Back to Journals » Clinical Epidemiology » Volume 14
Acute Kidney Injury in Patients with Non-Valvular Atrial Fibrillation Treated with Rivaroxaban or Warfarin: A Population-Based Study from the United Kingdom
Authors González-Pérez A , Balabanova Y, Sáez ME, Brobert G, García Rodríguez LA
Received 27 July 2022
Accepted for publication 5 October 2022
Published 2 November 2022 Volume 2022:14 Pages 1281—1291
DOI https://doi.org/10.2147/CLEP.S383996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Antonio González-Pérez,1 Yanina Balabanova,2 María E Sáez,1 Gunnar Brobert,3 Luis A García Rodríguez1
1Pharmacoepidemiology, Spanish Centre for Pharmacoepidemiologic Research (CEIFE), Madrid, Spain; 2Integrated Evidence Generation, Bayer AG, Berlin, Germany; 3Integrated Evidence Generation, Bayer AB, Stockholm, Sweden
Correspondence: Antonio González-Pérez, Pharmacoepidemiology, Spanish Centre for Pharmacoepidemiologic Research, c/ Almirante 28, 2°, Madrid, 28004, Spain, Tel +34 915 313 404, Fax +34 915 312 871, Email [email protected]
Purpose: To compare the risk of acute kidney injury (AKI) among users of rivaroxaban vs warfarin.
Patients and Methods: We identified two cohorts of patients with non-valvular atrial fibrillation (NVAF) who initiated rivaroxaban (15/20 mg/day, N = 6436) or warfarin (N = 7129) excluding those without estimated glomerular filtration rate values recorded in the year before oral anticoagulant (OAC) initiation and those with a history of end-stage renal disease or AKI. We used two methods to define AKI during follow-up (mean 2.5 years): coded entries (method A) and the Aberdeen AKI phenotyping algorithm (method B) using recorded renal function laboratory values during the study period to identify a sudden renal deterioration event. Cox regression was used to calculate hazard ratios (HRs) for AKI with rivaroxaban vs warfarin use, adjusted for confounders.
Results: The number of identified incident AKI cases was 249 (method A) and 723 (method B). Of the latter, 104 (14.4%) were also identified by method A. After adjusting for age, sex, baseline renal function and comorbidity, HRs (95% CIs) for AKI were 1.19 (0.92– 1.54; p=0.18) using method A and 0.80 (0.68– 0.93; p< 0.01) using method B. Estimates stratified by baseline level of chronic kidney disease were largely consistent with the main estimates.
Conclusion: Our results support a beneficial effect of rivaroxaban over warfarin in terms of AKI occurrence in patients with NVAF. More research into how best to define AKI using primary care records would be valuable for future studies.
Keywords: oral anticoagulation, cohort study, database, renal injury
Graphical Abstract:
Plain Language Summary
Some people with atrial fibrillation who use oral anticoagulants for stroke prevention may experience acute kidney injury (AKI), and some studies suggest this AKI risk may vary by type of oral anticoagulant. Investigating AKI risks using healthcare databases can be challenging because it is not always coded accurately, and kidney function test results are not always recorded. Primary care databases that do record kidney test results are potentially good data sources for investigating AKI, and we used one such database to compare AKI risk in patients taking rivaroxaban with those taking warfarin. After taking into account differences in the characteristics of patients who use these two different drugs, we found evidence of a 20% reduced AKI risk among rivaroxaban users vs warfarin users when AKI was identified from kidney test results. This finding was not seen when AKI was identified using AKI diagnostic codes – these AKI cases totalled around a third of those identified by kidney function values. Our findings suggest that doctors should consider these different effects on renal function when making decisions on which oral anticoagulant to prescribe for their patients. However, more research into how best to identify AKI using primary care records is needed.
