Back to Journals » Infection and Drug Resistance » Volume 16
Acute Hepatitis E Induced the First Episode of Immune-Mediated Thrombotic Thrombocytopenic Purpura: The First Case Report
Authors Lv F, Zhao Y, Yang XD, Chen HZ, Ren WY, Chen LX, Yi QQ, Zheng W, Pan HY
Received 4 May 2023
Accepted for publication 22 July 2023
Published 9 August 2023 Volume 2023:16 Pages 5149—5154
DOI https://doi.org/10.2147/IDR.S418430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Fei Lv,1,2 Yue Zhao,2 Xing-Di Yang,2 Han-Zhu Chen,2 Wen-Ya Ren,2 Ling-Xia Chen,2 Qiao-Qiao Yi,2 Wei Zheng,2 Hong-Ying Pan2
1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, People’s Republic of China; 2Department of Infectious Diseases, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, 310014, People’s Republic of China
Correspondence: Hong-Ying Pan, Tel/Fax +86-571-8589-3603, Email [email protected]
Purpose: Hepatitis E virus infection mainly presents with liver-related symptoms, and multiple studies have shown that hepatitis E virus infection can also induce extrahepatic-related symptoms. Thrombotic thrombocytopenic purpura is an uncommon and fatal thrombotic microangiopathy characterized by severe thrombocytopenia, organ damage, and microangiopathic haemolytic anaemia. We report the first case in which acute hepatitis E induced the first episode of immune-mediated thrombotic thrombocytopenic purpura.
Patients and Methods: A 53-year-old male was admitted to our emergency department with fever, thrombocytopenia, and abnormal liver function. Laboratory tests revealed significant bilirubin, AST, and ALT elevations, renal impairment, positive anti-HEV IgM and IgG antibody results, schistocytes on the blood smear, 0% ADAMTS-13 activity, and positive ADAMTS13 inhibitor results. He was diagnosed with acute hepatitis E, which induced the first episode of immune-mediated thrombotic thrombocytopenic purpura.
Results: After receiving treatment with plasmapheresis, glucocorticoid medication, rituximab, and other supportive medicines, the patient’s physiological circumstances and laboratory indicators improved, and a 4-month follow-up revealed no abnormalities.
Conclusion: This is a unique case report of an acute hepatitis E-induced immune-mediated thrombotic thrombocytopenic purpura initial episode. This case report offers evidence that hepatitis E virus infection can cause thrombotic thrombocytopenic purpura. In patients with abnormal liver function and thrombocytopenia, we advise screening for hepatitis E or thrombotic thrombocytopenic purpura.
Keywords: extrahepatic manifestations related to hepatitis E, thrombocytopenia, abnormal liver function, case report
Introduction
Hepatitis E virus (HEV) infection is not only limited to the liver but also associated with many extrahepatic manifestations. Recent studies have found that HEV infection can affect the nervous system, blood system, reproductive system, kidney, pancreas and autoimmunity. Therefore, HEV infection should be considered a systemic disease. In the haematologic system, the effects of HEV mainly include anaemia, decreased platelet count, haematopoietic syndrome, pure erythrocyte hypoplasia, and monoclonal gammaglobulinemia of unknown significance.1 Thrombotic thrombocytopenic purpura (TTP) is a rare, life-threatening disease that is divided into immune and congenital categories and is a recognized medical emergency. TTP results from either a congenital or acquired absence/decrease of the von Willebrand factor-cleaving protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13). Low levels of ADAMTS13 activity result in microthrombi formation, which leads to end-organ ischaemia and damage.2 Viral infection, immunosuppressants, HIV, oestrogen-containing birth control pills, and pregnancy are common trigger risk factors for ADAMTS-13 antibody formation in immune-mediated TTP. Only 10% of patients with immune-mediated TTP (iTTP) survive without proper treatment.3 Notably, thrombocytopenia can result from HEV infection.4–7 However, no cases of acute hepatitis E inducing the first episode of iTTP have been reported. Here, we describe the first case in which acute hepatitis E induced the first episode of iTTP, which was promptly identified and successfully treated.
