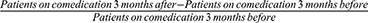Back to Journals » Therapeutics and Clinical Risk Management » Volume 20
Acthar Gel in African Americans versus Non-African Americans with Symptomatic Sarcoidosis: Physician Assessment of Patient Medical Records
Authors Bindra J, Chopra I, Hayes K , Niewoehner J, Panaccio MP, Wan GJ
Received 18 October 2023
Accepted for publication 24 January 2024
Published 9 February 2024 Volume 2024:20 Pages 83—94
DOI https://doi.org/10.2147/TCRM.S438174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jas Bindra,1 Ishveen Chopra,2 Kyle Hayes,3 John Niewoehner,3 Mary Prince Panaccio,3 George J Wan3
1Falcon Research Group, North Potomac, MD, USA; 2Manticore Consultancy, Bethesda, MD, USA; 3Mallinckrodt Pharmaceuticals, Bridgewater, NJ, USA
Correspondence: George J Wan, Mallinckrodt Pharmaceuticals, 440 Route 22 East, Bridgewater, NJ, USA, Email [email protected]
Introduction: Sarcoidosis is common among African Americans in the United States. Acthar® Gel is a viable option for the treatment of advanced symptomatic sarcoidosis. This study examined patient characteristics, Acthar Gel utilization, co-medication use, and treatment response based on physicians’ assessments among African Americans versus non-African Americans with advanced symptomatic sarcoidosis.
Methods: Data from the medical charts of patients were used. During data collection, patients had either completed ≥ 1 course or received treatment with Acthar Gel for ≥ 6 months.
Results: This study comprised 168 African Americans and 104 non-African Americans. On average, the time since the first diagnosis of sarcoidosis was slightly longer among African Americans than non-African Americans (5.2 versus 4.3 years). Skin, heart, eyes, and joints were the most common extrapulmonary sites involved among both race groups. Shortness of breath, fatigue, bone and joint pain, and wheezing/coughing were the most frequent symptoms among both race groups. A higher proportion of African Americans versus non-African Americans were first-time Acthar Gel users and had not completed treatment during data collection. Patients in both race groups with higher starting doses of Acthar Gel therapy had a shorter treatment duration and vice-versa. A significantly lower proportion of patients among both race groups were on any co-medication after Acthar Gel initiation (p< 0.0001). Further, a higher proportion of African Americans versus non-African Americans had a reduction in any co-medication use after Acthar Gel initiation. The mean daily dose of prednisone decreased among African Americans (18.5 to 10.1 mg) and non-African Americans (17.6 to 10.0 mg) after Acthar Gel initiation. Improvement in patient health status and overall symptoms was similar for both race groups.
Conclusion: Findings suggest that Acthar Gel improves health outcomes for patients with sarcoidosis, which could help to alleviate health disparities among African Americans, who are disproportionately affected by this disease.
Keywords: Acthar® Gel, African Americans, medical chart review, sarcoidosis, treatment utilization, treatment response
Introduction
Sarcoidosis is an inflammatory granulomatous condition with unknown etiology that affects multiple organs.1–4 Patients experience a broad spectrum of clinical manifestations of varying severity that impair both mental and physical functioning.5 The disability from sarcoidosis can result in a considerable direct and indirect economic burden on patients.5 More than 25,000 patients are diagnosed with sarcoidosis each year in the United States (US).6 Sarcoidosis-related hospitalization rates in the US have increased by about 26% from 2005 to 2014.7 Advanced sarcoidosis results in considerable morbidity and some mortality; one or more types of advanced disease are reported in about 25% of patients with sarcoidosis that requires close monitoring and multiple treatments.8 The African American population in the US is more frequently affected; 4 to 17 times more likely to develop sarcoidosis as compared to Caucasians.9 Further, these patients may experience a worse sarcoidosis prognosis, lower health-related quality-of-life (QoL), and higher hospitalization and mortality rates.5,10–12 In general, African Americans are underrepresented in research on rheumatic-related diseases, despite experiencing the disproportionate effect of these disorders.13,14 Therefore, it is important to close the gaps in healthcare disparities through the provision of effective interventions to underserved populations with sarcoidosis.
