Back to Journals » Journal of Pain Research » Volume 14
Acute Effects of a Brief Physical Exercise Intervention on Somatosensory Perception, Lumbar Strength, and Flexibility in Patients with Nonspecific Chronic Low-Back Pain
Authors Sitges C , Velasco-Roldán O , Crespí J, García-Dopico N, Segur-Ferrer J , González-Roldán AM, Montoya P
Received 1 September 2020
Accepted for publication 14 November 2020
Published 18 February 2021 Volume 2021:14 Pages 487—500
DOI https://doi.org/10.2147/JPR.S274134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Carolina Sitges,1,2 Olga Velasco-Roldán,1,3 Jaume Crespí,1 Nuria García-Dopico,1 Joan Segur-Ferrer,1 Ana María González-Roldán,1,2 Pedro Montoya1,2
1Research Institute of Health Sciences (IUNICS) and Balearic Islands Health Research Institute (IdISBa), University of the Balearic Islands (UIB), Palma, Spain; 2Departament of Psychology, UIB, Palma, Spain; 3Departament of Nursing and Physiotherapy, UIB, Palma, Spain
Correspondence: Carolina Sitges
Research Institute of Health Sciences (IUNICS), University of Balearic Islands (UIB), Carretera de Valldemossa km 7.5, Palma, 07122, Spain
Tel +34 971-259-885
Fax +34 971259501
Email [email protected]
Background: Evidence-based clinical guidelines consider physical exercise one of the best nonpharmacological interventions for low-back pain (LBP), but it is necessary to clarify the exercise-induced hypoalgesia effect of different modalities of exercise in chronic pain populations.
Purpose: This study focused on exploring acute changes in tactile and pressure-pain perception and lumbar strength and flexibility in patients with nonspecific chronic LBP (NSCLBP) after performing one of three 20-minute physical exercise modalities.
Methods: A total of 81 patients with NSCLBP were pseudorandomly distributed into three groups of 20-minute physical exercise — 1) aerobic (n=21, mean age 42± 9.72 years, nine men), 2) stretching (n=21, mean age 40± 11.37 years, ten men), and 3) strengthening (n=20, mean age 35.80± 11.56 years, ten men) — and 4) a control group (n=19, mean age 38.64± 10.24 years, eight men), and completed self-reported questionnaires during the same period. Tactile and pressure-pain thresholds and isometric lumbar muscle endurance and flexibility were assessed before and after this brief exercise-based intervention.
Results: All groups were comparable in terms of sociodemographic and clinical data, cardiovascular capacity, and self-reported data onphysical disability, mood, motivation, psychological response to stimulus properties of physical exercise, and physical activity enjoyment. Our analyses revealed higher tactile sensitivity (p< 0.001) and pressure-pain thresholds (p< 0.001) at the forefinger than other body locations. We also found lower pain sensitivity (p=0.010) and pressure pain–intensity ratings (p=0.001) and higher lumbar flexibility (p< 0.001) after intervention. After calculation of absolute pre–post differences, higher tactile sensitivity was observed at the gluteus medius muscle than the erector spinal muscle only after aerobic intervention (p=0.046).
Conclusion: These results add some evidence about different modalities of exercise-induced hypoalgesia in NSCLBP. However, the fact that we also found improvements in the control group limits our conclusions.