Introduction
Individuals with atrial fibrillation (AF) have a five-fold increased risk of stroke compared with those without AF1 and most require long-term prophylaxis with an oral anticoagulant (OAC) to effectively mitigate this risk. Although direct oral anticoagulants (DOACs) are recommended first-line OAC therapy in these patients,2–4 vitamin K antagonists (VKAs) remain extensively used in clinical practice.5
The last decade has seen emerging evidence of deterioration in renal function in patients receiving oral anticoagulants. Initially reported in patients receiving warfarin, this clinical syndrome has been called warfarin-related nephropathy (WRN). It is defined as an acute increase in international normalized ratio (INR) to above 3.0, with evidence of acute kidney injury (AKI) within a week of this increase that is not explained by other factors, but is seemingly related to anticoagulant overdosing.6 Reports also suggest that patients with chronic kidney disease (CKD), which is prevalent in over a third of patients with AF,7 are particularly susceptible to WRN.6 Furthermore, WRN has been associated with accelerated CKD progression and as a significant risk factor for short- and long-term mortality.6,8 There have also been reports of AKI associated with DOACs,8 although the evidence suggests that risks are greater with warfarin.9–12 Large population-based longitudinal healthcare databases are suitable data sources for conducting pharmacoepidemiological research. However, AKI can be challenging to identify from many secondary data sources due to limitations related to coding and availability of reliable laboratory data. In a study of AKI incidence using electronic health record (EHR) data from the United Kingdom, Sawhney et al13 found that while the majority of individuals developing AKI could be identified from hospital tests alone, around half were identified when using an alternative method of case identification based on the Kidney Disease Improving Global Outcomes phenotyping algorithm code. Additionally, several claims database studies on this topic have had limited information on some confounders. The primary objective of this study was to compare the incidence of AKI between new users of rivaroxaban, the most widely prescribed DOAC during the study period, and new users of warfarin in UK primary care. The secondary objective was a stratified analysis according to baseline renal function.
Materials and Methods
Study Design and Data Source
We undertook a population-based cohort study using anonymized longitudinal primary care electronic health records (EHRs) from the IQVIA Medical Research Data-UK (IMRD-UK). The database covers approximately 6% of the UK population,14 is representative of the UK demographic,15 and has been validated for pharmacoepidemiology research.16 The data captured are those that are entered in routine clinical practice, including coded diagnoses (Read codes),17 referrals, results of laboratory tests and all prescriptions issued. Primary care practitioners (PCPs) can also enter information manually in a free text field. Data communicated back from secondary care are entered in the patient’s primary care record retrospectively. The study protocol was approved by an independent scientific research committee (reference SRC-20SRC023). Data collection for IMRD-UK was approved by the South East Multicentre Research Ethics Committee in 2003. Individual studies using IMRD-UK data do not require separate ethical approval if only anonymized data are used.
Source Population and Study Cohorts
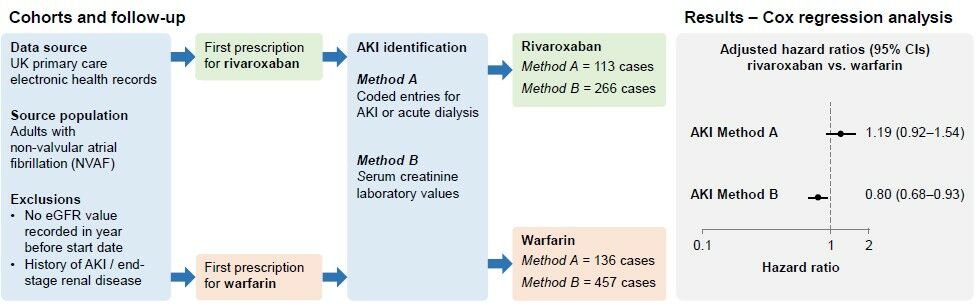
Identification of the study cohorts is shown in Figure 1. The source population comprised all individuals aged ≥18 years with non-valvular atrial fibrillation (NVAF) and a first prescription (start date) for rivaroxaban (15 or 20 mg/day) or warfarin between 1 January 2014 and 31 March 2019. Individuals could only enter the source population if they were OAC naïve, and if they had been registered with their participating practice with a first prescription for any medication at least 1 year previously. Owing to the absence of a Read code specifically for NVAF, to identify patients with NVAF, we identified those with a Read code for AF any time before the start date or within the 2 weeks after, excluding those with a code for heart valve replacement or mitral stenosis (ie, valvular AF) during the same time period. For this present study, we excluded individuals without an estimated glomerular filtration rate (eGFR) value recorded in the year before the start date and those with a history of AKI (using method A and/or B [ie, either AKI codes or AKI based on recorded laboratory measurements of renal parameters], as described later) or end-stage renal disease (ESRD, including individuals with baseline eGFR below 15mL/min/1.73m2).
