Back to Journals » International Journal of General Medicine » Volume 15
Acute Kidney Injury among Hospital-Admitted COVID-19 Patients: A Study from Jordan
Authors Oweis AO, Alshelleh SA, Hawasly L, Alsabbagh G, Alzoubi KH
Received 13 February 2022
Accepted for publication 11 April 2022
Published 29 April 2022 Volume 2022:15 Pages 4475—4482
DOI https://doi.org/10.2147/IJGM.S360834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ashraf O Oweis,1 Sameeha A Alshelleh,2 Lubna Hawasly,3 Ghalia Alsabbagh,3 Karem H Alzoubi4,5
1Department of Internal Medicine, Nephrology Division, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Internal Medicine, Nephrology Division, University of Jordan, Amman, Jordan; 3Department of Internal Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Pharmacy Practice and Pharmacotherapeutics, University of Sharjah, Sharjah, United Arab Emirates; 5Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Ashraf O Oweis, Department of Internal Medicine, Nephrology Division, Jordan University of Science and Technology, Irbid, Jordan, Tel +962-79-145-5505, Email [email protected]
Objective: During the COVID-19 pandemic, many patients have been admitted to hospitals with severe respiratory disease and suffered complications. Acute kidney injury (AKI) is among the more dangerous complications contributing to morbidity and mortality among patients.
Methods: This retrospective study focused on all hospital-admitted COVID-19 patients between September and December 2020. A total of 1,044 patients were enrolled. Patient demographics, medical records, and laboratory data were gathered. Patients were split into two groups: AKI and non-AKI. Comparisons comprised demographics, labs, ICU transfer, need for ventilation and oxygen therapy, medications, hospital stay, and deaths.
Results: AKI incidence in the cohort was 25.3%, and a majority were stage 1 (53.3%). Among these, hemodialysis was started in 1.8%. Higher age (P< 0.001), diabetes mellitus (P=0.001), hypertension (P=0.001), ACEI/ARB use (P=0.008), erythrocyte-sedimentation rate (P=0.002), CRP (P< 0.0001), and ferritin (P=0.01) were predictors of AKI. Among all admitted COVID-19 patients, 30.2% died in hospital. Among those with AKI, 75.9% died in comparison to 24.1% of non-AKI patients (P< 0.001). Among COVID-19 patients admitted to the ICU, 80.5% died: 70.5% were from the AKI group and 29.5% from the non-AKI group (P< 0.001).
Conclusion: High mortality and morbidity is associated with COVID-19 infection, and AKI is contributing significantly to the outcomes of hospitalized patients with the infection. Early recognition of and treatment for AKI will decrease mortality and hospitalization in patients with COVID-19.
Keywords: acute kidney injury, COVID, mortality, dialysis
Introduction
In December 2019 in Wuhan, China, multiple unexplained lower respiratory tract infections were reported, which proved to be a viral infection related to SARS-CoV2. The World Health Organization named this viral infection COVID-19. Since then, the COVID-19 pandemic has overwhelmed health systems worldwide and been associated with high admission rates to hospitals and intensive care units (ICUs) and death. In March 2020 the first case of COVID-19 was recorded in Jordan, and until June of the same year, the country was under complete lockdown, with a few sporadic cases reported nationwide. The true COVID-19 first wave in Jordan started in September 2020 and lasted until December of that year.
Acute kidney injury (AKI) is a common complication in hospital- and ICU-admitted patients.1,2 It is associated with longer hospital stay and higher mortality, especially in those with sepsis.2–4 Renal replacement therapy (RRT), whether early or late, may alter outcomes and decrease complications.5,6 In COVID-19 infection, AKI incidence has been shown to increase, resulting in increased mortality.7,8 Direct involvement of the kidneys in the inflammatory process, complement activation, and coagulopathy plays a role in COVID-19 patients with AKI.9 The current study aimed to identify the incidence of AKI among COVID-19 patients, possible risk factors, the effect of RRT, and mortality.
Methods
This was a retrospective cohort study in which data were collected from all adult COVID-19 patients admitted to King Abdullah University Hospital in Irbid, Jordan, an urban academic tertiary referral hospital with 650 beds and 20 medical ICU beds that provides services to five provinces in Jordan. All patients’ medical records were examined between September and December 2020. For patients who had had more than one admission, only the first admission was analyzed. Approval of the study protocol was obtained from the King Abdullah University Hospital and Jordan University of Science and Technology institutional review boards (107/136/2020). Because of the retrospective study design and expected high mortality rates, we asked that consent be waived. This study was conducted in accordance with the Declaration of Helsinki. Patients’ data were kept confidential as per international standards.