Previous studies suggest that Acthar® Gel (repository corticotropin injection; Mallinckrodt Pharmaceuticals) is a viable option for the treatment of advanced symptomatic sarcoidosis.15–17 Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides that interacts with all five melanocortin receptors. Its therapeutic effects in sarcoidosis may thus be attributed to the activation of several potential anti-inflammatory pathways through both glucocorticoid-dependent and independent mechanisms.18 Acthar Gel is the only other medication, in addition to oral glucocorticoids, approved by the US Food and Drug Administration for the treatment of symptomatic sarcoidosis. It is only commercially available in the US.18 Acthar Gel is also referenced in the European Respiratory Society treatment guidelines, which list Acthar Gel among the various anti-inflammatory treatments for pulmonary sarcoidosis. Further, these guidelines note that Acthar Gel can be used on a case-by-case basis when other treatments are ineffective or not tolerated. The US Sarcoidosis Delphi Expert Panel Consensus Statement provides similar recommendations for Acthar Gel in the treatment of sarcoidosis.19,20
In light of the evidence that sarcoidosis is common among African Americans and may result in a poor prognosis, real-world data are needed to understand patient characteristics and obtain insights on the optimal applications of Acthar Gel therapy for this vulnerable population in comparison to other races. Further, there has been increased awareness recently of health disparities in the African American population about sarcoidosis disease patterns and outcomes (eg, increased risk of mortality, hospitalizations, lower health-related QoL). To date, data on Acthar Gel utilization patterns and outcomes in sarcoidosis comparing African Americans with other races are limited.
To fill this knowledge gap, this study described patient characteristics, Acthar Gel utilization patterns, co-medication use, and treatment response based on the physicians’ assessments among African Americans versus non-African Americans with advanced symptomatic sarcoidosis who received treatment with Acthar Gel.
Methods
Study Design
A retrospective medical chart review study of a large case series of symptomatic sarcoidosis patients treated with Acthar Gel was conducted. A national database of Acthar Gel prescribers was merged with the American Medical Association Physician Masterfile listing to obtain a sample of physicians. A small randomized sample of physicians from the merged listing were contacted via telephone and email. From September 2017 to November 2017, 171 physicians were screened, and a total of 98 eligible physicians participated in the study. The physicians participating in this study specialized in cardiology, dermatology, gastroenterology, neurology, ophthalmology, primary care, pulmonology, and rheumatology. Physicians were eligible if they had treated ≥1 patient with symptomatic sarcoidosis. The patient should have received treatment with Acthar Gel within 36 months before the date of data collection.
Data Collection and Study Sample
The participating physicians provided data on the last 6 consecutive patients seen by the corresponding physician who met the study’s eligibility criteria. Each eligible physician could contribute data on a maximum of 6 patients to ensure that high-volume sites did not dominate the sample. The data from the medical records of qualified patients were extracted by physicians or their designated staff of qualified patients through a secure online system. The data collection process included a 5-minute physician screener form (for eligibility and consent to participate) and a 15-minute patient data collection form.
The medical records were extracted for adult patients (≥18 years) with a diagnosis of advanced symptomatic sarcoidosis, who had ≥1 symptom, and who had received Acthar Gel. Further, patients considered for this study had either completed ≥1 course of Acthar Gel or had received Acthar Gel for ≥6 months during data collection. Patients without any sarcoidosis-related symptoms were excluded from the study. Details on variables used for this study are provided in Table S1. For this study, patients were classified by race – African Americans versus non-African Americans (comprised Caucasians, Asians, American Indian/Alaska Natives, or Native Hawaiian/other Pacific Islanders).
This study used pre-existing and de-identified data that did not directly involve any human subjects and no personal health information was collected. This study was reviewed and verified as exempt by an Institutional Review Board (Solutions Institutional Review Board, Little Rock, AR, USA).