Follow-Up and AKI Identification
We followed the rivaroxaban and warfarin cohorts from the start date until the earliest of the following: a record of AKI, death, the last date of data collection for their practice, or the end of the study period (30 September 2019). We used two approaches to identify AKI regardless of whether it was a nosocomial or a community-acquired event. The first (method A) was based on Read code entries in the patient’s EHR indicating AKI or acute dialysis (defined as a code for dialysis and non-continuation of dialysis from 30 days after the initial dialysis code) along with a record of an outpatient visit to secondary care/hospitalisation (see Supplementary Table 1 for codes). The second (method B) was based on recorded serum creatinine (SCr) values using the validated Aberdeen AKI phenotyping algorithm developed by Sawhney et al.13 This method is potentially more accurate because it uses all recorded renal function laboratory values during the study period to identify a sudden renal deterioration event. Using method B, an AKI event was determined if any of the following three criteria were met: criterion 1, serum creatinine ≥1.5 times higher than the median of all creatinine values 8–365 days ago; criterion 2, serum creatinine ≥1.5 times higher than the lowest creatinine within 7 days; criterion 3, serum creatinine >26 μmol/L higher than the lowest creatinine within 48 hours). We manually reviewed the EHRs for a random sample of 100 cases and the AKI event was confirmed in all cases.
Covariates
We extracted data on age, sex, and Townsend index score of deprivation,15 comorbidities and frailty (using an index developed for research using primary care databases18) on, or any time before, the start date. Comedications were determined from prescription data on/in the year before the start date, and polypharmacy was ascertained as the number of different medications prescribed in the month before (but not including) the start date. Healthcare use (number of primary care visits, referrals and hospitalisations) and lifestyle variables (body mass index, smoking status and alcohol consumption; using the most recent value/status) were ascertained in the year before the start date. To determine renal function at baseline, we used recorded eGFR values, expressed as mL/min/1.73m2, and used the most recent valid SCr value recorded in the year before the start date to apply the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation,19 with the omission of ethnicity as this is not routinely recorded. We also used coded clinical entries indicating CKD stage, and acute/chronic dialysis.
Statistical Analysis
Baseline characteristics were described using counts and percentages for categorical variables and mean with standard deviation for continuous variables. Incidence rates of AKI, for both case definitions, were calculated using the number of incident cases during follow-up as the numerator and total person-years as the denominator, with 95% confidence intervals (CIs) assuming a Poisson distribution. Incidence rates were stratified by baseline renal function (>50 and ≤50 mL/min/1.73m2). Cox proportional hazards regression was used to calculate hazard ratios (HRs) for AKI with rivaroxaban vs warfarin use, adjusted for confounders, both overall and stratified by baseline renal function. We performed several sensitivity analyses. Firstly, we censored patients at the date of OAC discontinuation (>30 days after the end of the last consecutive prescription of the starting OAC; on-treatment analysis). Secondly, patients were eligible to contribute person-time to different OAC exposure categories according to their current exposure irrespective of the starting drug (as-treated analysis). Thirdly, we restricted the analysis to cases of severe AKI (defined as stage 2 or 3 AKI; this was only performed for Method B because there are no Read codes that specify AKI severity). Fourthly, we repeated the ITT analysis adjusting for death as a competing risk using Fine and Gray models.20 Lastly, we used two more stringent definitions of the Aberdeen algorithm to identify AKI cases: i) meeting criterion 2 or 3, and ii) meeting criterion 3.