Sex, age, reason for admission, comorbidities, laboratory-test data, medications, and ICU length of stay were obtained from medical records of the patients. To define AKI and determine its stage, the AKI Network (AKIN) classification was used.10 The Chronic Kidney Disease Epidemiology Collaboration equation was used for estimated glomerular filtration rate (eGFR).11 Chronic kidney disease (CKD) was defined as an eGFR <60 mL/min.12 As per accepted procedures, COVID-19 patients were diagnosed based on positive PCR tests.
Statistical Analysis
Stata/SE 14.2 (StataCorp, College Station, TX, USA) was used for data analysis. Percentages were utilized to express categorical variables, the χ2 test to test for significant associations. Means ± SD indicate continuous variables, while either unpaired t-tests or the Mann–Whitney U tests were used to test for significant differences. Regression analyses, both univariate and multivariate, were done to capture independent predictors of AKI. Outcomes related to mortality were assessed using Kaplan–Meier survival analysis.
Results
Of the 1,044 patients reviewed, 117 were excluded for missing data or being on chronic hemodialysis. Mean age was 60.3±17.1 years, and male patients made up 55.1% of the sample. Demographic and basic disease variables of patients are shown in Table 1. AKI incidence was 25.3%, with a majority of patients in stage1 (53.3%, Figure 1). For the AKI group, mean arterial pressure on admission was 91.7 mmHg, while that for the non-AKI group was 92.3 mmHg (P=0.56). Among patients who developed AKI, hemodialysis was initiated in 1.8% for such reasons as hyperkalemia (57.1%), fluid overload (7.1%), or a combination of both (21.4%). Mean eGFR on admission was 78.7±55.5 mL/min: 59.3±40.6 mL/min in the AKI group and 85.3±58.3 mL/min in the non-AKI group (P<0.001). Mean eGFR on discharge was 78.5±49.4 mL/min: 33.2±354.2 mL/min in the AKI group and 94.9±43.2 mL/min in the non-AKI group (P<0.001; Figure 2).
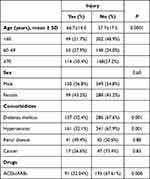 |
Table 1 Baseline characteristics based on AKI status |
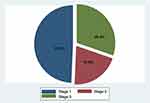 |
Figure 1 Pie chart of incidence of AKI stage based on AKIN stage. |
 |
Figure 2 Box plot of eGFR on admission and discharge based on AKI status. |
In terms of COVID-19, 45.4% of admitted patients were on oxygen therapy with face masks on admission, 12.6% on noninvasive ventilation, 9.6% on nonrebreather masks, and 7.2% were intubated. Most (76.2%) were started on dexamethasone, while only only 5.9% received remdesivir. Of patients who received dexamethasone, 28.5% developed AKI and 71.5% did not (P<0.001), while 42% of those who received remdesivir developed AKI and 58% of those who did not receive this therapy did not (P=0.005). The univariate analysis showed that hypertension (P=0.001), increasing age (P<0.001), diabetes mellitus (P=0.001), use of ACEIs/ARBs (P=0.008), erythrocyte-sedimentation rate (P=0.002), CRP (P<0.0001), and ferritin (P=0.01) were predictors of AKI, while white blood cells (P=0.25) and D-dimer (P=0.09) did not (Table 2). On multivariate analysis, age (OR 1.1, 95% CI 1.1–1.2; P=0.001), hypertension (OR 1.8, 95% CI 1.0–3.5; P=0.04), and a history of renal disease (OR 2.35, 95% CI 1.1–4.6; P=0.014) were strong predictors of AKI, but inflammatory markers were not.
 |
Table 2 Laboratory outcomes |
Mean time to discharge was 8.7±7.7 days: 11.2±7.8 days for the AKI group and 7.9±7.5 days for the non-AKI group (P<0.001). Mean time to transfer to the ICU was 5.9±6.9 days: 6.5±6.9 days for the AKI group and 4.8±6.7 days for the non-AKI group (P=0.002). In terms of ICU transfer, 31.2% of patients with AKI were transferred to the ICU and 68.9% of the non-AKI group. Regarding overall mortality, 30.2% died in hospital (Figure 3). Among patients with AKI, 75.9% died in comparison to 24.1% among non-AKI group (P<0.001). For overall ICU mortality, 80.5% of ICU admissions died: 70.5% from the AKI group and 29.5% from the non-AKI group (P<0.001). Mean time to death was 11.4±8.9 days. For the AKI group, it was 11.5±7.9 days and for the non-AKI group 11.4±10.6 days (P=0.12). It is worth mentioning that the COVID-19 strain at the time of our research was the β variant.