Statistical Analyses
Data were summarized using descriptive statistics; mean and standard deviation (SD) were reported for continuous variables and frequency counts and proportions were reported for categorical variables. To compare African Americans versus non-African Americans for patient-related characteristics, patterns of Acthar Gel utilization, and treatment response, the chi-square test was used for categorical variables; Fisher’s exact test was used if 20% of cells had expected frequencies <5. For continuous variables, the independent sample t-test was used. McNemar test was used to examine change in co-medication use after Acthar Gel initiation. A priori two-sided α of 0.05 was used to test the statistical significance of the relationship. Data were tabulated using IBM® SPSS Statistics version 27.0 (IBM Corporation, Armonk, NY, USA) for all analyses.
Results
Patient Demographic and Clinical Characteristics
A total of 168 African Americans and 104 non-African Americans with advanced symptomatic sarcoidosis were included in the study (Table 1). A majority of patients were 45–64 years of age. Among African Americans, more women than men had sarcoidosis. Overall, there were no statistically significant differences in age, gender, and geographic region.
 |
Table 1 Demographic Characteristics of African Americans versus Non-African Americans with Advanced Symptomatic Sarcoidosis Treated with Acthar Gel |
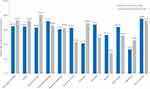
The distribution of both race groups across different clinical criteria used for confirming advanced symptomatic sarcoidosis was similar (Table 2). Most patients in both race groups had stage 3 or 4 sarcoidosis confirmation based on chest imaging and biopsy. There was no statistically significant difference between both race groups regarding time since diagnosis. On average, the time since the first diagnosis of sarcoidosis was slightly longer among African Americans than non-African Americans (mean ± SD: 5.2 ± 7.6 years versus 4.3 ± 5.1 years; p>0.05). A statistically significantly lower proportion of African Americans had ≥1 comorbidity versus non-African Americans (72.0% versus 83.7%; p=0.0280). Skin, heart, eyes, and joints were the most common extrapulmonary sites involved among both race groups; no statistically significant differences in extrapulmonary organ involvement were observed between both race groups. Shortness of breath, fatigue, bone and joint pain, and wheezing/coughing were the most frequent signs and symptoms reported among both race groups who initiated their most recent Acthar Gel treatment. The distribution of severity of symptoms varied across both race groups and by type of symptom; reported symptoms mainly tended to be moderate-to-severe for both race groups (Figure 1).
 |
Table 2 Clinical Characteristics of African Americans versus Non-African Americans with Advanced Symptomatic Sarcoidosis Treated with Acthar Gel |
Acthar Gel Treatment Patterns
A higher proportion of African Americans versus non-African Americans were first-time users of Acthar Gel (82.1% versus 76.0%) (Table 3). Further, a lower proportion of African Americans versus non-African Americans had completed a course of Acthar Gel therapy (44.0% versus 55.8%) during the data collection. However, there was no statistically significant difference in the utilization of Acthar Gel and completion of a course of Acthar Gel therapy. On average, the duration of the Acthar Gel treatment was slightly longer among African Americans versus non-African Americans (mean ± SD: 31.7 ± 32.0 versus 29.0 ± 27.4 weeks; p>0.05). The distribution of patients on Acthar Gel treatment over time was similar for both race groups (Figure 2).
 |
Table 3 Acthar Gel Treatment Patterns Among African Americans versus Non-African Americans |
 |
Figure 2 Cumulative number of African Americans versus non-African Americans continuing on Acthar Gel treatment over time. |
Most of the patients in both race groups initiated Acthar Gel at 41–80 U/week, followed by >80 U/week. Acthar Gel dosing varied across both groups as these patients had individualized dosing and dose adjustments. Patients in both race groups on higher starting doses (>80 U/week) had a shorter treatment duration whereas those on lower starting doses (≤80 U/week) had a longer treatment duration. For African Americans, the treatment regimen observed was 28.1 weeks on >80 U/week and 33.4 weeks on ≤80 U/week (34.7 weeks on 41–80 U/week and 31.7 weeks on ≤40 U/week). For non-African Americans, the treatment regimen observed was 28.8 weeks on >80 U/week and 31.8 weeks on ≤80 U/week (23.7 weeks on 41–80 U/week and 40.0 weeks on ≤40 U/week). Acthar Gel dose adjustment patterns were similar for both race groups.
