Back to Journals » Infection and Drug Resistance » Volume 16
Acute Porphyromonas gingivalis Subdural Abscess with Brain Abscess in the Left Temporal Lobe: A Case Report and Review of Literature
Authors Huang G, Zhou X, Zhang Z, Lai W, Zhong Q, Wu D , Ye X
Received 24 May 2023
Accepted for publication 21 September 2023
Published 29 September 2023 Volume 2023:16 Pages 6487—6491
DOI https://doi.org/10.2147/IDR.S422691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Guanlin Huang,* Xiaoping Zhou,* Zhenyu Zhang,* Wentao Lai, Qi Zhong, Daxing Wu, Xinyun Ye
Department of Neurosurgery, Ganzhou People’s Hospital, Ganzhou, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinyun Ye, Department of Neurosurgery, Ganzhou People’s Hospital, Ganzhou, Jiangxi, 341000, People’s Republic of China, Email [email protected]
Background: Brain abscesses are a rare but serious complication of focal intracerebral infection.
Case Description: We present a patient of acute subdural abscess with brain abscess in the left temporal lobe. After craniotomy, combined with the Third Next Generation Sequencing and Gene Diagnosis (TNGS & GD) of abscess, we prescribed sensitive antibiotics; the patient recovers well and the abscess did not recur.
Conclusion: For patients with acute subdural abscess, combined craniotomy and the TNGS & GD of abscess could achieve good results.
Keywords: intracerebral infection, brain abscess, acute subdural abscess, craniotomy, the Third Next Generation Sequencing and Gene Diagnosis, TNGS & GD
Introduction
Brain abscesses are a rare but serious complication of focal intracerebral infection.1–3 The most common mechanism of brain abscess infection is that bacteria spread to the elderly through adjacent tissues1 (mastoiditis, nasosinusitis, craniocerebral injury). When the brain abscess is adjacent to the ventricle or the surface of the brain, it can suddenly break down due to force, radiography, or improper puncture, forming ventriculitis or acute subdural abscess, meningitis, etc. However, it is rare for the brain abscess to break down and form acute subdural abscess.4 We present a patient of acute subdural abscess with brain abscess in the left temporal lobe.
Case Report
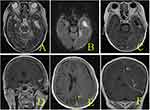
A 62-year-old female patient presented to us with headache on November 13, 2022, having had headache for 3 days. She had no clear history of trauma before the onset of the disease, while she had a history of rheumatoid arthritis. She was treated with long-term oral hormone. The physical examination on admission showed that the body temperature was 36.8 °C, the BP was 132/75 mmHg, and she was clear in mind. There was an abscess about 5 cm×5 cm in size on her left face, in which we could reach the sense of wave motion, and she did not present other positive signs. MRI showed that multiple intracranial abnormalities were enhanced, meningitis was considered, and brain abscess in the left temporal lobe was formed, bilateral frontal and left temporo-occipital regions were filled with pus under the skull inner plate and the dura mater of the longitudinal fissure pool, multiple swelling of the left temporal region, facial region, internal pterygoid muscle and external pterygoid muscle was considered, inflammatory lesions were considered, and left middle ear mastoiditis (Figure 1). In that case, we suggested craniotomy to deal with the subdural abscess and brain abscess. The patient and her family members refused craniotomy. Blood routine examination: white blood cells 7.56×109/L, neutrophils accounted for 88.1%. After admission, the patient was treated with vancomycin and meropenem for anti-infection, and the cerebrospinal fluid examination was conducted. The cerebrospinal fluid results are shown in Table 1. At the same time, the patient was punctured in the left facial abscess, and the yellow–green pus was punctured, and the bacterial culture and the Third Next Generation Sequencing and Gene Diagnosis (TNGS & GD) was performed. On the fifth day after admission (2022-11-18), the patient had a deeper consciousness disorder and was in a coma state. CT examination showed that the left frontotemporal crest, right frontal part, and left subdural low density shadow of the longitudinal fissure pool were significantly larger than before, and cerebral hernia was formed (Figure 2). We suggested craniotomy to deal with the subdural abscess and brain abscess again, and her family members chose craniotomy. In that case, she got the operation, the left frontotemporal and parietal subdural abscess was removed, the left temporal brain abscess was removed, with decompression. The images of intraoperation were as shown in Figure 3. The patient’s consciousness gradually improved after the operation. The TNGS & GD results of the left facial abscess and intraoperative pus showed the total length of the Porphyromonas gingivalis covering the genome was 2933 bp, the coverage was 3.21% (Figure 4), while the result of bacterial culture was nothing. The patient was diagnosed with Porphyromonas gingivalis, and treated with meropenem and metronidazole. On the second day after operation, the CT scan of the skull showed that the arc density shadow under the skull inner plate of the left frontotemporal part disappeared, the compression of the adjacent sulcus and gyrus was slightly reduced, and the structure is centered (Figure 5). Postoperative blood routine examination: white blood cells 19.68×109/L, neutrophils accounted for 94.3%. On the 3rd day after operation, there was no obvious fluid outflow from the drainage tube, and the drainage tube was removed. The body temperature remained normal until 2 weeks after the operation. The results of cerebrospinal fluid examination before discharge were normal. At the time of discharge, the patient was clear, the tension of the flap in the original operation area was not high, and the abscess did not recur.