Keywords: low-back pain, exercise therapy, aerobic exercise, flexibility
Introduction
With a vital prevalence of 70%–80%, low-back pain (LBP) is one of the ailments most experienced by the general population and the main cause of disability in industrialized countries, leading to significant public-health expenditure in terms of care and labor.1,2 About 90%–95% are of nonspecific origin3 and 90% acute (for less than 6 weeks),1 while 24%–87% will be recurrent and 50%–70% will become chronic (from 12 weeks).1,4
Evidence-based clinical guidelines consider physical exercise one of the best nonpharmacological interventions for LBP.5–7 However, is difficult to draw clear conclusions about efficiency, due to the heterogeneity of studies in terms of exercise modalities, intensity, duration, dose response, frequency, or supervision.7,8 Although several exercises, such as lumbar stabilization and core strengthening, have been widely considered in clinical practice good interventions to manage chronic LBP (CLBP), recent systematic reviews have found strong evidence that they are not more effective than any other form of active exercise in the long term.9,10 It is widely accepted that aerobic exercise, such as walking, is a good choice for LBP, as it strengthens the back muscles and reduces joint stiffness. Specifically, walking quickly activates the lumbar multifidus, and prolonged activation of paraspinal muscles is related to increases in muscle strength.9 Likewise, strength training produces changes in both facilitator and inhibitory stimuli, because activation of fundamental muscles involved in movement is increased, coactivation of antagonist muscles reduced, and coactivation of synergistic muscles improved.11 Furthermore, while stretching is the main intervention of physiotherapists to treat and prevent contractures, there is strong scientific evidence that this exercise does not have short-term effects on pain and joint mobility, and moderate evidence that it does not improve quality of life.12 Instead, other authors have suggested that flexibility exercises can improve postural stability and muscle balance, especially when combined with resistance exercises or muscle relaxation.13 As stated in a recent umbrella overview, the most effective form of exercise as a method of rehabilitation for CLBP is still unknown.14
Additionally, clinically important pain reductions might be observed after a single session of exercise: exercise-induced hypoalgesia (EIH).8 Numerous studies have examined effects of acute exercise on responses to experimentally induced noxious stimulation in healthy and clinical populations.15 A meta-analytic review showed that perception of experimentally induced pain in healthy participants for aerobic exercise was moderate, and for isometric and dynamic resistance exercises it was large.15 However, although EIH has been consistently demonstrated in pain-free participants, the acute effect of exercise on pain sensitivity is more variable in chronic pain populations, where increased hypoalgesia, reduced hypoalgesia, or even hyperalgesia has been observed.8,16 Current research supports the view that EIH can be impaired in different musculoskeletal pain disorders, which can explain the varied response to exercise in this population and influence the results of exercise prescription.17 In fact, EIH may not even be observed in chronic widespread-pain patients when exercising at moderate–high intensity, with exercise often exacerbating experimental pain.15
Considering CLBP is a highly prevalent and costly condition for which exercise management is frequently recommended, it is important to highlight that the evidence on EIH in this population is scarce and inconclusive. To the best of our knowledge, only two studies have examined EIH in a reduced sample of CLBP patients after submaximal aerobic exercise, showing a small effect on pressure-pain thresholds (PPTs)18 and a large effect on pressure pain–intensity ratings (PPIRs)19 after submaximal aerobic exercise. Another study examined EIH after a repeated lifting task, andno changes were detected over the lumbar erector spinae muscles in quantitative sensory tests, supporting impaired EIH in CLBP patients.17 However, effects on tactile and pain sensitivity of other exercise modalities in nonspecific CLBP (NSCLBP) have not been examined yet, so further research is needed to unravel the immediate analgesic response to exercise and whether this is affected in NSCLBP patients. As such, the main objective of this study was exploring acute effects of 20-minute exercise interventions (aerobic, strengthening, stretching) on tactile sensitivity, PPTs, and lumbar strength and flexibility in patients with NSCLBP. We hypothesized that exercise interventions would show higher tactile sensitivity and increased PPTs thanh a control group. Furthermore, we did not expect exercise modality–dependent effects on tactile sensitivity or PPTs.
Methods
Participants
The sample size was calculated using GRANMO (https://www.imim.cat/ofertadeserveis/software-public/granmo). Accepting an α risk of 0.05 and a β risk of 0.2 in a two-sided test, 25 subjects were necessary in each group to recognize as statistically significant a minimum difference of 1.5 units between any pair of groups, assuming that four groups existed and a common deviation of 1.5 and anticipating no dropouts. Inclusion criteria were subjects aged 18–59 years with NSCLBP >6 weeks or with at least three episodes of LBP (lasting >1 week) during the year prior to the study. Exclusion criteria were high functional impairment compromising such activities as walking, sitting, or getting up from a chair at both evaluation and intervention, pain at time of evaluation and/or intervention >5 (out of 10) on a visual analogue scale (VAS), and history or presence of sciatic radiating pain, referred pain, or osteoarthritis to lower extremities, spine surgery, spinal or pelvic fracture, hospitalization for serious trauma, injuries, or traffic accidents, and systematic diseases affecting the locomotor system. Finally, 81 patients with NSCLBP were pseudorandomly distributed using minimization (ie, stratifying patients according to age and sex) to ensure that treatment arms were balanced with respect to predefined factors into three groups of 20-minute physical exercise — 1) aerobic (n=21, mean age 42±9.72 years, nine men), 2) stretching (n=21, mean age 40±11.37 years, ten men), and 3) strengthening (n=20, mean age 35.80±11.56 years, ten men) — and 4) a control group (n=19, mean age 38.64±10.24 years, eight men), and questionnaires were completed during the same period (see Figure 1).
 |
Figure 1 Flowchart of participants during the selection and analysis phases. |
Both the protocol and information sheet were approved by the Research Ethics Committee of the Balearic Islands (IB 3186/16 PI), and the study complied with the Declaration of Helsinki and registered on the Australian New Zealand Clinical Trials Registry (ACTRN12618000997257).