Co-Medication Utilization Patterns
The use of co-medications for symptomatic sarcoidosis was examined 3 months before, at the time of, and 3 months after Acthar Gel initiation. A statistically significantly lower proportion of patients among both race groups were on any co-medication after Acthar Gel initiation (p<0.0001) (Figure 3). A higher proportion of African Americans versus non-African Americans had a reduction in any co-medication use after Acthar Gel initiation (Figure 4).
After Acthar Gel initiation, statistically significantly fewer African Americans (before: 59.5% [n=100] versus after: 11.9% [n=20]) and non-African Americans (before: 65.4% [n=68] versus after: 14.4% [n=15]) were on glucocorticoids. Prednisone dose was reported only for patients on glucocorticoids before and after Acthar Gel initiation and with dosing information available. Overall, the mean prednisone dose reduced after Acthar Gel initiation among African Americans (before: 18.5 mg/day versus after: 10.1 mg/day) and non-African Americans (before: 17.6 mg/day versus after: 10.0 mg/day). The proportion of patients on prednisone daily dose of <10 mg increased after Acthar Gel initiation among African Americans (before: 27.8% [27/97] versus after: 31.6% [6/19]) and non-African Americans (before: 13.6% [9/66] versus after: 60.0% [9/15]).
Physicians’ Assessments of Improvement
The assessment of improvement in the health status of the patient and overall symptoms was based on the assessment provided by the physician. Regarding the change in patients’ health status following Acthar Gel treatment, a majority of African Americans (n = 160, 95.2%) and non-African Americans (n = 101, 97.1%) had improved.
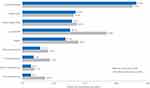
Treatment response to most clinical aspects was not statistically significantly different for both race groups. Reduction in inflammation, improvement in QoL, improvement in lung function, and reduction in fatigue were the most frequently reported changes in symptoms following Acthar Gel treatment among both race groups (Figure 5).
Discussion
The findings from this retrospective analysis comparing African Americans versus non-African Americans with advanced symptomatic sarcoidosis offer an understanding of patient characteristics and real-world clinical practice regarding the patterns of the use of Acthar Gel in these populations. This study captured primary data on the Acthar Gel utilization patterns and outcomes using the largest case series of patients with sarcoidosis.
Overall, the patient demographic and clinical profile as well as the Acthar Gel utilization pattern was similar for both race groups. The time since the first diagnosis of sarcoidosis was slightly longer among African Americans than non-African Americans, suggesting gaps in timely diagnosis among African Americans. Further, a higher proportion of African Americans versus non-African Americans were first-time Acthar Gel users and were continuing on Acthar Gel at the time of data collection, suggesting gaps in timely treatment among African Americans.
A higher proportion of African Americans versus non-African Americans had a reduction in co-medication use. Further, based on the physician’s assessment, African Americans showed improvement in patient health status and overall symptoms similar to non-African Americans. The patterns of reduction in co-medication use and improvement in patient’s health status after Acthar Gel initiation suggest that Acthar Gel may be a viable treatment option in both African Americans and non-African Americans with symptomatic sarcoidosis. Improvement in different treatment response aspects was also similar across both race groups. A statistically significantly higher proportion of non-African Americans had improvement in lung function and pulmonary fibrosis as well as a reduction in granuloma size. The probable reason for this difference could be that most of the African American patients had longer time since diagnosis as well as were first-time Acthar Gel users and were continuing on therapy.
Despite the availability of treatments, health disparities with regard to timely diagnosis and lack of use of improved treatment options persist among these underserved and vulnerable populations. Patient navigators and patient services (eg, injection training services) can be an option to enable improved healthcare quality and access for underserved segments of the population,21 thereby alleviating persistent health disparities in these populations. This study adds to the emerging literature on the effectiveness of Acthar Gel for African Americans and non-African Americans with sarcoidosis. These findings also serve as a blueprint for generating new hypotheses for further understanding of the patient profiles of vulnerable and underserved populations with advanced symptomatic sarcoidosis who are anticipated to achieve greater benefit from treatment with Acthar Gel. Finally, the study used a probability sampling technique, and thus the findings reflect the nationally representative sample and can be generalized to a broader African American population in the US diagnosed with symptomatic sarcoidosis.