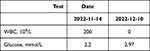 |
Table 1 Laboratory Tests for CSF in the Period of Treatment |
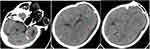 |
Figure 2 CT examination on the fifth day after admission. |
 |
Figure 3 Images of intraoperation: (A) pus collected during surgery, (B) pus discharged after craniotomy, (C) pus after opening the dura. |
 |
Figure 4 The Third Next Generation Sequencing and Gene Diagnosis. |
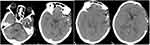 |
Figure 5 The CT of the second day after operation. Diagnosis (TNGS & GD) result of the patient. |
Discussion
The most common mechanism of brain abscess infection is that bacteria spread to the elderly through adjacent tissues.1 The imaging examination of this case showed left otitis media and multiple inflammation on the left face. Without obvious inducement, acute subdural abscess was caused by ulceration. Acute subdural abscess caused by brain abscess rupture is rare and difficult to diagnose, and is easily confused with glioma, metastatic tumor, and subdural effusion.5 If coma, chills, high fever, convulsions, and even gongs occur suddenly on the basis of clear intracranial space occupying lesions, the possibility of brain abscess rupture should be highly suspected.4 The disease can evolve rapidly, including decreased consciousness level, focal nerve defect, cranial nerve paralysis, vomiting, and even infectious shock.6 MRI diffusion imaging of brain abscess and subdural abscess showed high signal, apparent diffusion coefficient image showed low signal, and ADC value was lower than normal cortical gray matter.7 MRI combined with diffusion weighted and apparent diffusion coefficient images is a valuable method to distinguish brain abscess from primary, cystic, or necrotic tumors.8
In order to effectively clear the focus and control the increase of intracranial pressure, drilling drainage and craniotomy are mainly used at present.2,4,9,10 Craniotomy for abscess removal is more effective in the treatment of subdural abscess.11 After surgery, sensitive antibiotics should be continuously applied intravenously.12–16 When pathogens of infection are isolated by culture, drug-sensitive antibiotics can be selected to achieve the most effective treatment.4,17 In this case, there was pus in the temporal lobe and subdural region. Craniotomy was performed to remove the abscess, we took the bacterial culture and the TNGS & GD of the pus, but the result of bacterial culture was nothing, while the result of TNGS & GD was Porphyromonas gingivalis, and postoperative treatment with sensitive antibiotics achieved good results. In this case, the TNGS & GD help us find the Porphyromonas gingivalis, and played an important role in the subsequent treatment. We took the TNGS & GD as a strategy for confirming the bacterium, the TNGS & GD proving rapid, cheap, accurate, and portable real-time sequencing.18–22
Porphyromonas gingivalis is an oral, Gram negative, rod-shaped anaerobic pathogen. In this case, the abscess formed after periodontal infection can erode the lower wall of the maxillary sinus and cause skull destruction, pus protruding into the maxillary sinus, causing suppurative infection that passes through the sinuses. The passage between the two spreads to other paranasal sinuses, causing bone erosion to cause skull destruction. In that case, the Porphyromonas gingivalis intracranial abscess grew up. While there were no clear origins of intracranial abscess, oral infections should be taken into consideration,13–16 and this was the limitation.
Conclusion
For patients with acute subdural abscess, if surgery is necessary, combined craniotomy and the TNGS & GD of abscess could achieve good results.
Ethics Approval and Informed Consent
The patient provided informed consent for the case details and images to be published. No ethics committee approval was required for this study as the data had been analyzed in a retrospective manner.
Disclosure
The authors report no conflicts of interest in this work.