 |
Figure 3 Kaplan–Meier survival curve of survival probability by time of discharge based on AKI status. |
Discussion
AKI is among the most common reasons for mortality and morbidity in hospitalized patients, imposing a financial burden and prolonging hospitalization time.10 The incidence of AKI vary between community-acquired (4.3%) and hospital-acquired (2.1%), with total incidence of 6.4% for hospitalized patients.1 This incidence and risk of mortality and long-term morbidity increases in patients in ICUs and those with sepsis.13–15Increased age can impact AKI incidence in admitted patients. This could be related to functional and anatomical changes in the kidney that can happen with aging.16–18 Different classifications for AKI are used, and incidence can vary based on the system employed.10
Since the COVID-19 pandemic started in 2019, association of the disease with AKI has become well known. Prevalence in patients hospitalized with COVID-19 can reach 28%.19 It seems that positive COVID-19 infection in patients increase the risk of AKI and CKD and the need for RRT, regardless of the severity of the disease.20 Higher accuracy has been suggested for AKI-incidence reporting when using AKIN criteria, whereas other studies have reported better prediction of mortality when using the RIFLE criteria.21,22
Data from studies that evaluated kidney biopsies from patients with COVID-19 infections or postmortem biopsies with or without AKI showed effects that varied from mild tubular injury or cortical necrosis to disseminated intravascular coagulation.23,24 Several factors can contribute to direct damage of the kidneys with variable severity following SARS-CoV2 infection, such as angiotensin II overactivity, increased inflammatory markers with or without cytokine storm, activation of the immune system (lymphocytes and macrophages), and activation of complement pathways and the coagulation system.25 Though some studies failed to isolate SARS-CoV2 mRNA from urine of infected patients with AKI, others were able to identify SARS-CoV2–like particles on electron microscopy of renal biopsies.26,27 The use of nephrotoxic drugs in COVID-19 patients will contribute to AKI and increase its incidence and severity.28
In the current study, AKI incidence was 25.3% and a majority of patients were stage 1. In fact, a recent meta-analysis of 51 studies by Yang et al showed a pooled incidence of AKI of 12.3%. This differed among transplant patients, ICU patients, and deceased patients: 38.9%, 39%, and 42%, respectively. In the same meta-analysis, the pooled incidence of RRT was 5.4%, but higher in transplant and ICU patients: 15.6% and 16.3%, respectively.29 In our study, RRT need was only for 1.8% of patients. This can be explained by the higher mortality rate among patients with AKI.
Time of initiation of RRT remains debatable, due to a lack of data. Still, early initiation of RRT in septic patients and ICUs has better effects on treatment outcomes. Continuous RRT (CRRT) is mostly used for sick, unstable patients with COVID-19 in the ICU setting, with incidence of 5%–52%. In our cohort, we used sustained low-efficiency dialysis (SLED) in 99% of cases and CRRT in only 1%. Because of small numbers of patients started on dialysis, we were unable to observe a significant difference between the two modalities. If CRRT is not available or infeasible because of a hypercoagulable state, peritoneal dialysis can be used.30
As with risks of developing AKI in patients with COVID-19, comorbidities like hypertension and diabetes and increasing age increases the risk of AKI. Patients with AKI from the current study were 9 years older than those without. Previous kidney disease was a major risk, and patients with AKI had lower eGFR on admission than the other group. This would likely impact their discharge eGFR, which was significantly lower in patients who survived COVID-19 infection. Degree of inflammation and rise in CRP were risks on univariate analysis, but not significant on multivariate analysis in the current study. Kumar et al found similar risk factors like comorbidities, yet inflammatory markers were associated with more AKI in India.31 Other risks found in the current study included previous use of ACEIs and ARBs, in accordance with an Italian study by Russo et al, who found that other risks like comorbidities, age, CKD, and high CRP were associated with higher AKI and mortality risks.32 These data do not necessarily suggest that the use of ACEIs or ARBs contributes to disease severity, as the literature suggests that these drugs do not predispose patients with COVID-19 to AKI.33,34 However, their use could reflect underlying renal disease. Using medications in the treatment of patients with COVID-19 has an impact on AKI incidence and subsequently mortality. Patients who use steroids and/or remdesivir in the current study had lower incidence of AKI than those who were not treated with these medications. In concordance, this was also reported by a UK study.35 Although decreased mortality with remdesivir was not found, it decreased the incidence of AKI.36