Results
Baseline characteristics of the study cohorts are shown in Table 1. Mean age was 74 years in both cohorts, and over half were male (57% rivaroxaban cohort, 56% warfarin cohort). Individuals in the warfarin cohort more likely to have a higher level of deprivation and history of ischaemic heart disease, while individuals in the rivaroxaban cohort were more likely to be severely frail and have a higher number of recent referrals/hospitalisations (in the previous year).
 |
Table 1 Baseline Characteristics of the Study Cohorts |
After a mean follow-up of 2.5 years, the number of incident AKI cases identified using coded entries (Method A) was 136 (rivaroxaban cohort) and 113 (warfarin cohort), and using the Aberdeen algorithm (Method B) was 266 (rivaroxaban cohort) and 457 (warfarin cohort). Of these 723 AKI cases identified by the Aberdeen algorithm, 104 (14.4%) were also identified by codes (Supplementary Figure 1); of the 249 AKI cases identified by codes, 145 (58.2%) were not identified using the Aberdeen algorithm. Incidence rates of AKI using cases identified from coded entries (Method A) were 81.2 per 10,000 person-years (95% CI: 66.9–97.6, rivaroxaban cohort) and 67.9 per 10,000 person-years (95% CI: 57.0–80.3, warfarin cohort), and using the Aberdeen algorithm (Method B) they were 194.4 per 10,000 person-years (95% CI: 171.7–219.2) and 234.5 per 10,000 person-years (95% CI: 213.5–257.0), respectively. Kaplan–Meier plots can be found in Supplementary Figure 2.
In the Cox regression analysis, after adjusting for confounders, we found no clear evidence for a difference in AKI risk between rivaroxaban and warfarin users when using coded entries to identify AKI (HR 1.19, 95% CI: 0.92–1.54; p=0.18), but there was evidence that rivaroxaban users had a reduced risk of AKI vs warfarin users when using the Aberdeen algorithm (HR 0.80, 95% CI: 0.68–0.93; p<0.01) (Table 2). Estimates stratified by baseline CKD (Table 3), and results of the sensitivity analyses were largely consistent with the overall estimates (Supplementary Tables 2–14).
 |
Table 2 Incidence Rate per 10,000 Person-Years of AKI, and HRs (95% CIs) Comparing AKI in the Rivaroxaban vs Warfarin Cohorts (ITT Analysis) |
 |
Table 3 Incidence Rate per 10,000 Person-Years of AKI and HR (95% CI) Comparing AKI in the Rivaroxaban vs Warfarin Cohorts, Stratified by Baseline Renal Function (ITT Analysis) |
Discussion
In this large population-based EHR study of patients with NVAF, we found evidence of a 20% reduced risk of AKI among users of rivaroxaban compared with users of warfarin when using a definition of AKI that involved recorded laboratory renal function values. This finding was not seen when using an AKI definition based on coded database entries; however, as laboratory values are regarded as critical in AKI diagnosis, the results using the Aberdeen algorithm are likely to be more accurate. Only 14.4% of the AKI cases identified by the Aberdeen algorithm were also identified by coded AKI entries, while only 58.2% of AKI cases identified by codes were also identified by the Aberdeen algorithm, revealing poor agreement between the two case ascertainment methods. While neither of the two methods is perfect, the Aberdeen algorithm seems to be more successful in capturing incident AKI events. Thus, although on some occasions coded AKI entries might be able to identify true events that the Aberdeen algorithm does not, our results suggest that the opposite situation (an event detected by recorded SCr values without the corresponding diagnostic code) is more likely. This might be partly due to the exclusion of individuals without SCr values recorded at baseline (a proxy for under-recording of SCr values during follow-up). Furthermore, a great strength of SCr values is that they are precisely dated, while diagnostic codes for acute events are not always recorded on the date the event occurred, making it more difficult to differentiate incident events from old events.
Our findings, using the Aberdeen algorithm case ascertainment method, are consistent with several previous studies on this topic, among patients with NVAF using rivaroxaban compared with those using warfarin9,10,12,21 or phenprocoumon,11 including among high-risk groups such as those with CKD,11,12,22 diabetes,9,12 heart failure9 or the elderly.9,10,21 Some have also shown similar effect sizes (15–19% reduced AKI risk), although some have reported a larger, ~30% lower risk with rivaroxaban9,22 vs warfarin, including an even larger ~50% lower risk seen in one study.22 Furthermore, a recent large meta-analysis of data from randomized controlled trials (RCTs) and observational studies reported a 30% reduced risk of AKI among users of DOACs vs warfarin.23 Consistent with most other studies,9,12,21,23 our results did not infer any materially different effects between patients with baseline CKD and those without CKD. It is noteworthy that the AKI incidence rates in our study were much lower than those reported by Yao et al in their United States claims database study where AKI was based on primary and secondary diagnoses of AKI from linked hospitalisation data. However, as IMRD UK does include data communicated back from secondary care, we believe only a small number of cases would not have been captured in the database. Our analysis focused on rivaroxaban vs warfarin only; however, some other studies,9,11 albeit not all,21–23 which have evaluated different DOACs with VKAs have reported significantly reduced risks of AKI among rivaroxaban or dabigatran users that were not seen for apixaban users. In this present study, we have also demonstrated the importance of an accurate case definition for capturing AKI events shown by the divergent results produced by the two case definitions. This also highlights the potential limitations of some databases when relying on coded entries only for clinical events that are largely defined by laboratory parameters in clinical practice.
