Back to Journals » Journal of Inflammation Research » Volume 16
Active Fraction of Polyrhachis Vicina Roger (AFPR) Ameliorate Depression Induced Inflammation Response by FTO/miR-221-3p/SOCS1 Axis
Authors He J, Xie J, Zhou G , Jia C, Han D, Li D, Wei J, Li Y, Huang R, Li C, Wang B, Wei C, Su Q, Lai K, Wei G
Received 7 October 2023
Accepted for publication 12 December 2023
Published 23 December 2023 Volume 2023:16 Pages 6329—6348
DOI https://doi.org/10.2147/JIR.S439912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Junhui He,1,* Jiaxiu Xie,1,* Guili Zhou,2,* Chunlian Jia,2 Dongbo Han,2 Dongmei Li,1 Jie Wei,1 Yi Li,1 Renshan Huang,1 Chunlian Li,1 Bo Wang,3 Chao Wei,3 Qibiao Su,4 Kedao Lai,1 Guining Wei1
1Department of Pharmacology, Key Laboratory of Quality Standards, Guangxi Institute of Chinese Medicine & Pharmaceutical Science, Nanning, 530022, People’s Republic of China; 2Department of Pharmacology, Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 3Guangxi Shuangyi Pharmaceutical Co., Ltd, Nanning, Guangxi, 530021, People’s Republic of China; 4College of Health Science, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guining Wei, Department of Pharmacology, Guangxi Institute of Chinese Medicine & Pharmaceutical Science, Nanning, 530022, People’s Republic of China, Email [email protected] Qibiao Su, College of Health Science, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China, Tel +8618376664440, Email [email protected]
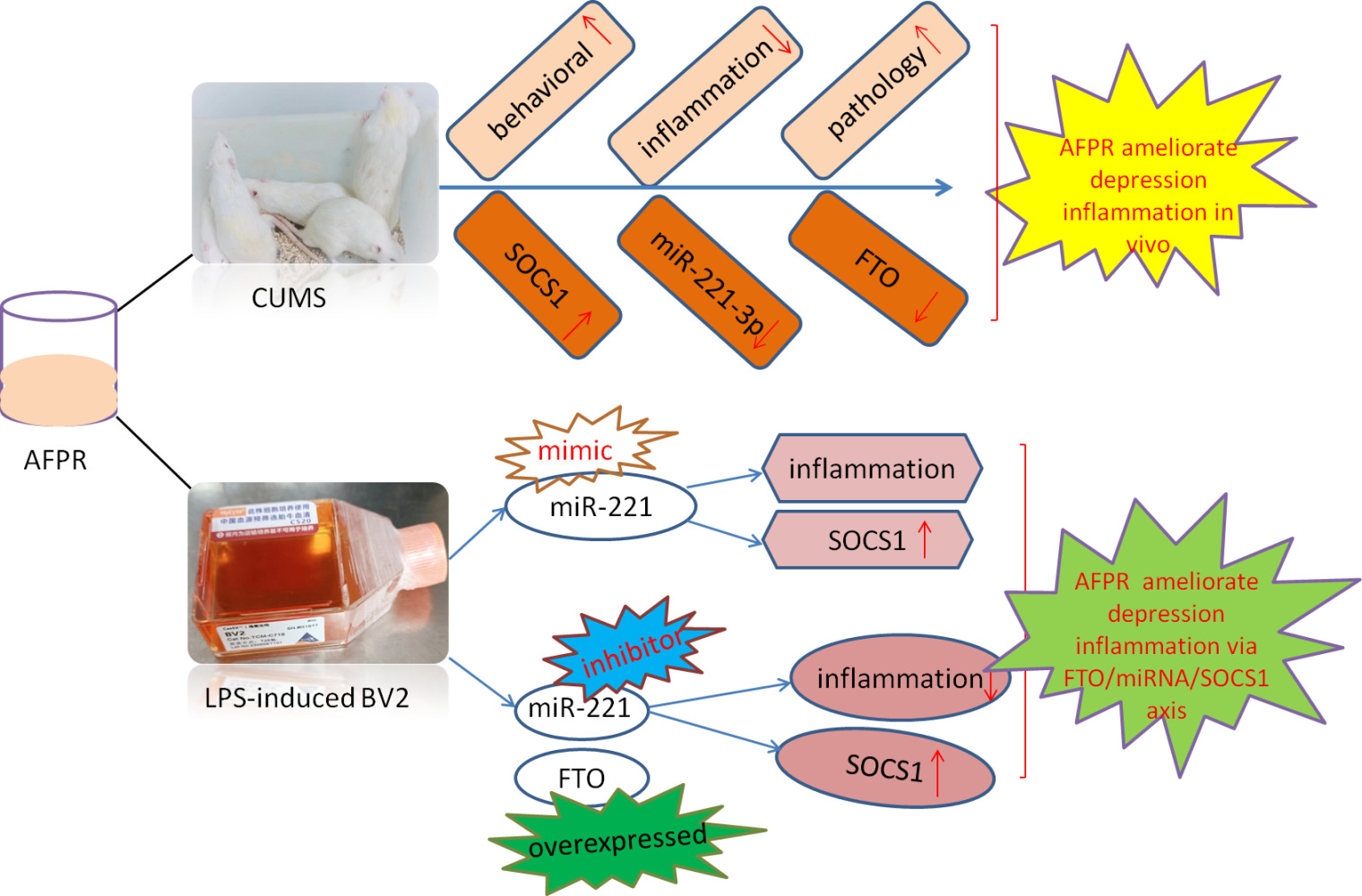
Purpose: Neuroinflammation is a significant etiological factor in the development of depression. Traditional Chinese medicine (TCM) has demonstrated notable efficacy in the treatment of inflammation. Our previous study surfaces that the active fraction of Polyrhachis vicina Roger (AFPR) has antidepressant and anti-neuroinflammatory effects, but the specific mechanisms remain to be elucidated. The objective of this study was to examine the impact of AFPR on inflammation in depression via the FTO/miR-221-3p/SOCS1 axis.
Methods: Chronic unpredictable stress (CUMS)-induced rats and LPS-induced BV2 cells were employed to simulate depression models in vivo and in vitro. The levels of inflammatory factors were detected using the ELISA assay. The expression of genes and proteins was detected using qRT-PCR and Western blot. Gene interactions were detected using the dual luciferase reporter gene. Protein-RNA interactions were investigated using RNA methylation immunoprecipitation (MeRIP) and RNA immunoprecipitation (RIP). Neuroinflammation in the brain was examined through H&E staining, while neuronal apoptosis was assessed using TUNEL staining.