Exercise Interventions
Aerobic intervention consisted of walking on a treadmill at low–moderate intensity (65.85%±7% of maximum heart rate and 3.02±1.04 using the Borg Scale of Perceived Exertion), measured as mean values collected every minute for 20 minutes. Stretching intervention consisted of stretching exercises of hip flexors, spinal extenders, quadratus lumborum, hamstrings, and external rotators in a supine position. Strengthening intervention consisted of preparatory exercises of pelvic tilt and abdominal drawing-in maneuver (bringing navel towards lumbar region), exercises of spinal extenders on a fitness ball, and other exercises to strengthen the gluteus, hamstrings, and abdominals in a supine position (for more details, see Supplementary material).
Sociodemographic, Clinical, and Self-Reported Data
Sociodemographic, substance-use, and medical and family-history data were collected using a semistructured interview. Participants rated their pain intensity marking a cross on a drawn line, and located their pain using a body map on paper. Then, assuming a very small redraw-error rate,20 we digitalized pain drawings (Navigate Pain, Aalborg University, Denmark), in order to build digital body maps of pain location (see Figure 2).
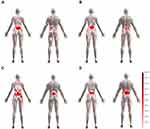 |
Figure 2 Digital pain chart of the body for aerobic (A), stretching (B), strengthening (C), and control (D) groups. |
Physical disability, mood, physical activity motivation, and psychological response to stimulus properties of physical exercise were measured using the Spanish versions of the Oswestry Disability Index,21 Profile of Mood States,22 Behavioural Regulation in Exercise Questionnaire,23 and Subjective Exercise Experience Scale,24 respectively. Cardiovascular capacity was measured using walking distance (m) during the 6-minute walking test25 and percentage predicted value using Casanova et al’s equation.26 Finally, the Spanish version of the Physical Activity Enjoyment Scale27 was used to assess enjoyment after intervention.
Tactile and Pressure-Pain Thresholds
We used the method of limits to determine tactile thresholds using manual von Frey filaments at one unilateral LBP location (spinal erector muscle), two locations proximal to the low back (gluteus medius muscle and sacrum), and one distal from the low back (forefinger) three consecutive times and in counterbalanced order to measure the presence of generalized pain. Individual PPTs (maximum pressure 5 kg/cm2) were also measured with a digital algometer (FPIX 50; Wagner Instruments, Greenwich, CT, USA) at the same body sites and in counterbalanced order. Subjective PPIRs were also measured using a VAS (0–10). Previously to PPTs, all participants received unpainful established pressure (2 kg/cm2) to the same body sites to evaluate the presence (or not) of hyperalgesia, and PPIRs were also measured using the VAS. Algometry was always conducted by the same researcher and according to the method recommended by Fischer.28 The mean of three measurements of both measures was used for statistical analysis.
Isometric Lumbar Muscle Endurance and Flexibility
Isometric lumbar muscle endurance was evaluated using the Ito test.29 From a prone position, participants had to maintain their self-perceived maximum lumbar extension (maximum 5 minutes). Time was used for statistical analysis. In order to ensure the maintenance of lumbar extension, we positioned a 9 cm object between the stretcher and the sternal manubrium during the evaluation. A small pillow was placed under the lower abdomen to decrease lumbar lordosis. We also controlled participant execution by active surveillance, detailed description of expected performance (extensor of lumbar spine, with feet off the stretcher, arms held parallel to the body, maximum neck flexion and gluteus contraction during the test), and forbidding them to use their arms or raise their legs during the evaluation. Back flexibility was also measured using the bilateral back-saver sit-andreach test,30,31 and the mean of two attempts for each leg was used for statistical analysis.
Procedure
First, participants were verbally informed about the aim of the study, asked to sign an informed-consent form, and pseudorandomly assigned to groups. Sociodemographic, self-reported, and clinical data were collected on paper or electronically (via LimeSurvey version 2.67.3+170,728) the same day or during the same week of the intervention. Tactile sensitivity and PPTs, isometric lumbar muscle endurance and flexibility, and cardiovascular capacity were measured before the intervention. Thereafter, participants performed the intervention individually under supervision (or if assigned to control group, completed questionnaires). Finally, tactile sensitivity and PPTs, isometric lumbar muscle endurance, and flexibility tests were repeated after the intervention.
Data Analyses
One-way analyses of variance (ANOVAs) with between-subject factors for group (aerobic, stretching, strengthening, and control) were used to examine differences in age, clinical data, cardiovascular capacity, and self-reported data. The X2 test was used for testing sex distribution. Lumbar endurance was analyzed using a mixed-model ANOVA with group as the between-subject factor and time (before and after) as the within-subject factor. Tactile sensitivity and PPTs and lumbar flexibility were analyzed using the same factors plus body location (spinal erector muscle, gluteus medius muscle, sacrum, and forefinger) and laterality (right and left).