A differential effect on AKI between rivaroxaban and warfarin is plausible due to their different mechanisms of action although the exact mechanistic pathway(s) are unclear. Warfarin affects all vitamin K dependent proteins, not just those in anticoagulation, and it has been shown to increase medial and intimal vascular calcification.24 Also, it is possible that rivaroxaban – a direct factor Xa inhibitor – could help renopreservation through a reduction in protease-activated receptor-mediated inflammation.24 Other possible pathophysiological pathways for WRN are associated with reductions in activated protein C and endothelial protein C receptor signalling.8
Direct oral anticoagulants are excreted by the kidneys to different degrees (80% for dabigatran, 65% for rivaroxaban and 25% for apixaban), whereas VKAs are largely metabolized through the liver,24 with relatively more harmful renal affects only being reported in the last decade. The significantly reduced AKI risk seen among rivaroxaban vs warfarin users in our study and previous investigations is clinically meaningful considering the life-long nature of anticoagulation in patients with NVAF and the ageing population. Patients with NVAF should be managed in a way that not only effectively reduces their risk of stroke while minimising bleeding but also best preserves renal function.
Strengths of the study include the large sample of patients enabling the calculation of precise estimates of effect, and the use of a database representative of the UK general population, meaning our findings our generalisable to the UK as a whole. We were able to control for a wide range of potential confounders including comorbidities, co-medications, demographics and lifestyle factors, although we acknowledge the possibility of residual or unmeasured confounding (potentially due to missing information on environmental or genetic factors, among others). Furthermore, sensitivity analyses showed the results of the main analyses to be robust. A limitation of our study is the potential for some non-differential outcome misclassification due to unrecorded/incorrect laboratory measurements, biasing the risk estimates towards the null. A detection bias in the opposite direction could operate if patients on warfarin were subject to greater monitoring including more laboratory investigations, thereby increasing the likelihood of AKI diagnosis. Additionally, we did not investigate INR levels among warfarin users and were therefore unable to account for potential overdosing that could lead to renal damage in these patients, which could have led to residual confounding. Also, medication use was based on prescriptions issued that did not capture over-the-counter drug use or lack of compliance. Finally, our study included a population from the UK with mostly European ancestry. Generalisability of our results to other populations (eg, Asian) should be undertaken with caution.
Conclusions
In conclusion, our results build on the existing evidence to support a beneficial effect of rivaroxaban over warfarin in terms of AKI occurrence in patients with NVAF. Clinicians should consider effects on renal function when making individualized decisions on the most appropriate OAC for their patients. Further evidence to support a causal association from RCTs and well-designed observational studies in other settings would help prescribers make more informed benefit–risk decisions regarding choice of long-term OAC therapy for their patients. More research into how best to define AKI using primary care EHRs would also be of value for future studies.
Abbreviations
AF, atrial fibrillation; AKI, acute kidney injury; chronic kidney disease (CKD); CKD, chronic kidney disease; CIs, confidence intervals; CKD-EPI, Chronic Kidney Disease Epidemiology; DOACS, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; EHRs, electronic health record; ESRD, end-stage renal disease; HRs, hazard ratios; INR, international normalized ratio; ITT, intention-to-treat; IMRD-UK, IQVIA Medical Research Data-UK; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulant; PCP, primary care practitioners; SCr, serum creatinine; WRN, warfarin-related nephropathy.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Ethics Approvals and Informed Consent
The study protocol was approved by the Independent Scientific Research Committee for IMRD-UK (reference number SRC-20SRC023). Data collection for IMRD-UK was approved by the South East Multicentre Research Ethics Committee in 2003, and individual studies using IMRD-UK data do not require separate ethical approval if only anonymized data are used. All data remained anonymised.
