can improve diagnostic accuracy. Only a very small number of cases can be diagnosed by culture because of insufficient culture time, use of antibiotics before culture, or multi-microbial growth. Typical histopathological manifestations include actinomycosis, suppurative granuloma, and sulfur granules. The abscess contained yellowish particles with a diameter of <1 mm. Microscopically, actinomyces were chrysanthemum-shaped, with dense centers composed of interwoven branched mycelia surrounded by long, radially eosinophilic mycelia, surrounded by a glial sheath at the end of the mycelia, bulked up in rod-like shape, and gram-positive. Epithelioid cells, macrophages, lymphocytes, and other inflammatory cells were found in the periphery of the granules surrounding the fibrous tissue. Silver hexamine and Schiff periodate staining were positive, and antacid staining was negative.5 However, due to the rarity of the disease, the lack of awareness in pathology and microbiology departments in most hospitals leads to a low rate of missed diagnoses.
Recently, molecular biology detection technology has developed rapidly, and most hospitals can rely on third-party experimental platforms to apply second-generation microbial sequencing, greatly improving the diagnostic positive rate of respiratory pathogenic microorganisms. The patient was initially identified using tNGS to identify the pathogen and was immediately confirmed using mNGS, a process that fully reflects the difference between mNGS and tNGS. Both mNGS and tNGS are pathogen detection methods based on high-throughput sequencing technology combined with other molecular detection techniques. mNGS enables unbiased testing of all pathogens in the sample (20,000–30,000 species); however, the technology is complex, challenging to interpret, expensive, and takes a long time. Studies have performed mNGS in 240 patients with suspected lung infections. The results depicted that the mNGS positivity rate was 88.30%, whereas the traditional pathogen detection method had a rate of 25.73%.6,7 tNGS detects pre-designed common respiratory pathogens (50–200 species), low abundance of pathogens with high sensitivity, reducing the analysis workload and host gene interference and price and shortening the detection time; however, tNGS cannot detect new or rare pathogens or undertake whole genome analysis of pathogens.6 Several studies have verified the pathogen detection performance of these two methods.2 The overall accuracy of the tNGS process was 65.6%, with a positive predictive value (PPA) of 45.9% and a negative (NPA) predictive value of 85.7%. The overall accuracy of the mNGS process was 67.1%, 56.6% PPA and 77.2% NPA. However, mNGS and tNGS may be missed for pathogens that are relatively difficult to break cell walls, such as fungi or tuberculosis, or the pathogen load is too low.1
The advantage of tNGS is that it can detect low-abundance pathogens with high sensitivity, reduce the amount of sequencing data and analysis workload, and improve detection efficiency and cost-effectiveness. However, the limitation of tNGS is that it can only detect pre-designed pathogens, cannot identify novel or rare pathogens, and cannot perform whole-genome analysis of pathogens. The advantage of mNGS is that it can detect all pathogens in samples without bias, find new or rare pathogens, and provide whole-genome information of pathogens, which is conducive to traceability, typing, and drug resistance evaluation. However, the limitations of mNGS are that it requires a large amount of sequencing data and analysis workload, high detection cost and time, and low sensitivity to pathogens with low abundance. In the field of clinical microbiology, tNGS is more suitable for the rapid screening and diagnosis of common or known pathogens, whereas mNGS is more suitable for the exploration and identification of unknown or rare pathogens. Therefore, in clinical practice, tNGS and mNGS are not substitutes for each other but are complementary. The selective use of these two technologies in different clinical needs can achieve more economical and efficient pathogen detection.