Absolute pre–post differences (values before minus after intervention) for tactile sensitivity and PPTs were also calculated and tested using mixed-model ANOVAs with group as the between-subject factor and body location as the within-subject factor. These absolute differences in lumbar endurance and flexibility were also calculated and tested using one-way ANOVAs.
Finally, we computed the pressure pain–sensitivity index (ratio between subjective PPIRs and PPTs) and tested this using mixed-model ANOVAs with group as the between-subject factor and time and body location as within-subject factors. We also calculated absolute pre–post differences in pressure pain–sensitivity index values and tested these using mixed-model ANOVAs with group as the between-subject factor and body location as the within-subject factor.
For all ANOVAs, normal distribution and homoscedasticity were tested using Kolmogorov–Smirnov and Levene tests, respectively, and Welch and Kruskal–Wallis tests were used in cases of violation of these assumptions, respectively. Greenhouse–Geisser corrections were also applied to control for violation of the sphericity assumption. Additionally, extreme-outlier values (more than three times interquartile range) were dropped. When significant effects were found (p<0.05), post hoc analyses were performed using Bonferroni correction for multiple comparisons. We also computed effect sizes for ANOVA (ηp2, see Table 2) and independent t-test comparisons between all groups (Cohen’s d) of the absolute pre–post differences for each body location (ie, spinal erector muscle, gluteus medius muscle, sacrum, and forefinger) of tactile thresholds, pain-intensity ratings of unpainful pressure, PPTs, PPITs, pain-sensitivity index, and isometric lumbar muscle endurance and flexibility measures (see Supplementary Table 1). All statistical analyses were performed using SPSS version 25.0.
 |
Table 1 Sociodemographic, clinical, cardiovascular capacity, and self-reported data |
Results
Sociodemographic, Clinical, and Self-Reported Data
All groups were comparable in sociodemographic and clinical data, cardiovascular capacity and self-reported data (see Table 1). Furthermore, 23.33% of medication consumed were analgesics, 27.78% nonsteroidal anti-inflammatory drugs, 11.11% anxiolytics, 6.67% antidepressants, and 31.11% other medications or supplements (eg, antithyroid, β-blockers, bronchodilators, and antihistamines).
Tactile Thresholds
Mixed-model ANOVA revealed significant main effects of body location on tactile thresholds (F3, 231=13.768, p<0.001, Greenhouse-Geisser correction — ηp2=0.152). Bonferroni post hoc analyses showed differences between the forefinger and spinal erector muscle (5.839±1.602 vs 6.707±1.710, p=0.008) and gluteus medius muscle (5.839±1.602 vs 7.154±1.620, p<0.001), and between the gluteus medius muscle and sacrum (7.154±1.620 vs 6.115±1.647, p<0.001; see Figure 3). Regarding absolute pre–post differences, mixed-model ANOVA revealed a trend only for a body location × group interaction effect, but with a medium effect size (F9, 231=1.808, p=0.068, ηp2=0.066). Bonferroni post hoc analyses showed higher tactile sensitivity at the gluteus medius muscle than the erector spinal muscle only after aerobic intervention (−0.429±3.924 vs 0.810±9.347, p=0.046, see Table 2).
Pain-Intensity Ratings for Unpainful Pressure
Mixed-model ANOVA revealed significant main effects of body location on PPIRs for unpainful established pressure (2 kg/cm2 — F3, 231=26.293, p<0.001, Greenhouse–Geisser correction — ηp2=0.255). Bonferroni post hoc analyses showed differences between the forefinger and spinal erector muscle (0.981±1.314 vs 1.663±1.782, p<0.001), sacrum (0.981±1.314 vs 1.924±2.043, p<0.001), and gluteus medius muscle (0.981±1.314 vs 2.430±2.034, p<0.001), between the spinal erector muscle and gluteus medius muscle (1.663±1.782 vs 2.430±2.034, p<0.001), and between the sacrum and gluteus medius muscle (1.924±2.043 vs 2.430±2.034, p=0.007). This analysis also showed a significant time × body location-interaction effect (F3, 231=3.608, p=0.017, Greenhouse–Geisser correction — ηp2=0.045). Bonferroni post hoc analyses showed a pre–post trend for thespinal erector muscle (1.471±1.782 vs 1.854±2.187, p=0.060; see Figure 4). Regarding absolute pre–post differences, mixed-model ANOVA also revealed significant main effects of body location (F3, 231=3.608, p=0.014, ηp2=0.045). Bonferroni post hoc analyses showed differences between the spinal erector muscle and gluteus medius muscle (−0.383±1.800 vs 0.218±1.764, p=0.042; see Table 2).