Results: The results showed that AFPR ameliorated depression induced inflammation by increasing SOCS1 expression. However, SOCS1 was identified as a target of miR-221-3p. Overexpression of miR-221-3p decreased the expression of SOCS1 and increased the levels of NF-κB, IL-7, and IL-6. In addition, we found that miR-221-3p was regulated by FTO-mediated m6A modification through MeRIP and RIP experiments. Interference with miR-221-3p and overexpression of FTO resulted in increased SOCS1 gene expression and decreased levels of NF-κB, IL-7, and IL-6, which were reversed by AFPR.
Conclusion: AFPR inhibits the maturation of pri-miR-221-3p through FTO-mediated m6A modification, reduces the production of miR-221-3p, increases the expression of SOCS1, and reduces the level of inflammation, thereby improving depressive symptoms.
Keywords: depression, FTO, SOCS1, inflammation, Polyrhachis vicina Roger
Graphical Abstract:
Introduction
Depression is a mental disorder that poses a serious risk to people’s physical and mental health. Among Americans, suicide caused by depression is the tenth leading cause of death.1,2 The problem should not be underestimated in China, where an increasing number of stars have reported suicide due to depression in recent years, and the disease has attracted great attention from the Chinese government. However, the pathophysiology of depression is still not fully understood. A better understanding of the molecular mechanisms underlying the onset and progression of depression is therefore still needed. In addition, traditional antidepressants are statistically ineffective in about 40% of patients worldwide. The search for more effective and less toxic antidepressants is therefore of great importance.
The Polyrhachis vicina Roger is a popular herb in southern China, which is known to tonify the kidneys and benefit the vital essence, detoxifying and eliminating swellings, and activating the meridians and collaterals. It is commonly used clinically for insomnia, neurasthenia, osteomyelitis, alopecia, rheumatism and paralysis, and other illnesses.3,4 AFPR was petroleum ether extract of it. In previous studies, we found that the main components of AFPR are hexadecenoic acid, hexadecanoic acid, E-Octadecenoic acid, etc., and have anti-inflammatory bone loss, anti-cancer, anti-pruritus, anti-depression, anti-fatigue, and other effects.5–9 We have demonstrated that AFPR can improve depression through neuroinflammation in our previous studies, but its mechanism of action to improve depression through neuroinflammation has not been clarified. Studies have shown that xiaoyao pills can improve depression by inhibiting lipopolysaccharide-induced inflammatory responses.10 Pulsatilla chinensis saponins inhibit intestinal inflammation-mediated IDO1 overexpression and rebalance tryptophan metabolism to improve depressive symptoms in mice.11 The above studies suggest that TCM can improve depression by suppressing inflammation and related indicators. However, there are still fewer studies on TCM regulating inflammation-related genes to improve depression through m6A modification. Therefore, this paper intends to explore the relevant mechanism of AFPR exerting antidepressant effects through inhibiting inflammation via m6A modification.
Research suggests that inflammation plays an important role in the maintenance and development of depression.12 Depressed patients and rats produced more pro-inflammatory cytokines,13,14 and inhibition of inflammation reduces symptoms associated with depression.15 It is important to note that inflammation is not unique to depression. Abnormal levels of inflammatory factors in the blood increase the likelihood of conditions such as depression, schizophrenia, and bi-directional affective disorder.16 Levels of IL-6, TNF, IL-1Rα, and SIL2R in the above disorders are significantly elevated during acute exacerbations and decreased after treatment, suggesting that an acute inflammatory state may be present in psychiatric patients in the acute phase. In addition, studies have shown that psychological stress (a risk factor for depression) induces an inflammatory response and that stress in healthy volunteers leads to elevated inflammatory markers.17 Therefore, understanding the role of inflammation in the treatment response to depression is crucial. Microglia are predominantly immune cells in the central nervous system. Upon injury and pathogen invasion, microglia are rapidly activated and release inflammatory cytokines to mediate the inflammatory response.18 Cytokine inhibitory signaling protein (SOCS) is specifically expressed on the cell surface, and the reduction of cellular inflammation is related to the activation or high expression of SOCS.19 Overexpression of SOCS1 in microglia and macrophages inhibits microglial hyperactivation.20
MiR-155-5p has been shown to promote inflammation in depression by down-regulating the expression of the target gene SOCS1, and shanzhiside methyl ester reverses this process.21 Interestingly, a single miRNA can regulate hundreds of target genes, and a single target can also be regulated by hundreds of miRNAs.22 A growing body of research suggests that miRNAs may influence the course of depression by regulating target genes.23–25 However, how miRNAs and target genes affect depression and whether drugs can directly or indirectly regulate the miRNA/target gene axis to ameliorate depression remains to be explored.
RNA m6A modifications have the effect of modifying eukaryotic RNA, are widespread in the brain, and are dynamically reversible in mammals.26 M6A methylation regulates mRNA splicing, decay, expression, and translation, and is important in various cellular pathways and processes including cell differentiation, development, and metabolism.27–30 Studies have shown that m6A is involved in key processes of neurobiological functions, including neurogenesis, synaptic plasticity, learning, and memory.26,31 However, the mechanism of action of m6A in regulating depression remains to be elucidated.
In this study, we used in vitro and in vivo models to determine the regulatory relationship of AFPR on SOCS1 and then identified the SOCS1 target gene miR-221-3p by bioinformatics analysis and qRT-PCR validation. In addition, we found that FTO was highly expressed in depression, while AFPR could decrease its expression; therefore, we speculated that FTO might be involved in miRNA maturation in depression, leading to increased inflammation levels. The results showed that FTO-mediated modification of m6A enhanced the recognition of pri-miR-221-3p by DGCR8 and promoted the maturation of miR-221-3p. The increased expression of miR-221-3p inhibited the expression of SOCS1, and AFPR was able to reverse the process and thus alleviate the inflammatory response in depression.
Materials and Methods
Reagents
Dried Polyrhachis vicina Rogers was provided by Nanning Zhenyuan Biotechnology Co., LTD (Nanning, Guangxi, China), and the AFPR was obtained according to the previous extraction method.4 Interleukin-6, Interleukin-7, and Interleukin-10 assay kits were provided by Shanghai Fanke Industrial Co., LTD (Shanghai, China). Beyotime Biotech inc. provides a step-by-step TUNEL apoptosis assays kit with green fluorescence (Shanghai, China). Anti-SOCS1 was provided by Abcam (UK). Trizol, the reverse transcription and amplification kit was provided by Vazyme Biotech Co., LTD (Nanjing, Jiangsu, China). The Mir-x miRNA First-Strand Synthesis Kit was provided by TaKaRa (Japan). Methylated RNA Immunoprecipitation (MeRIP) kit and RNA Immunoprecipitation (RIP) Kit were provided by Boxin Biotechnology Co., LTD (Guangzhou, China). RiboSCRIPTTM mRNA/lncRNA qRT-PCR Starter Kit was provided by Ruibo Biotechnology Co., LTD (Guangzhou, China).