Case Report
A previously healthy 53-year-old male was admitted to our emergency department on 2022-10-10 at 12:00 p.m. with fever, thrombocytopenia, and abnormal liver function.
The patient is a cook. In the past 20 years, he has smoked approximately 10 cigarettes a day and consumed approximately 80 grams of alcohol once a week. Before the onset of this disease, he had no underlying diseases, his annual check-ups had never indicated thrombocytopenia or abnormal liver function, he had never had any hepatitis or any surgery or any history of blood transfusion before and had not used any drugs or herbs recently.
Eight days prior, the patient displayed weakness and severe nausea without a clear cause. He started experiencing muscle aches all over his body, a 39 °C body temperature, a gradual darkening of his urine to a coffee colour, yellow skin, and sclera staining 5 days prior. He visited the neighbourhood hospital. The results of the tests at that time revealed severe thrombocytopenia, abnormal liver function, and inflammation. His symptoms grew worse after 4 days of treatment with piperacillin sodium and tazobactam sodium for injection 4.5 g q8h, compound glycyrrhizin, reduced glutathione, transmetil, ursodeoxycholic acid, and interleukin-11, and he visited our emergency room.
At admission, he was conscious, able to answer questions clearly, his body skin and sclera depth yellow staining, and had no obvious haemorrhagic points or purpura on his body. He also had no obvious cardiopulmonary abnormalities, light tenderness in his xiphoid process, no enlargement of his liver or spleen, Murphy’s sign (+), and no abnormalities in the nervous system physical examination. Urgent laboratory testing were as follows: PLT count 13 K/UL, Hb 104 g/L, Ret 52.4 K/UL, RBC count 3.47*10^6/UL, WBC count 10.94 K/UL, CRP 84.9 mg/L, ALT 288 U/L, AST 580 U/L, GGT 130 U/L, ALP 217 U/L, TB 15.76 mg/dL, DB 12.89 mg/dL, PT 14s, INR 1.22, and Cr 1.68 mg/dL. The direct Coombs test was negative, and the COVID-19 nucleic acid test was negative.
Therapies were initiated empirically with intravenous fluids. Glucocorticoids were given intravenously at a dose of 80 mg q12h and were combined with high-dose gamma globulin, meropenem 1 g q8h of anti-infection, ornithine aspartate and adenosine methionine for liver protection, and interleukin-11 to produce platelets. He also received 20 units of platelets and 300 mL of plasma.
On 2022-10-11 at 02:30, the patient suddenly displayed unconsciousness, an elevated heart rate, and limb convulsions, then developed decreased blood pressure and heart rate, and the carotid pulse was unreachable. Cardiopulmonary resuscitation was immediately performed. Approximately 1 minute later, the patient returned to sinus heart rate. After the rescue, we immediately performed blood tests on the patient, and the results showed that the levels of myocardial injury markers and blood potassium were normal. An urgent CT scan showed no obvious brain abnormalities, scattered cholecystitis or inflammation in the two lungs.