Pressure-Pain Thresholds, Pressure Pain–Intensity Ratings, and Pain Sensitivity Index
Mixed-model ANOVA revealed significant main effects of body location on PPTs (F3, 231=11.519, p<0.001, Greenhouse–Geisser correction — ηp2=0.130). Bonferroni post hoc analyses showed differences in PPTs between the forefinger and gluteus medius muscle (3.2401±1.053 vs 2.821±1.107, p<0.001), between the spinal erector muscle and sacrum (3.017±1.098 vs 3.220±1.089, p=0.039), the gluteus medius muscle (3.017±1.098 vs 2.821±1.107, p=0.002), and between the sacrum and gluteus medius muscle (3.220±1.089 vs 2.821±1.107, p<0.001; see Figure 5A). Regarding absolute pre–post differences, no statistically significant differences were observed (see Table 2).
Mixed-model ANOVA also revealed significant main effects of time on subjective PPIRs (F1, 77=13.142, p=0.001, ηp2=0.146). Bonferroni post hoc analyses showed lower PPIRs after intervention than before (2.581±1.584 vs 2.865±1.629, p=0.001; see Figure 5B). This analysis also showed significant main effects for body location (F3, 231=28.285, p<0.001, Greenhouse–Geisser correction — ηp2=0.269). Bonferroni post hoc analyses also showed lower PPITs after intervention than before (2.581±1.584 vs 2.0.865±1.629, p=0.001), and differences between the forefinger and spinal erector muscle (2.030±1.557 vs 2.757±1.728, p<0.001), sacrum (2.030±1.557 vs 3.043±1.818, p<0.001), and gluteus medius muscle (2.030±1.557 vs 3.064±1.764, p<0.001) and between the spinal erector muscle and gluteus medius muscle (2.757±1.728 vs 3.064±1.764, p=0.003). Regarding absolute pre–post differences, no statistically significant differences were observed (see Table 2).
Mixed-model ANOVA revealed significant main effects of time on the pressure pain–sensitivity index (F 1, 77=7.074, p<0.001, Greenhouse–Geisser correct — ηp2=0.084). Bonferroni post hoc analyses showed a reduction in pain sensitivity after intervention (1.217±0.945 vs 1.082±0.918, p=0.010). This analysis also showed significant main effects of body location (F3, 231=24.086, p<0.001, Greenhouse–Geisser correction — ηp2=0.238). Bonferroni post hoc analyses showed differences between the forefinger and spinal erector muscle (0.777±0.720 vs 1.171±0.954, p<0.001), sacrum (0.777±0.720 vs 1.250±1.170, p<0.001), and gluteus medius muscle (0.777±0.720 vs 1.401±1.098, p<0.001) and between the spinal erector muscle and gluteus medius muscle (1.171±0.954 vs 1.401±1.098, p<0.001; see Figure 6). Regarding absolute pre–post differences, no statistically significant differences were observed.
Isometric Lumbar Muscle Endurance and Flexibility
No statistically significant differences were observed forlumbar endurance (see Figure 7). Mixed-model ANOVA revealed significant main effects of time on lumbar flexibility (F1, 75=16.217, p<0.001, ηp2=0.178). Bonferroni post hoc analyses showed higher lumbar flexibility after intervention than before (20.294±8.018 vs 19.158±7.743, p<0.001). This analysis also revealed a significant laterality × time interaction effect (F1, 75=5.952, p=0.017, ηp2=0.074). Bonferroni post hoc analyses also showed that pre–post differences were maintained in both the right (19.014±7.672 vs 20.443±8.081, p<0.001) and left leg (19.301±8.072 vs 20.144±8.169, p=0.007; see Figure 8). Regarding absolute pre–post differences, no statistically significant differences were observed forlumbar endurance or lumbar flexibility (see Table 2).