Animals
The experiment was approved by the Animal Ethics Committee of the Guangxi Institute of Chinese Medicine & Pharmaceutical Science, and all animal testing procedures were carried out by the Regulations on the Administration of Experimental Animals in China (1988, as amended in 2017). Wistar rat, male, 8 weeks old, supplied by Tianqin Biotech Co., Ltd., Changsha (Changsha, Hunan, China). All animals are housed in a standard SPF-rated animal house and receive free food and water.
Drug Administration and Chronic Unpredictable Mild Stress (CUMS) Model
Animals were randomly divided control group, model group, positive group (fluoxetine 5 mg·kg−1), high dose group (AFPR 40 mg·mL−1, 30 times the human dose), and low dose group (AFPR 10 mg·mL−1, the human dose), with 10 rats in each group and administered by gavage. The CUMS model was as described previously.32 Briefly, rats were randomly given two stimuli daily: forced swimming in ice water for 6 min, noise for 2 h, day/night reversal, cage tilt at 45° for 12 h, tail clamping for 3 min, fasting for 24 h followed by water fasting for 2 h, wet bedding for 24 h, solitary caging for 48 h or crowding for 12 h. These stressors were randomly given for 6 weeks to prepare CUMS rat model. The drug was administered once daily before stress for 42 consecutive days. Control group rats were housed normally without being exposed to any of the above stressors.
Behavioral Tests
The ptosis test was as described previously.33 Briefly, the rats were placed on a fixed stent to observe the number of animals whose eyes could not open half and calculate the antagonistic rate, antagonistic rate (%) = (1− ptosis rats/total number of animals in the group) ×100%. The experiment simulates the droopy eyelid state caused by psychological disorders in depressed animals.
Motion inhibition test (MIT) was as described previously.33,34 In short, the animal was placed in the center of a circular whiteboard 40 cm in diameter and observed for 30s, and the time the animal left the circular whiteboard was recorded. The experiment simulated a state of reduced movement in depressed animals.
Cell Culture and Treatment
Mouse microglia BV-2 (Suzhou Starfish Biotechnology Co., LTD.) were cultured in a specific medium and placed in a cell culture incubator (37°C, 5% CO2). The medium was changed once every 1–2 days, depending on cell growth.
Lipopolysaccharide (LPS) was formulated at a concentration of 1 mg·mL−1 and dissolved in dimethyl sulfoxide (DMSO). AFPR was formulated at a concentration of 1 g·mL−1 and dissolved in phosphate-buffered saline (PBS).32,35 The in vitro model of LPS-induced BV2 is mainly based on reference,36 and was adapted accordingly. In brief, each well was implanted with 1 × 106 cells on the 6-well plate and divided into control, model (LPS 1 μg·mL−1), AFPR group (2 mg·mL−1 and 1 mg·mL−1) groups. BV2 cells were pretreated with LPS for 2 hours and then AFPR was added for 24 hours. The supernatant and cells were collected by centrifugation and used for subsequent experimental assays.
Cytotoxicity Assays
BV-2 cells were inoculated into 96-well plates and treated with AFPR (0.5–50 mg·mL−1) for 24 h. The old medium was discarded and 100 µL of freshly prepared CCK-8 solution was added to each well. After incubation for 1 hour, absorbance was measured at 450 nm with a microplate reader.
Cell Transfection
All plasmids used in this study were provided by Guangzhou RiboBio (Guangzhou, China). Cell transfection was performed using liposome 2000 reagent (Invitrogen, Carlsbad, CA). The methods of cell culture, drug administration, and modeling are the same as above, and the transfection process is according to kit instructions. Briefly, add transfection reagents and target plasmids respectively, and then mix gently followed by incubating for 5 min at room temperature, adding the corresponding mixture to the cells respectively, and incubating them in the incubator. After 8 h, replace a complete medium for another 72 h culture.
Dual-Luciferase Reporter Assay
HEK293T cells co-transfected with Lipofectamine 2000, miR-221-3p, NC, and the corresponding plasmids. After 48 h of transfection, cells were collected and analyzed for the double Luciferase activities of the double-Luciferase Reporter Assay Kit.
Inflammatory Factor Detection
The rat serum and cell supernatant were taken and placed at −20°C for later use. IL-6, IL-7, IL-10, and NF-ΚB protein levels were detected as per kit instructions. Briefly, 50 µL of different concentrations of standards were added to the standard wells; 40 µL of sample dilution was added to the sample wells first, and then 10 µL of the sample was added. Blank wells without adding sample and enzyme reagent, the rest of the steps are the same. Then, 100 µL of enzyme reagent was added to each well, incubated at 37°C for 1h, and the plate was washed with washing solution 5 times, each time for 30s, and then 50 µL of color developer A and 50 µL of color developer B were added successively after patting it dry, displayed at 37°C for 15 min away from light, added 50 µL of termination solution, and the absorbance was measured at 450 nm.
Pathological Detection
H&E, Nissl, and Tunnel staining were employed to measure the variations and apoptosis of neurons in rat brain tissue. Specific methods are described below.
- H&E staining: The rat brain tissue was fixed in 4% paraformaldehyde, embedded in paraffin, sectioned (5 μm), the slices were dewaxed in xylene for 10 min each, and then rehydrated in gradient ethanol for 5 min each, then stained with hematoxylin for 5 min, hydrochloric acid alcohol (75%) fraction, washed back to blue, eosin soaked for 3 minutes, rinsed water, ethanol dehydrated for 10s, transparent in xylene for 1 minute, air dried, sealed in neutral resin.
- Nissl staining: Paraffin sections were deparaffinized and rehydrated, washed in water, stained with toluidine blue for about 5 minutes, examined microscopically, washed in water, dried, and re-stained with the staining solution for 5 minutes, washed in water, differentiated with glacial acetic acid (0.01%), washed in water to terminate the reaction, examined microscopically, washed in water, dried, and transparent with xylene for 10 minutes, and sealed with a neutral gel. Microscopically, the nictitating bodies were purple and the nuclei were lavender.