Ten hours later, laboratory test results were returned as follows: anti-HEV-IgM antibodies (1.3 S/CO) and anti-HEV-IgG antibodies (5.9 S/CO) were positive, whole blood metagenomics for pathogen detection failed to find any pathogens’ DNA or RNA, anti-endothelial cell antibodies (AECA) +1:100. His laboratory tests did not support the diagnosis of hepatitis A or B or C, toxoplasmosis, Treponema pallidum, rubella, cytomegalovirus, herpesvirus and HIV. After a multidisciplinary discussion among doctors in the infectious, haematology and emergency departments, the preliminary diagnosis was 1. Acute hepatitis E;8 2. Non-exclusion of haematological diseases; 3. Possibility of autoimmune liver disease; and 4. Cholecystitis. Considering the haematological diseases, we immediately performed bone marrow aspiration and biopsy and peripheral blood smear cell morphology analysis. We found more than 1% schistocytes on the peripheral blood smear (shown in Figure 1). On that day, his haemoglobin level was 8.3 g/dL, and schistocytes on the bone marrow smear were also found, suggesting microangiopathy haemolytic anaemia (MAHA). Laboratory results on 2022-10-10 were used to evaluate the patient via the PLASMIC score: peripheral blood platelet counts 13 K/UL (1 score), indirect bilirubin 2.87 μmol/L (1 score), no advanced cancer (1 score), no history of solid organ transplantation or stem cell transplantation (1 score), International Normalized Ratio (INR) 1.22 (1 score), and Cr 1.68 mg/dL (1 score), with 6 scores in total. It was suggested that the patient had a high risk of TTP. The patient’s peripheral blood was sent for testing on 2022-10-12 to determine ADAMTS-13 activity and its inhibitor. The test results came back the following day, showing that ADAMTS-13 activity was 0% and ADAMTS-13 inhibitor was positive. The diagnosis of iTTP was clear.
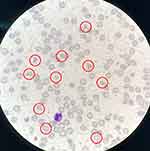 |
Figure 1 Schistocytes found in the peripheral blood smear on 2022-10-11 (in the red circle). |
We started the primary treatment plan immediately, which included intravenous administration of rituximab 0.6 g once a week, 80 mg of glucocorticoids every 12 hours, and daily plasma exchange. The time between plasmapheresis was gradually increased throughout the course of the treatment, and the dosage of glucocorticoids was decreased from 80 mg q12h (for 6 days) to 40 mg q12h (for 5 days) to 20 mg q12h (for 7 days) to 30 mg qd (for 5 days) to 20 mg qd (at discharge). The patient gradually became conscious, his platelet counts gradually increased, while bilirubin, AST, ALT, and Cr gradually decreased, ADAMTS-13 activity recovered to 100%, and the ADAMTS-13 inhibitor returned negative on 2022-10-18.
The patient’s platelet counts reached 254 K/UL on 2022-11-04 following the final plasma exchange and rituximab treatment on 2022-11-03, with a total of 12 plasma exchanges and 4 doses of rituximab. Liver function indicators were also much better than they were previously (shown in Figure 2). Schistocytes were still visible in peripheral blood smears that day, but they were considerably fewer than they had been earlier in the course of treatment. The patient was eligible for discharge and was discharged on 2022-11-05 with oral glucocorticoids (20 mg qd) and ursodeoxycholic acid.
 |
Figure 2 PLT count (K/UL), Hb value (g/L), ALT level (U/L) trends and anti-HEV IgM/IgG Ab titer throughout the course of plasma exchange, rituximab and glucocorticoids during hospitalization. |
The patient underwent routine follow-up exams at the hospital over the course of the following five months, which revealed no anomalies.
Discussion
HEV infection has many extrahepatic manifestations in addition to its liver-specific effects and should be viewed as a systemic illness as a result.1 There have been a few reports of thrombocytopenia linked to HEV infection4–7 but very few reports of acute HEV infection with TTP. To our knowledge, only Mar Rivero-Barciela et al reported one case of transfusion-induced acute hepatitis E with TTP relapse in 2018.9
TTP is a rare, life-threatening disease, and timely diagnosis and treatment are essential to save lives.10 Viral infection, immunosuppressants, HIV, oestrogen-containing birth control pills, and pregnancy are common trigger risk factors for ADAMTS-13 antibody formation in iTTP.2
In this case report, our patient was acutely infected with HEV. He was also diagnosed with iTTP. Other triggers of iTTP include pharmacological factors, inflammation, HIV, HAV, HBV, HCV, etc., were ruled out by the patient’s medical history, whole blood metagenomics for pathogen detection and other laboratory tests. This strongly suggests that the patient’s iTTP was caused by acute HEV infection. It should be noted that we found that the patient’s AECA was +1:100. Although AECA has occasionally been shown to be present in TTP, its role in the pathogenesis of the disease has never been ascertained.11 Another study found that AECA plays a role in the thrombocytopenia and vasculitis characteristic of TTP.12 Regarding the mechanism by which HEV induces iTTP, we did not find clear explanations in the available reports. According to our case report, HEV may be involved in the production of ADAMTS13 inhibitors and AECA, followed by TTP. The exact mechanism still needs more in-depth study.