 |
Figure 7 Isometric lumbar muscle endurance (means and SE), using the Ito test (seconds), before and after the 20-minute physical exercise. |
 |
Figure 8 Lumbar flexibility (means and SE), using the bilateral back-saver sit-and-reach test (cm), before and after the 20-minute physical exercise. *p<0.001. |
Discussion
Previous research about EIH in CLBP patients islimited to three studies focused on aerobic exercise. We consider that the results of our study could have clinical relevance, because all modalities of our exercise-based intervention (ie, aerobic, strengthening, and flexibility) caused improvements (ie, lower pain sensitivity and PPITs and higher lumbar flexibility) among NSCLBP patients. Partially agreeing with our hypothesis and previous research, our analyses revealed lower pain sensitivity and PPIRs and higher lumbar flexibility after intervention on any exercise modality. Meeus et al18 showed statistically significant differences in PPTs for the back (L3) and nonspecific locations for extremities after a submaximal cycle ergometer aerobic exercise protocol in CLBP patients. Hoffman et al19 also reported reduced PPIRs on a VAS at 10-second intervals during pressure-pain stimulus to the nondominant index finger following aerobic exercise (cycling) in this population. On the contrary, Kuithan et al17 found no changes forthe lumbar erector spinae muscles in quantitative sensory tests (including PPTs, thermal detection, pain thresholds, and temporal summation) after a repeated-lifting task, supporting impaired EIH in CLBP patients. However, obtaining improvements (ie, lower pain sensitivity and PPITs and higher lumbar flexibility) in both intervention and control groups suggests that some confounding variables could have been involved. A recent systematic review with meta-analysis32 showed that the mere presence of a stranger did not influence pain perception or expression, but decreased pain-related arousal. However, other research has shown that the presence of a stranger is associated with decreased pain.32 There is also evidence that in a threatening pain context, social presence appears to inhibit pain expression, supporting the idea that the observer acts as a safety cue.32 Moreover, individuals with high attachment avoidance or catastrophizing report more pain in the presence of a stranger.32 Despite being seemly contradictory, these findings are not mutually exclusive, and they suggest that social and temperamental factors should be controlled for in future studies.
After calculation of absolute pre–post differences, higher tactile sensitivity was observed at the gluteus medius muscle than theo erector spinal muscle only after aerobic intervention. Sensory sensitivity after exercise has been studied in animal models33–35 and humans.36 Treadmill training can improve sensory function, producing neurotrophins (ie, BDNF and NT3) in spinal cord and skeletal muscle33,35 and increasing endogenous opioid content (ie, β-endorphin and met-enkephalin) in brain-stem regions (ie, rostral ventromedial medulla and periaqueductal gray area).34 Nagi and Mahns36 showed that C-tactile fibers mediate allodynia, experimentally induced using high-frequency cutaneous vibration after eccentric exercise. Some studies have reported that aerobic exercise protocols (eg, 15-minute incremental bicycling) produces EIH in exercising and nonexercising muscles of healthy subjects, but only when performed at moderate–high intensity.37–39 Maybe our differences in tactile sensitivity did not reach statistical significance because of the intensity (low–moderate) of aerobic exercise. Moreover, a lack of immediate effects (or even increases in pain) after acute exercise could be explained by a loss of conditioned pain modulation present among several chronic pain conditions, which regular exercise could restore.40
We also found higher tactile sensitivity and PPTs at the forefinger than other body locations, consistent with a higher density of receptors and larger cortical representation. We consider that inclusion of a pain-related and pain-nonrelated body location in assessment could add some evidence about central mechanisms involved in chronic pain conditions. The observation of hypoalgesia after exercise in some groups with chronic pain conditions and the observation of hyperalgesia after exercise in other groups with chronic pain may be influenced by whether the exercise is performed using a painful or nonpainful body area.8 Nijs, Kosek, Van Oosterwijck, and Meeus41 concluded that exercising painful muscles does not change pain sensitivity neither in exercising muscle or at distant locations in patients with local muscular pain. Vaegter, Handberg, and Graven-Nielsen42 also showed that EIH is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Thus, future research should consider these variables to unravel this heterogeneity of results among CLBP patients.
Limitations
Both intervention and control groups showed improvements (ie, lower pain sensitivity and PPITs and higher lumbar flexibility) that could not be solely due to physical exercise interventions. Other factors, such as beliefs or fear about exercise and pain, and social and temperamental factors could be involved and should be considered in pain research. Moreover, a high risk of performance and detection bias (due to lack of blinding of participants and researchers and self-assessment outcomes) are present, and were unavoidable due to the nature of interventions.43,44 Additionally, some studies have suggested that low–moderate intensity exercise (50%–60% of maximum heart rate) tends to improve chronic pain symptoms,45 but acute effects perhaps are present only when performed at moderate–high intensities. Therefore, the intensity of exercises should also be explored in further research.
Conclusion
To the best of our knowledge, this is the first study to show changes in tactile and pain sensitivity and lumbar flexibility after a brief intervention based on different modalities of exercise (ie, aerobic, strengthening, and flexibility) in patients with NSCLBP. However, the fact that we also found improvements in the control group limits our conclusions.