This study provides real-world evidence suggesting an individualized Acthar Gel treatment (dose and duration) strategy for African Americans with advanced symptomatic sarcoidosis. African Americans initiating a higher dose (>80 U/week) had a shorter duration of treatment than those initiating a lower dose (≤80 U/week). This individualized approach showed improvement in health status for 95% of African Americans and 97% of non-African Americans based on the physicians’ assessments upon completion of Acthar Gel treatment. Based on the physician’s assessment, a majority of both race groups showed improvement in overall symptoms, inflammation, patient QoL, and lung function.
Reduction or discontinuation of background co-medications, specifically glucocorticoids is another crucial goal of treatment. Although glucocorticoids are considered the first-line therapy for sarcoidosis, their prolonged use can result in considerable toxicity.22,23 A statistically significant reduction in glucocorticoid use following Acthar Gel therapy was observed in African Americans than non-African Americans from 3 months before to 3 months after Achar Gel initiation. After Acthar Gel initiation, 80% of African Americans and 78% of non-African Americans discontinued glucocorticoids. Among those who had glucocorticoid dosing information available, there was a 45.3% and 43.2% reduction in glucocorticoid dose among African Americans and non-African Americans, respectively after Acthar Gel initiation. Acthar Gel may be a safe and effective treatment that can improve the patient’s overall health status and may serve as a viable treatment alternative to reduce the burden of high-dose and prolonged glucocorticoid use in this population.
The current study had the following limitations. First, data retrospectively collected from medical charts of patients may have omissions and errors. Completeness of information was assessed to the extent possible to minimize bias resulting from any missing data. In addition, only data available in medical charts or known to be complete to the respondents were extracted. Second, physicians’ standards for the interpretation of change in each patient’s health status vary which may result in bias due to over- or under-estimation of the effectiveness of Acthar Gel. Third, this study was unable to quantify clinical data such as diagnostic and safety measures, clinical and sustained response after treatment, and reasons for discontinuation or dose adjustments related to Acthar Gel. Fourth, this was an exploratory study on Acthar Gel treatment, and thus data were not collected for other medications. The statistical testing conducted was intended to test the statistical significance of the magnitude and directionality of the relationships; the analysis was not intended to present any causal association between these race groups. Finally, data on adverse reactions in this population, drivers of the decision to use Acthar Gel, and detailed information on prior therapies were not captured. Previous studies have provided information on adverse events. Baughman et al (2016) showed that within 33 months of Acthar Gel initiation, discontinuation of treatment due to agitation or peripheral edema was observed in five patients. Dose reduction was observed in six patients with the same adverse events.16 Further, no modification in Acthar Gel dosage was observed in one patient who had skin hyperpigmentation.16 Among 18 patients enrolled in the 24-week study of Acthar Gel treatment, treatment was discontinued for one patient due to toxicity, and Acthar Gel dose was reduced to 50% in seven patients.15 Adverse events reported comprised jitteriness (n = 6), headache (n = 3), edema (n = 2), and nausea (n = 1).15 The most frequent adverse events of Acthar Gel treatment noted in the prescribing information are behavioral and mood changes, changes in glucose tolerance, elevation in blood pressure, fluid retention, increased appetite, and weight gain.18 The mechanism of action of Acthar Gel involves multiple pathways and causes direct modulation of immune cells (eg, B cells and macrophages), independent of steroids.24 This mechanism of action is supported by a systematic review demonstrating distinct adverse event profiles of Acthar Gel and glucocorticoids in rheumatoid arthritis.25
Conclusions
Most African Americans and non-African Americans treated with Acthar Gel in this study showed improvement in health status. Further, African Americans were on an individualized Acthar Gel treatment that was associated with a significant reduction in the use of other sarcoidosis medications, especially glucocorticoids. In summary, findings indicate that Acthar Gel may be a viable treatment option that may improve health in African Americans with advanced symptomatic sarcoidosis, thereby alleviating persistent health disparities in these vulnerable and underserved populations.
