Back to Journals » Journal of Inflammation Research » Volume 14
Acute Inflammatory Edema: A Case Report with Histopathological and Immunohistochemical Findings
Authors Chirasuthat P, Chirasuthat S , Suchonwanit P
Received 12 August 2021
Accepted for publication 11 September 2021
Published 21 September 2021 Volume 2021:14 Pages 4877—4880
DOI https://doi.org/10.2147/JIR.S334051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Phatcharawat Chirasuthat, Suthep Chirasuthat, Poonkiat Suchonwanit
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Poonkiat Suchonwanit
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand
Tel +66-2-2011141
Fax +66-2-201-1211 ext 4
Email [email protected]
Abstract: Acute inflammatory edema (AIE) is a rare variant of pseudocellulitis characterized by blanchable, erythematous, and edematous plaques mainly on the thighs and abdomen and sparing areas of increased pressure. The condition occurs predominantly in critically ill patients with hypoalbuminemia, those with increased body mass index, and those with evidence of fluid overload. AIE was introduced in 2019; however, its histopathological picture has never been elucidated in the literature. We report a case of AIE in a 64-year-old Thai woman with several comorbidities and illustrate its histopathological and immunohistochemical features for the first time. Treatment with diuretics, fluid restriction, and adjuvant hemodialysis revealed marked improvement after ten days. Our report emphasizes that AIE is a distinct dermatosis with specific characteristics that help differentiate AIE from cellulitis and other pseudocellulitic conditions. Furthermore, our observations support the role of lymphatic alterations in the pathogenesis of the disease.
Keywords: cellulitis, dermis, edema-phage, lymphedema, pseudocellulitis, volume overload
Introduction
Acute inflammatory edema (AIE) is a non-infectious inflammatory skin disorder that is characterized by bilateral, erythematous, edematous plaques, most commonly observed on the dependent parts of the thighs and abdomen, sparing areas of skin subject to pressure.1 It is considered to be an under-recognized variant of pseudocellulitis that occurs primarily in critically ill patients. Factors associated with the development of AIE include hypoalbuminemia, obesity, fluid overload, and renal impairment.1 The pathogenesis of AIE remains unclear; however, acute fluid accumulation in connective tissues causing microtrauma with resultant inflammation is hypothesized.2 Since its first description in 2019, to the best of our knowledge, no demonstration of its histopathological picture exists in the literature. Herein, we describe a case of AIE and illustrate its histopathological and immunohistochemical features for the first time in a Thai patient with several comorbidities. Our microscopic findings help differentiate AIE from other conditions and highlight the role of the lymphatic system in the pathogenesis of the disease.
Case Presentation
A 64-year-old woman presented to the hospital with fever, low urine output, dramatic weight gain of 14 kg, and presence of erythematous, edematous rashes on her abdomen and legs for one week. The patient suffered from several comorbidities, including obesity, obstructive sleep apnea, non-alcoholic fatty liver disease, hypertension, dyslipidemia, chronic kidney disease, and type 2 diabetes mellitus. She was hospitalized, presumptively diagnosed with cellulitis, and administered empirical antibiotics with ceftriaxone for one week; however, the skin lesions did not respond to the treatment. On Day 9 of hospitalization, the dermatology department was consulted for evaluation of her skin lesions. On general examination, the patient was febrile (38.6°C); however, other hemodynamic parameters were normal. Dermatological examination revealed ill-defined, blanchable, erythematous, edematous, mildly tender, warm plaques on the abdomen and lower extremities (Figure 1). The skin folds were spared.
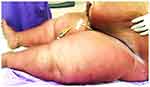 |
Figure 1 Acute inflammatory edema manifesting as erythematous, edematous plaques on the lower abdomen and dependent parts of the lower extremities with sparing of the skin folds. |
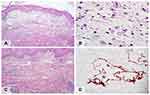
Laboratory investigations revealed leukocytosis (white blood cell count of 10,310/uL with a differential including 65% neutrophils, 29% lymphocytes, 4% monocytes, and 2% eosinophils), hypoalbuminemia (27.6 g/L), and elevated serum creatinine levels (1.52 mg/dL); these were indicative of acute kidney injury. The aspartate transaminase, alanine transaminase, gamma-glutamyl transferase, and globulin levels were within normal limits. The blood cultures were negative. Incisional biopsy was performed for microscopic examination, differentiating cellulitis and pseudocellulitic conditions, and further tissue culture for microorganisms. Histopathological sections showed perivascular and interstitial inflammatory cell infiltration, predominantly in the markedly edematous papillary dermis (Figure 2A). The inflammatory cells comprised lymphocytes, neutrophils, and scattered histiocytes, including edema-phages with bubbly cytoplasm (Figure 2B). The overlying epidermis showed irregular acanthosis with extensive ballooning and keratocyte necrosis in discrete foci. Vasodilation and moderate erythrocyte extravasation were observed. Furthermore, numerous, large, irregular, thin-walled vascular channels lined by a single layer of flattened endothelial cells were observed (Figure 2C). These vascular spaces appeared empty.
The immunohistochemical examination revealed positive staining of the ectatic vessel endothelial cells with monoclonal antibodies to D2-40 (podoplanin), which are highly selective lymphatic endothelial markers of lymphatic endothelium; hence, this indicates that the vessels were of a lymphatic nature (Figure 2D).3 Special stains for microorganisms and tissue cultures of the biopsy specimens were negative.