- Tunel staining: Paraffin sections were deparaffinized and rehydrated, and treated with Proteinase K working solution at 37°C for 25 min; washed with PBS, 50 μL of Tunel reaction mixture was added evenly dropwise onto the specimen, and coverslips were protected from light at 37°C for 1 h. The slides were dried, and the Converter-POD working solution was incubated at 37°C for 30 min. The specimens were incubated for 30 min at 37°C in a wet box protected from light. DAB substrate was used for color development for 10 min, hematoxylin was used for re-staining, xylene was used for transparency, and neutral gum was used to seal the slides. Light microscopy was used for photography.
Detection of m6A Modification
Total RNA from BV2 cells of about 2×107 was extracted with TRIzol according to the method provided by the manufacturer. The RNA was fragmented using the reagent fragmentation method and divided into IP and IgG groups. The m6A and IgG antibodies were added to the IP and IgG groups respectively and incubated at 4°C for 4 h. Then prepare Protein A/G beads and add them to the IP and IgG groups and incubate for 1 h at 4°C. Add the appropriate reagents and collect the supernatant for RNA extraction. Finally, qRT-PCR was used to detect co-precipitated RNA samples that will contain m6A modification sites.
RNA Immunoprecipitation
Collecting cells and added lysis solution followed by lysed at 4°C for 30 min, centrifuged, and then the supernatant was collected, 50 µL RNA was taken as input group, and the rest of the samples were conjugated with IgG antibody or DGCR8 antibody. The beads were then incubated for 4 h at 4°C, washed six times with buffer, eluted with buffer containing SDS (0.1%) and proteinase k, and incubated for 30 min at 55°C. RNA from the input and immunoprecipitation was extracted with TRIzol reagent and reverse transcribed into cDNA according to the instructions for qRT-PCR validation.
qRT-PCR
Rat brain tissue and BV2 cells were collected to extract total RNAs by TRIzol reagent. Then the total RNAs were reversed transcription by the HiScript III RT SuperMix for qPCR (+gDNA wiper) or the Mir-x miRNA First-Strand Synthesis Kit. qPCR was conducted by the ChamQ SYBR qPCR Master Mix or riboSCRIPTTM mRNA/lncRNA qRT-PCR Starter Kit in a LightCycler 480 II Real-Time PCR thermal cycler (Roche, Switzerland). The primer sequences were synthesized by Shanghai Sangon Biotech Co., Ltd (Table 1). Relative gene expression was calculated using 2−ΔΔCT with GAPDH and U6 as endogenous control genes.
 |
Table 1 The Sequences of the Primers for qRT-PCR |
Western Blot
BV2 cells were harvested and 100 mg rat brain tissue was weighed, then added 500 μL mixed lysate (RIPA lysate: phosphatase inhibitor: protease inhibitor = 50:1:1) to the cells and tissues, and ground the cells and tissue thoroughly using a grinding machine (FastPrep-24TM 5G, USA), and centrifuged at 4°C for 10 min, the supernatant was collected and stored at −80°C. Following the determination of protein concentration by the BCA method, proteins were denatured at 95°C. Aliquots of protein samples were loaded onto 12.5% sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and then transferred onto NC membranes.37 The membrane was then blocked with 5% skimmed milk powder for 1 h. Membranes were then washed with TBST for 5 min for three washes, and the SOCS1 primary antibody (1:1000) was incubated overnight at 4°C followed by incubation with the secondary antibody for 1 h at room temperature. The protein bands were stained with the ECL reagent (Vazyme, Nanjing, China). Image J software was used to analyze the grayscale values for each band.
Statistical Analysis
SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8.0 were used for statistical analyses. Data were expressed as mean ± standard deviation (SD) values and unpaired two-tailed Student’s T-test was used to determine the significance of the differences between the two groups; a two-way analysis of variance (ANOVA) was used for the multiple comparisons. P<0.05 indicated statistical significance. Graphs were plotted using the GraphPad Prism 8.0 software package.
Results
AFPR Alleviated Depressive Behavior and Inflammatory Response in CUMS Rats
First, we observed the effect of AFPR on depressive behavior in CUMS rats by motor inhibition test and ptosis test. The results of the motor inhibition test showed that CUMS increased the retention time of rats in the circle (P<0.01), whereas AFPR decreased the orbital retention time of rats (Figure 1A). As shown in Figure 1B, all rats in the control group did not develop eyelid ptosis, and all rats in the model group developed eyelid ptosis, and the confrontation rate was significantly compared with the control group (P<0.01). In the positive group, the high dose group, and the low dose group, 2, 0, and 2 eyelid ptosis were observed respectively, which have significant differences in confrontation rates compared with the model group (P<0.01, P<0.01, P<0.01). These results indicate that the CUMS was successfully modeled and that AFPR can improve depressive behavior in rats.
Next, to observe the effect of AFPR on the inflammatory response in rats, we performed ELISA and qRT-PCR experiments. Interestingly, CUMS was found to lead to an increase in serum levels of IL-6 (P<0.01) and IL-7 (P<0.01) by ELISA, as well as a decrease in the level of IL-10 (P<0.05) in the serum of the rats, while their levels were able to be reversed in the high dose AFPR group (Figure 1C-E). The result of qRT-PCR showed that CUMS led to increased relative mRNA expression of IL-6 (P<0.05), IL-7 (P<0.05), and NF-κB (P<0.01) in rats, and AFPR was able to reverse these phenomena (Figure 1F-H). These data indicate that AFPR can improve depressive behavior and reduce the inflammatory response in CUMS rats.
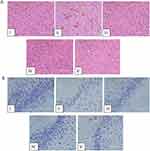
AFPR Improved Inflammatory Infiltration of the Prefrontal Cortex and Hippocampal Neuronal Damage in Rats
The results of H&E staining are shown in Figure 2A. Compared with the control group, the prefrontal cortex of rats in the model group showed obvious inflammatory infiltration, which was manifested by nuclear consolidation and necrosis, as shown by the yellow arrows; and glial cell differentiation was increased, as shown by the red arrows. Studies have shown that microglia are important for synaptic regulation, can be activated in neuropsychiatric disorders, and contribute to pathological processes by promoting neuroinflammation.10 While the AFPR group showed an improvement in the above symptoms. Figure 2B shows that the results of Nissl staining indicated that the neurons in the hippocampus of rats in the model group were injured, which manifested as a reduction in Nissl vesicles and lightening of color; whereas AFPR was able to ameliorate the above symptoms in rats. Together, these findings suggest that AFPR may ameliorate inflammatory infiltrate and hippocampal neuronal injury in the prefrontal cortex of CUMS rats.