Most of the patients who were transferred to the ICU died (80.5%), which can be expected due to severity of disease, higher chance of acute respiratory distress syndrome, sepsis, and AKI, as found in other studies.37 In the current study, 70.5% of patients who developed AKI in the ICU died. This could be related to the fact that the center where this study was carried out is a tertiary referral hospital and accepts/transfers patients from five provinces in Jordan. In fact, the incidence of AKI in our ICU (non–COVID-related) is 31.6%. Additionally, in Fominskiy et al, mortality in AKI patients was higher than those without AKI (52.9% vs 38.9%). In fact, most of these patients were older, had had previous CKD, or were in stage 1 AKI, similar to the characteristics found in the current study.38 Other studies have found higher mortality in patients with COVID-19 and AKI.39,40 Lastly, a study from a neighboring country, Saudi Arabia, showed that a cohort of COVID-19 patients had higher mortality associated with transfer to ICUs, severe disease, or AKI.41
We believe that the AKI in patients in the current study was multifactorial, ie, hemodynamic changes had a role, as well as cytokine storms, yet it is likely that some AKI developed in the hospital and was not present on admission. However, postmortem analysis of COVID-19 patients and obtaining renal biopsies for any of the admitted patients with COVID-19 are not routine hospital procedures. The standard protocol for all admitted patients with COVID-19 consists of dexamethasone, multivitamins, and oxygen therapy. At the time, data on the use of remdesivir was emerging, and some of the patients in this study received it. Prevention of AKI in these patients might be difficult, especially given that they present late to the hospital. In general, increased supportive care, especially fluid replacement, might be the course for these cases.
Conclusion
The current study showed higher risk of mortality and morbidity in COVID-19 patients with AKI. Identifying high-risk groups and earlier diagnosis of AKI in COVID-19 patients can improve results in patients and decrease mortality.
Acknowledgments
The abstract of this paper was presented at the World Congress of Nephrology in February 2022, organized by the International Society of Nephrology as a poster presentation with interim findings. Authors would like to thank the Deanship of Research-Jordan University of Science and Technology-Irbid-Jordan for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9(6):1007–1014. doi:10.2215/CJN.07920713
2. Santos RPD, Carvalho ARS, Peres LAB, Ronco C, Macedo E. An epidemiologic overview of acute kidney injury in intensive care units. AMB Rev Assoc Med Bras. 2019;65(8):1094–1101. doi:10.1590/1806-9282.65.8.1094
3. Pinheiro KHE, Azedo FA, Areco KCN, Laranja SMR. Risk factors and mortality in patients with sepsis, septic and non septic acute kidney injury in ICU. J Bras Nefrol. 2019;41:462–471. doi:10.1590/2175-8239-jbn-2018-0240
4. Jiang L, Zhu Y, Luo X, et al. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol. 2019;20(1):468. doi:10.1186/s12882-019-1660-z
5. Ahmed AR, Obilana A, Lappin D. Renal replacement therapy in the critical care setting. Crit Care Res Pract. 2019;2019:6948710. doi:10.1155/2019/6948710
6. Xiao L, Jia L, Li R, Zhang Y, Ji H, Faramand A. Early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: a systematic review and meta-analysis. PLoS One. 2019;14(10):e0223493. doi:10.1371/journal.pone.0223493
7. Shao M, Li X, Liu F, Tian T, Luo J, Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol Res. 2020;161:105107. doi:10.1016/j.phrs.2020.105107
8. Fabrizi F, Alfieri CM, Cerutti R, Lunghi G, Messa P. COVID-19 and acute kidney injury: a systematic review and meta-analysis. Pathogens. 2020;9(12):1052. doi:10.3390/pathogens9121052
9. Chueh TI, Zheng CM, Hou YC, Lu KC. Novel evidence of acute kidney injury in COVID-19. J Clin Med. 2020;9(11):3547. doi:10.3390/jcm9113547
10. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. doi:10.2215/CJN.02730313
11. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–1009. doi:10.2215/CJN.06870909
12. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
13. Wang X, Jiang L, Wen Y, et al. Risk factors for mortality in patients with septic acute kidney injury in intensive care units in Beijing, China: a multicenter prospective observational study. Biomed Res Int. 2014;2014:172620. doi:10.1155/2014/172620
14. Peng Q, Zhang L, Ai Y, Zhang L. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin Med J. 2014;127(10):1820–1826.