Acknowledgments
Thanks to Antònia Siquier, SOIB-Joves Qualificats Program, University of Balearic Islands, and Albert Cid Royo from SMI, Department of Health Science and Technology, Aalborg University for their support in building digital body maps of pain location and Lorenzo Mas and Juan José Castillo for their assistance during data acquisition.
Disclosure
This research was funded by the Spanish Ministerio de Economía, Industria y Competitividad (grants PSI2015-66295-R, PSI2016-78637-P, and PSI2017-88388-C4 AEI/FEDER, UE). The authors report no conflicts of interest in this work.
References
1. Van Tulder M, Becker A, Bekkering T, et al. Chapter 3: european guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(Suppl.2):169–191. doi:10.1007/s00586-006-1071-2
2. Delitto A, George SZ, van Dillen D, Whitman JM, Sowa GA. Low back pain: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the american physical therapy association. J Orthop Sports Phys Ther. 2012;42(4):1–81. doi:10.2519/jospt.2012.42.4.A1.Low
3. Bardin LD, King P, Maher CG. Diagnostic triage for low back pain: a practical approach for primary care. Med J Aust. 2017;206(6):268–273. doi:10.5694/mja16.00828
4. Violante FS, Mattioli S, Bonfiglioli R Low-back pain. In: Lotti M, Bleecker ML, eds. Handbook of Clinical Neurology. Vol 131 (
5. Geneen L, Smith B, Clarke C, Martin D, Colvin LA, Moore RA. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Libr. 2017;4. doi:10.1002/14651858.CD011279.pub3.www.cochranelibrary.com
6. National Institute for Health and Care Excellence (NICE). Low back pain and sciatica in low back pain and sciatica in over 16s: assessment and management NICE guideline; 2016 November. Avaialable from: https://www.nice.org.uk/guidance/ng59/resources/low-back-pain-and-sciatica-in-over-16s-assessment-and-management-pdf-1837521693637.
7. Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic pain. a national clinical guideline. Edinburgh; 2019. Avaialable from: http://www.sign.ac.uk/pdf/SIGN136.pdf.
8. Vaegter HB, Jones MD. Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. PAIN Reports. 2020;5(5):e823. doi:10.1097/pr9.0000000000000823
9. Suh JH, Kim H, Jung GP, Ko JY, Ryu JS. The effect of lumbar stabilization and walking exercises on chronic low back pain. Medicine. 2019;26. doi:10.1097/MD.0000000000016173
10. Smith BE, Littlewood C, May S. An update of stabilisation exercises for low back pain: A systematic review with meta-analysis. BMC Musculoskelet Disord. 2014;15:1–21. doi:10.1186/1471-2474-15-416
11. López-Chicharro J, Fernández-Vaquero A. Fisiología del ejercicio[Physiology of exercise]. 3rd ed. Madrid:M édica Panamericana. 2008. Spanish
12. Harvey LA, Katalinic OM, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures (Review). Cochrane Database Syst Rev. 2017;(1). doi:10.1002/14651858.CD007455.pub3.www.cochranelibrary.com
13. Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi:10.1249/MSS.0b013e318213fefb
14. Schroeder J, Otte A, Reer R, Braumann KM. Low back pain – an umbrella overview of exercise therapy in the general population and special demands in athletes. Dtsch Z Sportmed. 2015;66(10):257–262. doi:10.5960/dzsm.2015.191
15. Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139–1150. doi:10.1016/j.jpain.2012.09.006
16. Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20:11. doi:10.1016/j.jpain.2019.03.005
17. Kuithan P, Heneghan NR, Rushton A, Sanderson A, Falla D. Lack of exercise-induced hypoalgesia to repetitive back movement in people with chronic low back pain. Pain Pract. 2019;19(7):740–750. doi:10.1111/papr.12804
18. Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med. 2010;42(9):884–890. doi:10.2340/16501977-0595
19. Hoffman MD, Shepanski MA, MacKenzie SP, Clifford PS. Experimentally induced pain perception is acutely reduced by aerobic exercise in people with chronic low back pain. J Rehabil Res Dev. 2005;42(2):183–189. doi:10.1682/JRRD.2004.06.0065
20. Boudreau SA, Kamavuako EN, Rathleff MS. Distribution and symmetrical patellofemoral pain patterns as revealed by high-resolution 3D body mapping: A cross-sectional study. BMC Musculoskelet Disord. 2017;18(1):1–10. doi:10.1186/s12891-017-1521-5