AFPR Attenuated Neuronal Apoptosis in CUMS Rats
Tunnel staining was performed to further observe the effect of AFPR on neuronal apoptosis. Figure 3 showed that compared to the control group, neuron apoptosis was increased in the model group. It was shown that activation of microglia contributes to increased levels of pro-inflammatory cytokines and activation of inflammation-related classes, thus causing neuronal apoptosis.38 While in comparison to the model group, neuron apoptosis was reversed by the administration group. These results suggest that the CUMS model causes inflammation in rat brain tissue and induces neuronal apoptosis, while AFPR attenuates the process.
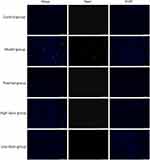 |
Figure 3 AFPR attenuated neuronal apoptosis in CUMS rats. Tunel staining (20×). |
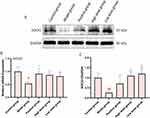
AFPR Increased SOCS1 Expression in CUMS Rat Brain
To study SOCS1 expression in CUMS rats as well as the relationship between SOCS1 and AFPR, SOCS1 expression at the gene and protein level was examined by qRT-PCR and Western blotting. Analysis of the qRT-PCR results (Figure 4B) showed that SOCS1 expression in the depressed rats was downregulated (P<0.05), while the high-dose and low-dose AFPR groups were able to upregulate SOCS1 expression (P<0.05, P<0.05). Interestingly, the Western blot plots (Figure 4A) and grey scale values (Figure 4C) showed the same trend as qRT-PCR. These findings suggest that SOCS1 may be implicated in the development of depression, and AFPR could increase SOCS1 expression in CUMS rat brains.
SOCS1 is a Target Gene of miR-221-3p
The Targetscan database was used to predict miRNAs with the potential to target SOCS1 and yielded a total of 8 miRNAs. Next, we selected 3 miRNAs for qRT-PCR validation based on their ability to bind from strong to weak. We found that CUMS led to a decrease in miR-30a-5p expression (P<0.05) (Figure 5A), and an increase in miR-221 3p expression (P<0.05) (Figure 5C), and no significant trends in miR-98-5p expression (Figure 5B). It is interesting to note that AFPR was able to reverse the expression of miR-30a-5p (P<0.05) and miR-221-3p (P<0.01). Based on the above findings, miR-30a-3p and miR-221-3p may be involved in the development of depression, and miR-221-3p conformed to our trend, so we selected it as the focus of study in our subsequent experiments.
To verify whether miR-221-3p targets SOCS1, Targetscan was used to predict the putative binding sites in 3’-UTR regions of SOCS1 for miR-221-3p (Figure 5D), then a luciferase reporter vector experiment was performed to confirm their targeting relationship. The result showed that after co-transformation with mmu-miR-221-3p, the reporter fluorophore expression of m-SOCS1-MUT was increased compared to that of m-SOCS1-WT (P<0.01) (Figure 5E). This result suggests that the mmu-miR-221-3p mimic may have significant interaction with the predicted site on the 3’-UTR of m-SOCS1.
AFPR Alleviated LPS-Induced Inflammatory Response in BV2 Cells
Next, to explore the antidepressant mechanism of action of the AFPR, we simulated an in vitro depression model in BV2 cells. The IC50 value of AFPR was determined to be 3.72 mg·kg−1 using the CCK8 assay (Figure 6A), then the cells were perturbed with AFPR and the expression of miR-221-3p and SOCS1 were detected by qRT-PCR. We found that LPS led to an increase in miR-221-3p expression (P<0.01) (Figure 6B) and a decreased SOCS1 expression (P<0.05) (Figure 6C) in BV2 cells, which was abrogated by AFPR. qRT-PCR was also used to detect levels of inflammatory factors, and there was an increase in the levels of IL-6 (P<0.05), IL-7 (P<0.05) and NF-κB (P<0.01), However, AFPR was able to decrease the levels of these (Figure 6D-F).
AFPR Improves LPS-Induced Inflammation in BV2 Cells via the miR-221-3p/SOCS1 Axis
To test the regulatory relationship between miR-221-3p and SOCS1, and to test whether AFPR ameliorates inflammatory responses induced by LPS via the miR-221-3p/SOCS1 axis. We overexpressed the miR-221-3p and detected SOCS1 expression by qRT-PCR and Western blot analysis. The qRT-PCR result showed that overexpression of miR-221-3p inhibited the expression of SOCS1 (P<0.05) (Figure 7A), while the AFPR could increase the expression of SOCS1. Western blot results showed the same trend as qRT-PCR (Figure 7B and C). We also detected inflammatory factors in cells by qRT-PCR and cell supernatants by ELISA, and the qRT-PCR results showed that the overexpression of miR-221-3p promoted the levels of IL-6 (P<0.01), IL-7 (P<0.01) and NF-κB (P<0.01) and AFPR was able to downregulate their levels (Figure 7D-F); the ELISA results showed that the overexpression of miR-221-3p promoted the levels of NF-κB (P<0.01) and IL-7 (P<0.01) and that the high dose AFPR group was able to downregulate their levels (Figure 7G and H). These findings suggest that AFPR may ameliorate the inflammatory response generated by BV2 cells induced by LPS via the miR-221-3p/SOCS1 axis.
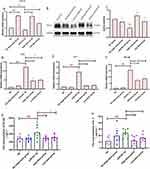
AFPR Regulates FTO to Ameliorate Aberrant m6A Modifications
Indeed, studies have shown that m6A modifications are tightly associated with all phases of RNA metabolism, including RNA folding, stability, nuclear export, splicing, regulation, and degradation of translation.39,40 Therefore, we hypothesize that m6A may play a role in regulating miR-221-3p in depression. We first examined the expression levels of m6A-related genes in depression in vivo and in vitro, and the results showed that FTO was significantly expressed both in vivo and in vitro (P<0.05, P<0.05) model of depression and that AFPR can downregulate its expression level (Figure 8A and F), whereas other genes related to m6A (METTL3, METTL14, YTHDF1, YTHDF2) in vivo (Figure 8B-E) and in vitro (Figure 8G-J) were not significantly different. This result suggests that FTO may be responsible for the abnormal m6A modification.