Acknowledgments
We thank Susan Bromley. EpiMed Communications, Abingdon, UK, for medical writing assistance, funded by Bayer AG and in accordance with Good Publication Practice. IQVIA provided the IMRD-UK incorporating data from THIN, a Cegedim Database. Reference made to THIN is intended to be descriptive of the data asset licensed by IQVIA.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; in the drafting, revising or critically review of the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Bayer AG (grant number not applicable). Bayer AG had no role in the design or conduct of the study aside from the roles carried out by YB (current employee of Bayer) and GB (former employee of Bayer) as described below.
Disclosure
A González Pérez, ME Sáez and LA García Rodríguez work for CEIFE, which has received research funding from Bayer AG. LA García Rodríguez has also received honoraria for serving on advisory boards for Bayer AG. Y Balabanova is an employee of Bayer AG. G Brobert was an employee of Bayer AB at the time of the study and is currently a paid consultant for Bayer. A González-Pérez and LA García Rodríguez report grants from Bayer during the conduct of the study. Y Balabanova reports Bayer AG has funded this piece of work. The authors report no other potential conflicts of interest in relation to this work.
References
1. Virani Salim S, Alonso A, Benjamin Emelia J, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9). e139–e596.
2. Kirchhof P, Benussi S, Kotecha D, et al. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
3. January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;2019:CIR0000000000000665.
4. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393.
5. Washam JB, Holmes DN, Thomas LE, et al. Pharmacotherapy for atrial fibrillation in patients with chronic kidney disease: insights from ORBIT-AF. J Am Heart Assoc. 2018;7(18):e008928.
6. Brodsky SV. Anticoagulants and acute kidney injury: clinical and pathology considerations. Kidney Res Clin Pract. 2014;33(4):174–180.
7. Fanikos J, Burnett AE, Mahan CE, Dobesh PP. Renal function considerations for stroke prevention in atrial fibrillation. Am J Med. 2017;130(9):1015–1023.
8. Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost. 2016;14(3):461–467.
9. Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–2632.
10. Coleman CI, Kreutz R, Sood N, et al. Rivaroxaban’s impact on renal decline in patients with nonvalvular atrial fibrillation: a Us marketscan claims database analysis. Clin Appl Thromb Hemost. 2019;25:1076029619868535.
11. Bonnemeier H, Kreutz R, Enders D, et al. P4749Renal function worsening in factor-xa inhibitors vs phenprocoumon in patients with non-valvular atrial fibrillation and renal disease - insights from the RELOADED study. Eur Heart J. 2019;40(Supplement_1):ehz745.
12. Hernandez AV, Bradley G, Khan M, et al. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcome. 2020;6(4):301–307.
13. Sawhney S, Robinson HA, van der Veer SN, et al. Acute kidney injury in the UK: a replication cohort study of the variation across three regional populations. BMJ Open. 2018;8(6):e019435.
14. THIN. The health improvement network. Available from: https://www.the-health-improvement-network.com/en/.
15. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255.
16. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401.
17. NHS Digital. Read codes. https://digital.nhs.uk/services/terminology-and-classifications/read-codes
18. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360.
19. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
20. Fine JP, Gray RJA. Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:446.
21. Harel Z, McArthur E, Jeyakumar N, et al. The risk of acute kidney injury with oral anticoagulants in elderly adults with atrial fibrillation. Clin J Am Soc Nephrol. 2021;16(10):1470–1479.
22. Chan Y-H, Yeh Y-H, Hsieh M-Y, et al. The risk of acute kidney injury in Asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. 2018;265:83–89.
23. Sitticharoenchai P, Takkavatakarn K, Boonyaratavej S, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. Non-vitamin K antagonist oral anticoagulants provide less adverse renal outcomes than warfarin in non-valvular atrial fibrillation: a systematic review and meta analysis. J Am Heart Assoc. 2021;10:e019609.
24. van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of Vitamin K antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients. 2015;7(11):9538–9557.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