21. Flórez-García MT, García-Pérez MA, García-Pérez F, Armenteros-Pedreros J, Álvarez-Prado A, Martinez-Lorente MD. Adaptación transcultural a la población española de la escala de incapacidad por dolor lumbar de Oswestry [Cross-cultural adaptation to the Spanish population of the Oswestry low-back pain disability scale]. Rehabilitación. 1995;29:138–145. Spanish
22. Andrade EM, Arce C, Seoane G. Adaptación al español del cuestionario “Perfil de los Estados de Ánimo” en una muestra de deportistas [Adaptation of the Profile of Mood States into Spanish with a sample of athletes]. Psicothema. 2002;14:708–713. Spanish
23. González-Cutre D, Sicilia Á. Hacia una mayor comprensión de la motivación en el ejercicio físico: medición de la regulación integrada en el contexto español [Towards a better understanding of motivation in physical activity: measuring integrated regulation in the Spanish context]. Psichothema. 2010;22:841–847. Spanish
24. De Gracia M, Marcó M. Adaptación y validación factorial de la “Subjetive Exercise Experiences Scale (SEES)” [Adaptation and Factorial Validation of the “Subjective Exercise Experiences Scale (SEES)”]. Rev Psciología Del Deport. 1997;6(1):60–68. Spanish
25. González-Mangado N, Rodríguez-Nieto MJ. Prueba de la marcha de los 6 minutos [6 minute walk test]. Med Respir. 2016;9(1):11–21. Spanish
26. Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156. doi:10.1183/09031936.00194909
27. Moreno JA, González-Cutre D, Martínez C, Alonso N, López M. Propiedades psicométricas de la Physical Activity Enjoyment Scale (PACES) en el contexto Español. Estud Psicol. 2008;29(2):173–180. doi:10.1174/021093908784485093.Spanish
28. Fisher AA. Algometry in diagnosis of musculoskeletal pain and evaluation of treatment outcome: an update. J Musculoskeletal Pain. 1998;6(1):5–32. doi:10.1300/J094v06n01_02
29. Ito T, Shirado O, Suzuki H, Takahashi M, Kaneda K, Strax TE. Lumbar trunk muscle endurance testing: an inexpensive alternative to a machine for evaluation. Arch Phys Med Rehabil. 1996;77(1):75–79. doi:10.1016/S0003-9993(96)90224-5
30. Plowman SA, Meredith MD, Eds. Fitnessgram/Activitygram Reference Guide.
31. Hui SS-C, Yuen PY, Validity of the modified back-saver sit-and-reach test: A comparison with other protocols. Med Sci Sports Exerc. 2000;32(9):1655–1659. doi:10.1097/00005768-200009000-00021
32. Che X, Cash R, Chung S, Fitzgerald PB, Fitzgibbon BM. Investigating the influence of social support on experimental pain and related physiological arousal: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;92:
33. Hutchinson KJ, Gómez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(6):1403–1414. doi:10.1093/brain/awh160
34. Stagg NJ, Mata HP, Ibrahim MM, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model. Anesthesiology. 2011;114(4):940–948. doi:10.1097/ALN.0b013e318210f880
35. Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010;90(5):714–725. doi:10.1021/acsnano.7b07248
36. Nagi SS, Mahns DA. C-tactile fibers contribute to cutaneous allodynia after eccentric exercise. J Pain. 2013;14(5):538–548. doi:10.1016/j.jpain.2013.01.009
37. Vaegter HB, Dørge DB, Schmidt KS, Jensen AH, Graven-Nielsen T. Test-retest reliability of exercise-induced hypoalgesia after aerobic exercise. Pain Med. 2018;19(11):2212–2222. doi:10.1093/pm/pny009
38. Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain. 2013;155(1):158–167. doi:10.1016/j.pain.2013.09.023
39. Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc. 2014;46(4):817–825. doi:10.1249/MSS.0000000000000143
40. Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol. 2017;595(13):4141–4150. doi:10.1113/JP273355
41. Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician. 2012;15:205–213.
42. Vaegter HB, Handberg G, Graven-Nielsen T. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain. 2016;32(1):58–69. doi:10.1097/AJP.0000000000000223
43. Polaski AM, Phelps AL, Kostek MC, Szucs KA, Kolber BJ. Exercise-induced hypoalgesia: A meta- analysis of exercise dosing for the treatment of chronic pain. PLoS One. 2019;14(1):1–29. doi:10.1371/journal.pone.0210418
44. van Middelkoop M, Rubinstein SM, Verhagen AP, Ostelo RW, Koes BW, Van TMW. Exercise therapy for chronic nonspecific low-back pain. Best Pract Res Clin Rheumatol. 2010;24(2):193–204. doi:10.1016/j.berh.2010.01.002
45. Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol. 2015;29(1):120–130. doi:10.1016/j.berh.2015.04.022
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