TTP used to be classified as a clinical diagnosis based on the typical clinical symptoms it exhibits, including fever, thrombocytopenia, MAHA, renal dysfunction, and neurological dysfunction.10 Pentalogy, however, only affects 5% of patients and is typically only fully apparent when the disease is advanced. To determine whether a patient had a severe ADAMTS-13 activity deficiency, the PLASMIC score was used.13
The symptoms of acute hepatitis E in this patient interfered with the diagnosis of TTP. He had fever, MAHA (schistocytes on the blood smear, decreased haemoglobin, elevated indirect bilirubin in peripheral blood smear), severely decreased platelet count, renal impairment (serum creatinine 1.68 mg/dL) and neurological symptoms (sudden loss of consciousness and convulsion in the emergency room). The clinical diagnosis of TTP was essentially confirmed by the PLASMIC score, which showed a high risk of TTP. We immediately began massive shock therapy, which included glucocorticoids, gamma globulin, and plasma infusion, based on the clinical diagnosis. Subsequent reports of ADAMTS-13 activity and its inhibitor confirmed our clinical diagnosis. According to a recent study, bilirubin and haemoglobin cause false low levels of ADAMTS-13 activity in FRET assays but not in VWF:CB assays and chromogenic assays. Lipids might block the device membrane in a semiquantitative assay.14 Our ADAMTS-13 activity tests used VWF:CB assays, and our patient did not have hyperlipidaemia. Despite the patient’s hyperbilirubinemia, the results of ADAMTS-13 activity are credible.
We therefore recommend that ADAMTS-13 testing be considered after the PLASMIC score to confirm or exclude TTP in patients with acute hepatitis E with unexplained severe thrombocytopenia and/or MAHA.
The primary recommendations for the emergency treatment of TTP are daily plasma exchange, glucocorticoid shock therapy, and rituximab once per week for a total of four weeks, per the TTP guidelines of China 2022,13 ISTH 202015 and Japan 2017.16 Our patient had a platelet infusion at the time of admission, but according to the recommendations, patients with a high suspicion of TTP who have not had plasmapheresis should not, in general, have a platelet infusion because it raises the risk of microthrombosis and organ damage. The recommendations state that platelet infusion after plasmapheresis should only be taken into account in cases of life-threatening bleeding in vital organs.
We should have carefully considered whether platelet transfusion was necessary because our patient did not have serious bleeding tendencies or bleeding in a vital organ at the time of admission. This should serve as a warning to other physicians. The patient experienced a complete clinical response, demonstrating the efficacy of our treatment from the standpoint of therapeutic effect.
Acute hepatitis E and TTP are both serious and life-threatening diseases, and the patient in this case report suffered from the first attack of TTP. It is more difficult to diagnose and treat these two diseases because their clinical symptoms overlap, and it is easy to miss the diagnosis of or misdiagnose TTP, which can be fatal. Diagnostic test results, such as those from ADAMTS-13, may also be delayed. When the health care team fails to suspect TTP, treatment may be delayed or even result in patient death.2
Conclusion
The diagnosis of acute hepatitis E and iTTP in our patient was clear, and other common causes of iTTP were excluded. We believe we have reported the first case of acute hepatitis E inducing the first episode of iTTP. This case provides evidence for TTP induced by HEV infection. We advise screening patients with abnormal liver function and thrombocytopenia for the presence of hepatitis E and/or TTP. When confirming or ruling out TTP in patients with acute hepatitis E who have unexplained severe thrombocytopenia and/or MAHA, ADAMTS-13 testing should be taken into account. More cases of TTP induced by HEV infection should be collected and integrated to explore the potential connection between HEV infection and TTP.