AFPR Regulates miR-221-3p Maturation via FTO-Dependent m6A Methylation in Depression
DGCR8 is a key enzyme in the m6A modification process, triggering the progression of pri-miRNAs to mature miRNAs. To test if FTO participates in the miR-221-3p maturation process, first, we examined pri-miR-221-3p abundance in depression using MeRIP analysis. The results showed that pri-miR-221-3p was enriched in depression (P<0.01) and that the AFPR was able to decrease its level (P<0.05) (Figure 9A). We then overexpressed FTO and examined the level of pri-miR-221-3p expression by qRT-PCR, and the results revealed that FTO overexpression decreased the expression of the pri-miR-221-3p (P<0.05) (Figure 9B). Finally, we investigated the extent of DGCR8 antibody binding to pri-miR-221-3p by RIP assay following FTO overexpression. We found that FTO overexpression increased the level of pri-miR-221-3p binding to DGCR8 (P<0.01) and that AFPR was able to reverse the process (Figure 9C). This suggests that FTO-dependent m6A methylation promotes the recognition of pri-miR-221-3p by DGCR8, thus promoting the generation of the miR-221-3p protein. However, AFPR can reverse this process.
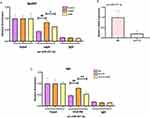
AFPR Reverses FTO-Mediated Pri-miR-221-3p Maturation to Attenuate Inflammatory Responses in Depression
Finally, to test whether AFPR could reverse FTO-mediated maturation of pri-miR-221-3p, thereby attenuating the inflammatory response, we first interfered with miR-221-3p, followed by overexpression of FTO, and then gave AFPR intervention. Interestingly, we found that IL-6 (P<0.01), IL-7 (P<0.01), and NF-κB (P<0.01) expression in cells were increased in both the interference and simultaneous overexpression groups and that the process could be reversed in the high dose group treated with AFPR (Figure 10A-C). However, the expression of SOCS1 was just opposite to them (Figure 10D). In addition, the levels of IL-7 and NF-κB in cell supernatants showed consistency with those in cells (Figure 10E and F). These results suggest that AFPR reverses FTO-mediated pri-miR-221-3p maturation, thereby attenuating the inflammatory response.
Discussion
Our results suggest that AFPR has an ameliorative effect on depression and enhances the expression of SOCS1, which inhibits the expression of downstream inflammatory factors. Next, through bioinformatics and experimental validation, we found that miR-221-3p could target SOCS1, that low expression of SOCS1 is associated with high expression of miR-221-3p, whereas AFPR could reverse the process. Combined with the study of m6A in neuropsychiatric disorders, we found that FTO expression is reduced in depression, suggesting that FTO may be involved in the regulatory process of depression. In the further study, we found that pri-miR-221-3p was significantly enriched in depression and that overexpression of FTO was able to reduce the expression of pri-miR-221-3, and the results of the RIP experiments also showed that FTO was able to bind to pri-miR-221-3p, which may affect the generation of miR-221-3p maturation. Finally, we overexpressed FTO while interfering with miR-221-3p and found SOCS1 expression to be decreased and the levels of downstream inflammatory factors to be increased, suggesting that FTO promotes the maturation of pri-miR-221-3p cells. However, the administration of AFPR reversed this process. The above results suggest that AFPR inhibits FTO-mediated maturation of pri-miR-221-3p and elevates the expression level of SOCS1, which reduces the inflammatory response and ameliorates depression-like symptoms.
Studies have shown that SOCS can inhibit cytokine signaling in a variety of cell types.41 Among them, SOCS1 and SOCS3 are the two best-studied SOCS genes,42,43 which play an important role in the regulation of inflammation and may be expressed in microglia. N6-methyladenosine from SOCS1 has been previously shown to modulate inflammatory responses of macrophages in different harsh environments.44 The SOCS1/JAK2/STAT3 pathway regulates early brain injury due to subarachnoid hemorrhage through inflammatory responses.41 SOCS1 mimetic peptide inhibits chronic intraocular inflammation (uveitis).45 MiR-155-5p regulates the inflammatory phenotype of fibroblasts by down-regulating SOCS1 expression.46 However, SOCS1 has been understudied in depression; therefore, we delved into the possible regulatory role of AFPR through the modulation of SOCS1-mediated pro-inflammatory and anti-inflammatory cytokines in the CUMS rat model. In summary, our results indicate that SOCS1 expression is decreased in depression, with increased IL-6 and IL-7 levels as well as decreased IL-10 levels. AFPR, on the other hand, reversed the levels of the aforementioned metrics. This result suggests that AFPR may ameliorate the inflammatory response in depression by upregulating the level of SOCS1.
miRNAs can regulate gene expression and participate in various cell physiological processes such as cell growth, differentiation, and motility.47 Several studies have shown that miRNAs have an important role in either promoting or inhibiting the development of depression.48–50 Inhibition of miR-221 has been shown to attenuate acute lung injury induced by LPS via SOCS1.51 The miR-221 exosome promotes inflammation by mediating the polarization of M1 macrophages via SOCS1/STAT.52 In this study, we investigated the role of miR-221-3p targeting SOCS1 to regulate the inflammatory response in depression. The result showed that miR-221-3p inhibited SOCS1 expression, thus promoting the inflammatory response, whereas AFPR was able to reverse the process.
Among eukaryotic messenger RNAs, the m6A modification is the most abundant transcriptomic internal epi modification.53 The study of RNA methylation modification has made the question of how this modification affects normal physiological functions and consequently causes disease a hot topic for researchers.54 Studies have shown that m6A is highly abundant in synapses and is involved in neuronal plasticity, learning and memory, and adult neurogenesis. In addition, m6A can respond to environmental stimuli, suggesting an important role in connecting molecular and behavioral stresses.55 In this study, we first investigated whether m6A-related gene expression levels were altered in depression. We found that the level of FTO expression was significantly increased in depression, whereas it decreased after AFPR treatment. Subsequently, detected by MeRIP assay, we found that pri-miR-221-3p was able to bind to the m6A antibody, indicating that pri-miR-221-3p could be altered by methylation of m6A. We found that overexpression of FTO led to a reduction in pri-miR-221-3p expression. Thus, we hypothesized that FTO may be involved in the miRNA maturation process in depression. Furthermore, using RIP experiments, we find that miR-221-3p is regulated by FTO-mediated m6A modification, and FTO enhances the recognition of pri-miR-221-3p by DGCR8 and promotes the maturation of miR-221-3p, which in turn affects the expression level of target genes. FTO deficiency has been shown to result in weight loss, reduction of anxiety and depressive-like behaviors, suppression of inflammation, and more.56 Our study also shows that FTO low expression attenuates depressive behaviors and suppresses inflammatory responses.