References
1. De Andres Crespo M, McKinnon C, Halliday J. What you need to know about brain abscesses. Br J Hosp Med. 2020;81(8):1–7. doi:10.12968/hmed.2020.0103
2. Kural C, Kırmızıgoz S, Ezgu MC, Bedir O, Kutlay M, Izci Y. Intracranial infections: lessons learned from 52 surgically treated cases. Neurosurg Focus. 2019;47(2):E10. doi:10.3171/2019.5.FOCUS19238
3. Suthar R, Sankhyan N. Bacterial Infections of the Central Nervous System. Indian J Pediatr. 2019;86(1):60–69. doi:10.1007/s12098-017-2477-z
4. Hakan T. Management of bacterial brain abscesses. Neurosurg Focus. 2008;24(6):E4. doi:10.3171/FOC/2008/24/6/E4
5. Kong F, Li L, Zhang D, et al. Healthy adults with Streptococcus pneumoniae meningitis and Streptococcus pneumoniae subdural abscess: two case reports and a literature review. J Int Med Res. 2022;50(11):3000605221137470. doi:10.1177/03000605221137470
6. Abdulrazeq H, Walek K, Sampath S, et al. Development of posttraumatic frontal brain abscess in association with an orbital roof fracture and odontogenic abscess: a case report. Surg Neurol Int. 2022;13:539. doi:10.25259/SNI_813_2022
7. Mueller-Mang C, Castillo M, Mang TG, Cartes-Zumelzu F, Weber M, Thurnher MM. Fungal versus bacterial brain abscesses: is diffusion-weighted MR imaging a useful tool in the differential diagnosis. Neuroradiology. 2007;49(8):651–657. doi:10.1007/s00234-007-0242-0
8. Fountas KN, Duwayri Y, Kapsalaki E, et al. Epidural intracranial abscess as a complication of frontal sinusitis: case report and review of the literature. South Med J. 2004;97(3):
9. Leavitt L, Baohan A, Heller H, Kozanno L, Frosch MP, Dunn G. Surgical management of an abscess of the insula. Surg Neurol Int. 2022;13:591. doi:10.25259/SNI_871_2022
10. Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment--78 years: aspiration versus excision. World Neurosurg. 2011;76(5):431–436. doi:10.1016/j.wneu.2011.03.048
11. Zhai Y, Wei X, Chen R, Guo Z, Raj Singh R, Zhang Y. Surgical outcome of encapsulated brain abscess in superficial non-eloquent area: a systematic review. Br J Neurosurg. 2016;30(1):29–34. doi:10.3109/02688697.2015.1109059
12. Akashi M, Tanaka K, Kusumoto J, Furudoi S, Hosoda K, Komori T. Brain abscess potentially resulting from odontogenic focus: report of three cases and a literature review. J Maxillofac Oral Surg. 2017;16(1):58–64. doi:10.1007/s12663-016-0915-5
13. Sakkas A, Nolte I, Heil S, et al. Eggerthia catenaformis infection originating from a dental abscess causes severe intestinal complications and osteomyelitis of the jaw. GMS Interdiscip Plast Reconstr Surg DGPW. 2021;10:Doc02. doi:10.3205/iprs000152
14. Pereira A, Tavares AT, Prates M, et al. Brain abscess: a rare clinical case with oral etiology. Case Rep Infect Dis. 2022;2022:5140259. doi:10.1155/2022/5140259
15. Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M. Intracranial bacterial infections of oral origin. J Clin Neurosci. 2015;22(5):800–806. doi:10.1016/j.jocn.2014.11.015
16. Sah R, Nepal G, Sah S, et al. A rare case of brain abscess caused by Actinomyces meyeri. BMC Infect Dis. 2020;20(1):378. doi:10.1186/s12879-020-05100-9
17. Corsini Campioli C, Castillo Almeida NE, O’Horo JC, et al. Bacterial brain abscess: an outline for diagnosis and management. Am J Med. 2021;134(10):1210–1217.e2. doi:10.1016/j.amjmed.2021.05.027
18. Loman NJ, Quick J, Simpson JT. A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat Methods. 2015;12(8):733–735. doi:10.1038/nmeth.3444
19. Zhang P, Jiang D, Wang Y, Yao X, Luo Y, Yang Z. Comparison of de novo assembly strategies for bacterial genomes. Int J Mol Sci. 2021;22(14):7668.
20. Molina-Mora JA, Campos-Sánchez R, Rodríguez C, Shi L, García F. High quality 3C de novo assembly and annotation of a multidrug resistant ST-111 Pseudomonas aeruginosa genome: benchmark of hybrid and non-hybrid assemblers. Sci Rep. 2020;10(1):1392. doi:10.1038/s41598-020-58319-6
21. De Maio N, Shaw LP, Hubbard A, et al. Comparison of long-read sequencing technologies in the hybrid assembly of complex bacterial genomes. Microb Genom. 2019;5(9). doi:10.1099/mgen.0.000294
22. Li C, Chng KR, Boey EJ, Ng AH, Wilm A, Nagarajan N. INC-Seq: accurate single molecule reads using nanopore sequencing. Gigascience. 2016;5(1):34. doi:10.1186/s13742-016-0140-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.