Abbreviation
MAHA, microangiopathy haemolytic anaemia.
Acknowledgments
We would like to acknowledge the patient and his family who gave informed consent for this publication. No Institutional approval was required for publication of this case report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Wu J, Xiang Z, Zhu C, et al. Extrahepatic manifestations related to hepatitis E virus infection and their triggering mechanisms. J Infect. 2021;83(3):298–305. doi:10.1016/j.jinf.2021.07.021
2. Stanley M, Killeen RB, Michalski JM. Thrombotic thrombocytopenic purpura. In: StatPearls. Treasure Island (FL): StatPearls PublishingCopyright © 2022, StatPearls Publishing LLC.; 2022.
3. George JN. TTP: the evolution of clinical practice. Blood. 2021;137(6):719–720. doi:10.1182/blood.2020009654
4. Yamazaki Y, Naganuma A, Arai Y, et al. Clinical and virological features of acute hepatitis E in Gunma prefecture, Japan between 2004 and 2015. Hepatol Res. 2017;47(5):435–445. doi:10.1111/hepr.12765
5. Woolson KL, Forbes A, Vine L, et al. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40(11–12):1282–1291. doi:10.1111/apt.12986
6. Masood I, Rafiq A, Majid Z. Hepatitis E presenting with thrombocytopaenia. Trop Doct. 2014;44(4):219–220. doi:10.1177/0049475514521610
7. Fourquet E, Mansuy JM, Bureau C, et al. Severe thrombocytopenia associated with acute autochthonous hepatitis E. J Clin Virol. 2010;48(1):73–74. doi:10.1016/j.jcv.2010.02.016
8. European Association for the Study of the Liver. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256–1271.
9. Riveiro-Barciela M, Bes M, Quer J, et al. Thrombotic thrombocytopenic purpura relapse induced by acute hepatitis E transmitted by cryosupernatant plasma and successfully controlled with ribavirin. Transfusion. 2018;58(11):2501–2505. doi:10.1111/trf.14831
10. Bull TP, McCulloch R, Nicolson PLR. Diagnostic uncertainty presented barriers to the timely management of acute thrombotic thrombocytopenic purpura in the United Kingdom between 2014 and 2019. J Thromb Haemost. 2022;20(6):1428–1436. doi:10.1111/jth.15681
11. Praprotnik S, Blank M, Levy Y, et al. Anti-endothelial cell antibodies from patients with thrombotic thrombocytopenic purpura specifically activate small vessel endothelial cells. Int Immunol. 2001;13(2):203–210. doi:10.1093/intimm/13.2.203
12. Wright JF, Wang H, Hornstein A, et al. Characterization of platelet glycoproteins and platelet/endothelial cell antibodies in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 1999;107(3):546–555. doi:10.1046/j.1365-2141.1999.01751.x
13. Yin J, Yu ZQ. 血栓性血小板减少性紫癜诊断与治疗中国指南 (2022年版) [Chinese guideline on the diagnosis and management of thrombotic thrombocytopenic purpura (2022)]. Zhonghua Xue Ye Xue Za Zhi. 2022;43(1):7–12. Chinese. doi:10.3760/cma.j.issn.0253-2727.2022.01.002
14. Woods AI, Paiva J, Dos Santos C, Alberto MF, Sánchez-Luceros A. From the discovery of ADAMTS13 to current understanding of its role in health and disease. Semin Thromb Hemost. 2023;49(3):284–294. doi:10.1055/s-0042-1758059
15. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496–2502. doi:10.1111/jth.15010
16. Matsumoto M, Fujimura Y, Wada H, et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. 2017;106(1):3–15. doi:10.1007/s12185-017-2264-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