In this study, the CUMS model leads to an inflammatory response in rat brain tissue, and inflammation further activates glial cells, which leads to the upregulation of pro-inflammatory cytokines, resulting in neuronal apoptosis. Next, we evaluated the antidepressant effect of AFPR by detecting the levels of relevant inflammatory factors and pathological examination, etc. We found that AFPR attenuated the activation of microglia in brain tissues, down-regulated the levels of inflammatory factors, and attenuated neuronal apoptosis. In addition, when exploring the related anti-inflammatory mechanism in an in vitro model, we used SOCS1 as the core target, and by detecting the expression of its upstream and downstream genes, we found that AFPR could inhibit the maturation of pri-miR-221-3p through FTO-mediated modification of m6A, and reduce the generation of miR-221-3p, which led to the elevation of the expression of its target gene SOCS1. However, elevated levels of the cytokine inhibitor SOCS1 reduce inflammation levels, which in turn improves depression symptoms. In summary, AFPR has the effect of improving inflammation in depression, suggesting that TCM can improve depression by anti-neuroinflammation and thus improve depression, and AFPR can be used as a potential antidepressant in the development of new drugs, and this study provides a reference for the idea of antidepressant research in TCM.
Conclusion
The current study suggests that the antidepressant effect of AFPR is associated with the upregulation of SOCS1 and the downregulation of miR-221-3p and FTO expression. AFPR reduces the recognition of pri-miR-221-3p by DGCR8 through FTO-mediated modification of m6A and inhibits the maturation of miR-221-3p, resulting in the upregulation of the target gene SOCS1 and the alleviation of the inflammatory response, thus improving depression-like symptoms. However, whether AFPR directly regulates FTO-mediated m6A modifications affecting downstream target gene alterations remains to be elucidated. Furthermore, there is a lack of in vivo experimental validation in this study. Our next step will be to explore the role of AFPR in ameliorating depression via FTO-mediated m6A modification in vivo through the AAV delivery system, to provide experimental evidence for the development of AFPR as a new antidepressant drug in TCM.
Funding
This research was funded in part by the National Natural Science Foundation of China (Grant No. 81960729), Guangxi Traditional Chinese Medicine Key Discipline Construction Project (GZXK-Z-20–75).
Disclosure
The authors report no conflicts of interest in this work.
References
1. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville (MD); 2017.
2. Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. doi:10.1002/mpr.1359
3. Huang X, Wei J, Lu M. Guangxi standard of traditional Chinese medicinal materials (volume Ii). Ecotoxicol Environ Safety. 1996;255–258.
4. He J, Han D, Jia C, et al. Integrating Network Pharmacology, Molecular Docking and Pharmacological Evaluation for Exploring the Polyrhachis vicina Rogers in Ameliorating Depression. Drug Des Devel Ther. 2023;17:717–735. doi:10.2147/DDDT.S399183
5. Feng X, Wei G, Liu Z, Gao Y, Zhou R, Song F. Effect of active fraction of Polyrhachis vicina Rogers on LPS-induced inflammatory bone loss in mice. J Guangxi Med Univ. 2023;40(2):182–188.
6. Wei X, Li D, He J, et al. Effects of active components of Polyrhachis vicina on apoptosis activation in colorectal cancer SW116 cells via miR-186-5p /Cx43. Chinese Traditional Patent Med. 2022;44(6):1783–1791.
7. He F, Li D, Su Q, Zeng X, Wei G. Experimental study on the treatment of pruritus skin in mice by the active components of Polyrhachis vicina Roger. J Chinese Med Mater. 2018;41(5):1200–1203.
8. Zhang X, Long Q, Chu S, et al. Inhibitory effect of extratable petroleum ether of Polyrhachis vicina Roger on neuroinflammatory response in depressed rats. Acta Pharma Sin. 2018;53(7):1042–1047.
9. Li P, Wei G, Li D, He J, Wu L, Long T. Anti-fatigue effect of the extract of Polyrhachis vicina Roger and Ostreagigastnunb in mice. Guangxi Sci. 2019;26(5):583–587.
10. Yirmiya R, Rimmerman N, Reshef R. Depression as a Microglial Disease. Trends Neurosci. 2015;38(10):637–658. doi:10.1016/j.tins.2015.08.001
11. Wang T, Song Y, Ai Z, et al. Pulsatilla chinensis saponins ameliorated murine depression by inhibiting intestinal inflammation mediated IDO1 overexpression and rebalancing tryptophan metabolism. Phytomedicine. 2023;116:154852. doi:10.1016/j.phymed.2023.154852
12. Nowak W, Grendas LN, Sanmarco LM, et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine. 2019;50:290–305. doi:10.1016/j.ebiom.2019.10.063
13. Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. doi:10.1016/j.jad.2011.08.003
14. Zhao YT, Deng J, Liu HM, et al. Adaptation of prelimbic cortex mediated by IL-6/STAT3/Acp5 pathway contributes to the comorbidity of neuropathic pain and depression in rats. J Neuroinflammation. 2022;19(1):144. doi:10.1186/s12974-022-02503-0
15. Arioz BI, Tastan B, Tarakcioglu E, et al. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol. 2019;10:1511. doi:10.3389/fimmu.2019.01511
16. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. doi:10.1038/mp.2016.3
17. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi:10.1038/nri.2015.5
18. Xu L, He D, Bai Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol Neurobiol. 2016;53(10):6709–6715. doi:10.1007/s12035-015-9593-4
19. Kedzierski L, Tate MD, Hsu AC, et al. Suppressor of cytokine signaling (SOCS)5 ameliorates influenza infection via inhibition of EGFR signaling. Elife. 2017;6.