Acknowledgments
This study was presented, in part or full, at the 2022 American Thoracic Society conference in San Francisco, United States on May 17, 2022.
Author Contributions
All authors made a significant contribution to the work reported, including study conception, design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Mallinckrodt Pharmaceuticals.
Disclosure
JB is an employee of Falcon Research Group. IC was a research collaborator for the duration of this study. GJW, KH, and JN are employees at Mallinckrodt Pharmaceuticals. MP is a consultant providing services on behalf of Mallinckrodt Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi:10.1164/ajrccm.160.2.ats4-99
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155–1167. doi:10.1016/S0140-6736(13)60680-7
3. Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and management of sarcoidosis. Am Fam Physician. 2016;93(10):840–848.
4. Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician. 2004;70(2):312–322.
5. Sharp M, Eakin MN, Drent M. Socioeconomic determinants and disparities in sarcoidosis. Curr Opin Pulm Med. 2020;26(5):568–573. doi:10.1097/MCP.0000000000000704
6. Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc. 2016;13(8):1244–1252. doi:10.1513/AnnalsATS.201511-760OC
7. Patel N, Kalra R, Doshi R, et al. Hospitalization rates, prevalence of cardiovascular manifestations, and outcomes associated with sarcoidosis in the United States. J Am Heart Assoc. 2018;7(2). doi:10.1161/JAHA.117.007844.
8. Baughman RP, Wells A. Advanced sarcoidosis. Curr Opin Pulm Med. 2019;25(5):497–504. doi:10.1097/MCP.0000000000000612
9. Rybicki BA, Kirkey KL, Major M, et al. Familial risk ratio of sarcoidosis in African-American sibs and parents. Am J Epidemiol. 2001;153(2):188–193. doi:10.1093/aje/153.2.188
10. Judson MA, Baughman RP, Thompson BW, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(3):204–211.
11. Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):119–127.
12. Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med. 2020;383(10):958–968. doi:10.1056/NEJMra2005230
13. Davis CM, Apter AJ, Casillas A, et al. Health disparities in allergic and immunologic conditions in racial and ethnic underserved populations: a Work Group Report of the AAAAI Committee on the Underserved. J Allergy Clin Immunol. 2021;147(5):1579–1593. doi:10.1016/j.jaci.2021.02.034
14. Lima K, Phillip CR, Williams J, Peterson J, Feldman CH, Ramsey-Goldman R. Factors associated with participation in rheumatic disease-related research among underrepresented populations: a qualitative systematic review. Arthritis Care Res. 2020;72(10):1481–1489. doi:10.1002/acr.24036
15. Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313–322. doi:10.1007/s00408-017-9994-4
16. Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72. doi:10.1016/j.rmed.2015.11.007
17. Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1753466619888127. doi:10.1177/1753466619888127
18. Mallinckrodt Pharmaceuticals. Acthar® Gel [package insert]. Available from: https://www.acthar.com/pdf/Acthar-PI.pdf. 2021.
19. Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
20. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
21. Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(15 Suppl):3541–3550. doi:10.1002/cncr.26264
22. Khan NA, Donatelli CV, Tonelli AR, et al. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir Med. 2017;132:9–14. doi:10.1016/j.rmed.2017.09.003
23. Entrop JP, Kullberg S, Grunewald J, Eklund A, Brismar K, Arkema EV. Type 2 diabetes risk in sarcoidosis patients untreated and treated with corticosteroids. ERJ Open Res. 2021;7(2):00028–2021. doi:10.1183/23120541.00028-2021
24. Mirsaeidi M, Baughman RP. Repository corticotropin injection for the treatment of pulmonary sarcoidosis: a narrative review. Pulm Ther. 2022;8(1):43–55. doi:10.1007/s41030-022-00181-0
25. Fleischmann R, Furst DE. Safety of repository corticotropin injection as an adjunctive therapy for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2020;19(8):935–944. doi:10.1080/14740338.2020.1779219
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.