Based on her clinical presentation and investigation findings, a diagnosis of AIE was made. We ensured fluid restriction and administered diuretics and adjuvant hemodialysis. No antibiotics were administered. Ten days following this treatment regimen, the cutaneous lesions showed marked response, and her condition was greatly improved.
Discussion
AIE is an under-recognized variant of pseudocellulitis that is characterized by bilateral, erythematous, edematous plaques on the dependent parts of the body.1 The principal differential diagnosis of AIE includes cellulitis and other pseudocellulitic conditions.4,5 The pathogenesis of AIE is proposed to be an acute volume overload with impaired lymphatic drainage, leading to microtrauma of the affected connective tissues with the subsequent release of inflammatory cytokines.2 Stain and culture of the specimens from skin lesions are recommended to help differentiate cellulitis from its mimicking conditions.5,6
AIE was first described by Marchionne et al in a case series of 15 patients; furthermore, they reported that the disease predominantly occurred in patients with a low serum albumin level, fluid overload, a body mass index ≥25 kg.m−2, and renal dysfunction.1 They described the skin biopsy findings of three patients, which revealed marked papillary edema and mixed inflammatory cell infiltration comprising neutrophils, lymphocytes, and histiocytes with bubbly cytoplasm (edema-phages) in all specimens; however, they did not provide representative histopathological pictures of these findings. Hence, our case report has demonstrated, for the first time, histopathological images of edema-phages that are described as swelling or ballooning of histiocytes, containing either small or large clear vacuoles in their cytoplasm. Unlike foamy histiocytes (lipid-laden macrophages), they do not contain cholesterol, which is observed as abundant fine granular and/or foamy content in the cytoplasm. We hypothesize that edema-phages are histiocytes engulfing excess protein and/or fluids in the extracellular space and could be categorized as pathognomonic cells under AIE.
Additionally, we observed extensive proliferation of abnormal dilated vascular channels that were later identified as lymphatic vessels, as they showed positive staining with monoclonal antibodies of D2-40 (podoplanin). This finding is crucial for differentiating AIE from cellulitis and other conditions of pseudocellulitis.7,8 Abnormal lymphatic channels may play an important role in the pathogenesis of AIE. Following development of volume overload, the impairment of lymphatic drainage may play a role in synergistically promoting extracellular fluid retention in the papillary dermis. In cases where the process occurs abruptly, microtears may develop in the surrounding connective tissue that may activate the process of inflammation.
The primary focus in AIE management is to reduce the excessive amount of body fluid. Hence, fluid restriction, diuretics, hemodialysis, local compression, frequent repositioning, and increased mobility are the recommended treatment modalities. In the absence of infection, antibiotics are not recommended in AIE. Misdiagnosis of AIE leads to inappropriate antibiotic administration, increased morbidity, and poor distribution of medical resources.9
Conclusion
We describe, for the first time, a case of AIE with histopathological and immunohistochemical findings. Despite the rarity of this condition, our report highlights that AIE is a distinct dermatosis with specific characteristics that help differentiate AIE from other conditions. Our observations also reinforce the role of lymphatic abnormalities in the pathogenesis of the disease.
Ethics Approval and Consent to Participate
This article was performed in accordance with the principles of Declaration of Helsinki. Ethical review and approval was not required to publish the case details in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the patient for publication of this case report and any accompanying images as per our standard institutional rules.
Funding
No sources of funding were used to prepare this manuscript.
Disclosure
The authors declare that this manuscript was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors report no conflicts of interest in this work.
References
1. Marchionne EM, McCalmont TH, Pincus LB, LeBoit PE, Fox LP. Acute inflammatory edema: a mimicker of cellulitis in critically ill patients. J Am Acad Dermatol. 2019;81(4):931–936. doi:10.1016/j.jaad.2019.05.083
2. Heymann WR. Acute inflammatory edema: a swell concept. J Am Acad Dermatol. 2019;81(4):906–907. doi:10.1016/j.jaad.2019.07.096
3. Kong LL, Yang NZ, Shi LH, et al. The optimum marker for the detection of lymphatic vessels. Mol Clin Oncol. 2017;7(4):515–520. doi:10.3892/mco.2017.1356
4. Korniyenko A, Lozada J, Ranade A, Sandhu G. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19(4):e141–142. doi:10.1097/01.mjt.0000522273.81146.49
5. Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73(1):70–75. doi:10.1016/j.jaad.2014.11.012
6. Surovy AM, Pelivani N, Hegyi I, Buettiker U, Beltraminelli H, Borradori L. Giant cellulitis-like Sweet syndrome, a new variant of neutrophilic dermatosis. JAMA Dermatology. 2013;149(1):79–83. doi:10.1001/2013.jamadermatol.548
7. Singer S, Li DG, Gunasekera N, et al. The ALT-70 cellulitis model maintains predictive value at 24 and 48 hours after presentation. J Am Acad Dermatol. 2019;81(6):1252–1256. doi:10.1016/j.jaad.2019.03.050
8. Suchonwanit P, Triamchaisri S, Wittayakornrerk S, Rattanakaemakorn P. Leprosy Reaction in Thai Population: a 20-Year Retrospective Study. Dermatol Res Pract. 2015;2015:253154. doi:10.1155/2015/253154
9. Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatology. 2017;153(2):141–146. doi:10.1001/jamadermatol.2016.3816
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.