20. Zhang S, Gao L, Liu X, Lu T, Xie C, Jia J. Resveratrol Attenuates Microglial Activation via SIRT1-SOCS1 Pathway. Evid Based Complement Alternat Med. 2017;2017:8791832. doi:10.1155/2017/8791832
21. Sun Z, Zhan H, Wang C, Guo P. Shanzhiside methylester protects against depression by inhibiting inflammation via the miRNA-155-5p/SOCS1 axis. Psychopharmacology (Berl). 2022;239(7):2201–2213. doi:10.1007/s00213-022-06107-7
22. Fang L, Sorensen P, Sahana G, et al. MicroRNA-guided prioritization of genome-wide association signals reveals the importance of microRNA-target gene networks for complex traits in cattle. Sci Rep. 2018;8(1):9345. doi:10.1038/s41598-018-27729-y
23. Xian X, Cai LL, Li Y, et al. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20(1):122. doi:10.1186/s12951-022-01332-w
24. Fan C, Li Y, Lan T, Wang W, Long Y, Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol Ther. 2022;30(3):1300–1314. doi:10.1016/j.ymthe.2021.11.006
25. Fiori LM, Kos A, Lin R, et al. miR-323a regulates ERBB4 and is involved in depression. Mol Psychiatry. 2021;26(8):4191–4204. doi:10.1038/s41380-020-00953-7
26. Livneh I, Moshitch-Moshkovitz S, Amariglio N, Rechavi G, Dominissini D. The m(6)A epitranscriptome: transcriptome plasticity in brain development and function. Nat Rev Neurosci. 2020;21(1):36–51. doi:10.1038/s41583-019-0244-z
27. Kasowitz SD, Ma J, Anderson SJ, et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14(5):e1007412. doi:10.1371/journal.pgen.1007412
28. Zhang G, Xu Y, Wang X, et al. Dynamic FMR1 granule phase switch instructed by m6A modification contributes to maternal RNA decay. Nat Commun. 2022;13(1):859. doi:10.1038/s41467-022-28547-7
29. Zhang T, Zhang SW, Zhang SY, Gao SJ, Chen Y, Huang Y. m6A-express: uncovering complex and condition-specific m6A regulation of gene expression. Nucleic Acids Res. 2021;49(20):e116. doi:10.1093/nar/gkab714
30. Sun R, Tian X, Li Y, et al. The m6A reader YTHDF3-mediated PRDX3 translation alleviates liver fibrosis. Redox Biol. 2022;54:102378. doi:10.1016/j.redox.2022.102378
31. Widagdo J, Anggono V. The m6A-epitranscriptomic signature in neurobiology: from neurodevelopment to brain plasticity. J Neurochem. 2018;147(2):137–152. doi:10.1111/jnc.14481
32. Wang H, Yang Y, Yang S, et al. Ginsenoside Rg1 Ameliorates Neuroinflammation via Suppression of Connexin43 Ubiquitination to Attenuate Depression. Front Pharmacol. 2021;12:709019. doi:10.3389/fphar.2021.709019
33. He J, Zhu F, Li D, Wei J, Fan J, Wei G. Effect of Mahonia bealei Leaf Extract on Inflammation in Depression of Rats via NF-κB/NLRP3 Signaling Pathway. Chin J Exp Traditional Med Formulae. 2022;28(17):67–74.
34. He J, Li D, Wei J, et al. Mahonia Alkaloids (MA) Ameliorate Depression Induced Gap Junction Dysfunction by miR-205/Cx43 Axis. Neurochem Res. 2022;47(12):3761–3776. doi:10.1007/s11064-022-03761-3
35. Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients. 2019;11(11):2794. doi:10.3390/nu11112794
36. Li W, Ali T, Zheng C, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021;18(1):38. doi:10.1186/s12974-021-02091-5
37. Yang J, Zhang M, Yang D, et al. m(6)A-mediated upregulation of AC008 promotes osteoarthritis progression through the miR-328-3p‒AQP1/ANKH axis. Exp Mol Med. 2021;53(11):1723–1734. doi:10.1038/s12276-021-00696-7
38. Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, et al. The Immune System and the Role of Inflammation in Perinatal Depression. Neurosci Bull. 2016;32(4):398–420. doi:10.1007/s12264-016-0048-3
39. Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42. doi:10.1038/nrm.2016.132
40. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169(7):1187–1200. doi:10.1016/j.cell.2017.05.045
41. Wang Y, Kong XQ, Wu F, et al. SOCS1/JAK2/STAT3 axis regulates early brain injury induced by subarachnoid hemorrhage via inflammatory responses. Neural Regen Res. 2021;16(12):2453–2464. doi:10.4103/1673-5374.313049
42. Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–3448. doi:10.4049/jimmunol.1201168
43. Wang D, Wen X, Zhang X, et al. Molecular characterization and expression of suppressor of cytokine signaling (SOCS) 1, 2 and 3 under acute hypoxia and reoxygenation in pufferfish, Takifugu fasciatus. Genes Genomics. 2018;40(11):1225–1235. doi:10.1007/s13258-018-0719-8
44. Hu Z, Li Y, Yuan W, et al. N6-methyladenosine of Socs1 modulates macrophage inflammatory response in different stiffness environments. Int J Biol Sci. 2022;18(15):5753–5769. doi:10.7150/ijbs.74196
45. He C, Yu CR, Mattapallil MJ, Sun L, Larkin Iii J, Egwuagu CE. SOCS1 Mimetic Peptide Suppresses Chronic Intraocular Inflammatory Disease (Uveitis). Mediators Inflamm. 2016;2016:2939370. doi:10.1155/2016/2939370
46. Cheng J, Zhang Y, Yang J, Wang Y, Xu J, Fan Y. MiR-155-5p modulates inflammatory phenotype of activated oral lichen-planus-associated-fibroblasts by targeting SOCS1. Mol Biol Rep. 2022;49(8):7783–7792.
47. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486
48. Wei ZX, Xie GJ, Mao X, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. 2020;45(6):1050–1058. doi:10.1038/s41386-020-0622-2
49. Liu Z, Yang J, Fang Q, et al. MiRNA-199a-5p targets WNT2 to regulate depression through the CREB/BDNF signaling in hippocampal neuron. Brain Behav. 2021;11(8):e02107. doi:10.1002/brb3.2107
50. Guan W, Xu DW, Ji CH, et al. Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacol Res. 2021;174:105932. doi:10.1016/j.phrs.2021.105932
51. Wang T, Jiang L, Wei X, et al. Inhibition of miR-221 alleviates LPS-induced acute lung injury via inactivation of SOCS1/NF-kappaB signaling pathway. Cell Cycle. 2019;18(16):1893–1907. doi:10.1080/15384101.2019.1632136
52. Cai M, Shi Y, Zheng T, et al. Mammary epithelial cell derived exosomal MiR-221 mediates M1 macrophage polarization via SOCS1/STATs to promote inflammatory response. Int Immunopharmacol. 2020;83:106493. doi:10.1016/j.intimp.2020.106493
53. Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi:10.1038/nature12730
54. Wang J, Ishfaq M, Xu L, Xia C, Chen C, Li J. METTL3/m(6)A/miRNA-873-5p Attenuated Oxidative Stress and Apoptosis in Colistin-Induced Kidney Injury by Modulating Keap1/Nrf2 Pathway. Front Pharmacol. 2019;10:517. doi:10.3389/fphar.2019.00517
55. Mitsuhashi H, Nagy C. Potential Roles of m6A and FTO in Synaptic Connectivity and Major Depressive Disorder. Int J Mol Sci. 2023;24(7):6220. doi:10.3390/ijms24076220
56. Sun L, Ma L, Zhang H, et al. Fto Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice via Alterations in Gut Microbiota. Theranostics. 2019;9(3):721–733. doi:10.7150/thno.31562
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.