Actinomyces infections are most sensitive to antibiotics, such as penicillin G, penicillin VK, amoxicillin, ampicillin, clindamycin, and erythromycin, making these agents the first-line treatment options. Actinomycetes are the most sensitive to penicillin G, penicillin VK, amoxicillin, ampicillin, clindamycin, and erythromycin, which are the first-line treatment drugs. Piperacillin tazobactam, imipenem cilastatin sodium, and ceftriaxone are candidates.8 The initial treatment regimen for this patient was piperacillin-tazobactam. Throughout this treatment, the patient continued to experience intermittent fever, and there was an ongoing rapid progressive cavity formation in the right lung. It is worth noting that the patient’s subjective well-being improved. This suggests that the initial treatment may not have been entirely ineffective. Piperacillin tazobactam has also played a therapeutic role as a second-line drug, but its effect is not immediate. Furthermore, the use of imipenem-cilastatin was relatively brief, making it challenging to definitively assess treatment efficacy within this limited timeframe. Importantly, it should be noted that later in the treatment course, the patient was transitioned to a combination of clindamycin (first-line drug) and ceftriaxone (potential treatment option), which led to significant clinical improvement. This evidence strongly supports the recommendation that severe infections should be managed using first-line antimicrobial agents. For complex and invasive cases, extension to 12–18 months is recommended for immunocompromised patients.9 There are no randomized controlled studies evaluating antimicrobial regimens for different actinomycosis. Alternative antibiotic treatment regimens for penicillin-allergic patients include ceftriaxone, doxycycline, macrolides, and carbapenems. Surgical management could be considered in obvious dense necrotic abscess, empyema, fistula/sinus tract, and refractory hemoptysis.10
In conclusion, pulmonary actinomycosis is a rare chronic granulomatous infectious disease that lacks clinical specificity, is often misdiagnosed, and can present with an acute course in an immunocompromised population. Clinicians should be aware of the different manifestations of pulmonary actinomycosis, including pulmonary masses, cavities, sinus tract, and poor response to antimicrobials; then actinomycosis should be suspected. Previous gold standard diagnoses included lung or excision biopsy, including bacterial culture, histology, and gram staining, to determine the presence of pathological sulfur particles.11 Due to harsh pathogen culture conditions and difficult histopathological evidence, second-generation sequencing can be used as a valid method for precise diagnosis. Actinomycetes are sensitive to various antimicrobial drugs; however, the course of antimicrobial treatment is long. It is recommended to provide 6–12 months according to different clinical characteristics and extend the effective antibiotic treatment to 18 months in some cases. With the development and popularization of molecular biological detection technology, the diagnostic efficiency of rare respiratory tract infections has significantly improved, and increasingly rare infections can be clearly diagnosed by tNGS and mNGS at early admission, which greatly improves the efficiency of our diagnosis and treatment by strengthening clinicians’ understanding of rare clinical infections.
Ethics and Consent Statement
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images, and our study was approved by the Ethics Committee at Affiliated Banan Hospital of Chongqing Medical University, to publish the case details.
Funding
No public or private funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183–197. doi:10.2147/IDR.S39601
2. Gaston DC, Miller HB, Fissel JA. Evaluation of metagenomic and targeted next-generation sequencing workflows for detection of respiratory pathogens from bronchoalveolar lavage fluid specimens. J Clin Microbiol. 2022;60(7):e0052622. doi:10.1128/jcm.00526-22
3. Suzuki M, Araki K, Matsubayashi S, et al. A case of recurrent hemoptysis caused by pulmonary actinomycosis diagnosed using transbronchial lung biopsy after bronchial artery embolism and a brief review of the literature. Ann Transl Med. 2019;7(5):108. doi:10.21037/atm.2019.02.11
4. Han JY, Lee K-N, Lee JK, et al. An overview of thoracic actinomycosis: CT features. Insights Imag. 2013;4(2):245–252. doi:10.1007/s13244-012-0205-9
5. Cruz Choappa R, Vieille Oyarzo P. Diagnóstico histológico de actinomicosis [Histological diagnosis of actinomycosis]. Rev Argent Microbiol. 2018;50(1):108–110. Portugues. doi:10.1016/j.ram.2017.05.005
6. Minogue TD, Koehler JW, Norwood DA. Targeted next-generation sequencing for diagnostics and forensics. Clin Chem. 2017;63(2):450–452. doi:10.1373/clinchem.2016.256065
7. Huang J, Jiang E, Yang D. Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect Drug Res. 2020;13:567–576. doi:10.2147/IDR.S235182
8. Gilbert D. The Sanford guide to antimicrobial therapy 2020. Antimicrob Therapy. 2020;2020:81–85.
9. Kolditz M, Bickhardt J, Matthiessen W, et al. Medical management of pulmonary actinomycosis: data from 49 consecutive cases. J Antimicrob Chemother. 2009;63(4):839–841. doi:10.1093/jac/dkp016
10. Endo S, Murayama F, Yamaguchi T, et al. Surgical considerations for pulmonary actinomycosis. Ann Thorac Surg. 2002;74(1):185–190. doi:10.1016/S0003-4975(02)03616-0
11. Boot M, Archer J, Ali I. The diagnosis and management of pulmonary actinomycosis. J Infect Public Health. 2023;16(4):490–500. doi:10.1016/j.jiph.2023.02.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.