15. Oweis AO, Alshelleh SA, Momany SM, Samrah SM, Khassawneh BY, Al Ali MAK. Incidence, risk factors, and outcome of acute kidney injury in the intensive care unit: a single-center study from Jordan. Crit Care Res Pract. 2020;2020:8753764. doi:10.1155/2020/8753764
16. Alshelleh SA, Oweis AO, Alzoubi KH. Acute kidney injury among nonagenarians in Jordan: a retrospective case-control study. Int J Nephrol Renovasc Dis. 2018;11:337–342. doi:10.2147/IJNRD.S186121
17. Oweis AO, Alshelleh SA. Incidence and outcomes of acute kidney injury in octogenarians in Jordan. BMC Res Notes. 2018;11(1):279. doi:10.1186/s13104-018-3397-3
18. Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–29. doi:10.1159/000445450
19. Silver SA, Beaubien-Souligny W, Shah PS, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med. 2021;3(1):83–98.e81. doi:10.1016/j.xkme.2020.11.008
20. Moledina DG, Simonov M, Yamamoto Y, et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am. J Kidney Dis. 2021;77(4):490–499.e491. doi:10.1053/j.ajkd.2020.12.007
21. Xiong J, Tang X, Hu Z, Nie L, Wang Y, Zhao J. The RIFLE versus AKIN classification for incidence and mortality of acute kidney injury in critical ill patients: a meta-analysis. Sci Rep. 2015;5(1):17917. doi:10.1038/srep17917
22. Allen JC, Gardner DS, Skinner H, Harvey D, Sharman A, Devonald MAJ. Definition of hourly urine output influences reported incidence and staging of acute kidney injury. BMC Nephrol. 2020;21(1):19. doi:10.1186/s12882-019-1678-2
23. Kudose S, Batal I, Santoriello D, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi:10.1681/ASN.2020060802
24. Santoriello D, Khairallah P, Bomback AS, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158–2167. doi:10.1681/ASN.2020050744
25. Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi:10.1681/ASN.2020040419
26. Enikeev D, Taratkin M, Efetov S, et al. Acute kidney injury in COVID-19: are kidneys the target or just collateral damage? A comprehensive assessment of viral RNA and AKI rate in patients with COVID-19. Curr Opin Urol. 2021;31(4):363–368. doi:10.1097/MOU.0000000000000901
27. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi:10.1016/j.kint.2020.04.003
28. Mohamadi Yarijani Z, Najafi H. Kidney injury in COVID-19 patients, drug development and their renal complications: review study. Biomed Pharmacother. 2021;142:111966. doi:10.1016/j.biopha.2021.111966
29. Yang X, Tian S, Guo H. Acute kidney injury and renal replacement therapy in COVID-19 patients: a systematic review and meta-analysis. Int Immunopharmacol. 2021;90:107159. doi:10.1016/j.intimp.2020.107159
30. Shemies RS, Nagy E, Younis D, Sheashaa H. Renal replacement therapy for critically ill patients with COVID-19-associated acute kidney injury: a review of current knowledge. Ther Apher Dial. 2021;26(1):15–23.
31. Kumar S, Hanumaiah H, Rajiv A, et al. Incidence, risk factors and outcome of COVID-19 associated AKI: a study from South India. J Assoc Physicians India. 2021;69(6):11–12.
32. Russo E, Esposito P, Taramasso L, et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2021;34(1):173–183. doi:10.1007/s40620-020-00875-1
33. Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22(5):31. doi:10.1007/s11886-020-01291-4
34. Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi:10.1016/j.phrs.2020.104927
35. Lumlertgul N, Pirondini L, Cooney E, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11(1):123. doi:10.1186/s13613-021-00914-5
36. Kuno T, Miyamoto Y, Iwagami M, Ishimaru M, Takahashi M, Egorova NN. The association of remdesivir and in-hospital outcomes for COVID-19 patients treated with steroids. J Antimicrob Chemother. 2021;76(10):2690–2696. doi:10.1093/jac/dkab256
37. Alser O, Mokhtari A, Naar L, et al. Multisystem outcomes and predictors of mortality in critically ill patients with COVID-19: demographics and disease acuity matter more than comorbidities or treatment modalities. J Trauma Acute Care Surg. 2021;90(5):880–890. doi:10.1097/TA.0000000000003085
38. Fominskiy EV, Scandroglio AM, Monti G, et al. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2021;50(1):102–109. doi:10.1159/000508657
39. Gutiérrez-Abejón E, Martín-García D, Tamayo E, Álvarez FJ, Herrera-Gómez F. Clinical profile, pharmacological treatment, and predictors of death among hospitalized COVID-19 patients with acute kidney injury: a population-based registry analysis. Front Med. 2021;8:657977. doi:10.3389/fmed.2021.657977
40. Alessandri F, Pistolesi V, Manganelli C, et al. Acute kidney injury and COVID-19: a picture from an intensive care unit. Blood Purif. 2021;50:1–5.
41. Al Sulaiman KA, Aljuhani O, Eljaaly K, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–187. doi:10.1016/j.ijid.2021.02.037
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
