Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Acne Vulgaris Relapse in Sudanese Patients Treated with Oral Isotretinoin: Rate and Predictive Factors
Authors Ibrahim S, Osman B, Awaad RM, Abdoon I
Received 20 January 2023
Accepted for publication 23 March 2023
Published 30 March 2023 Volume 2023:16 Pages 839—849
DOI https://doi.org/10.2147/JMDH.S405509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shimaa Ibrahim,1 Bashier Osman,2 Reela M Awaad,3 Iman Abdoon2
1Clinical Pharmacy Program, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan; 2Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan; 3Dermatology Department, Awaad Center for Dermatology and Venereology, Khartoum, Sudan
Correspondence: Iman Abdoon, Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Qassr Street, Khartoum, Sudan, Email [email protected]
Purpose: Acne vulgaris is a skin disorder primarily affecting teenagers and young adults. Acne relapse is the main drawback of oral isotretinoin (OI), which is the golden therapy for severe acne. This study aimed to assess the rate and predictive factors of acne relapse among Sudanese patients using OI.
Patients and Methods: A cross-sectional study was conducted in a dermatology and venereology clinic-Sudan, using a self-administered questionnaire and data collection sheet. Patients using OI for acne treatment were enrolled in the study. Chi-square test and logistic regression analysis were used to evaluate the association between variables. P-value < 0.05 was considered statistically significant.
Results: 225 acne patients (mean age: 26.0± 4.2 years, females: 88.9%) were included in this study. OI daily dose ranged from 0.25 to 1 mg/kg/day, with frequent daily doses of 40– 49 mg (57.3%) over 3– 6 months (81.8%). Around one-third of patients (36%) received maintenance therapy after completion of OI course. At a 2-year follow-up, approximately 36% of patients experienced acne relapse that commonly occurred within 6– 18 months after the last OI therapy. Early discontinuation of OI was a positive predictor of acne relapse which was 3.99 times greater in patients who had early discontinued OI than those completing the planned OI course (OR=3.99; p=0.002). OI cumulative doses of 120– 139 mg/kg and 140– 159 mg/kg were negative predictors of acne relapse (OR=0.23; p=0.001 and OR=0.15; p=0.02, respectively). Most patients (94.2%) received prescription OI, and 76.4% of women were advised to use contraceptives. About 69% of patients practiced skin care.
Conclusion: About one-third of patients experienced acne relapse. Early discontinuation and low cumulative doses of OI are the main risk factors for acne relapse. Long-term therapy of OI, with cumulative doses of 120– 159 mg/kg, would be beneficial to reduce acne relapse.
Keywords: acne relapse, oral isotretinoin, risk factors, cumulative dose
Introduction
Acne vulgaris is a chronic inflammatory disease affecting the pilosebaceous follicle. The prevalence of acne was about 9.4% of the global population, and it affects over 85% of adolescents.1 It is among the three dermatoses that affect the population in the world, and usually begins in adolescence and disappears in the mid-twenties.2
The pathophysiology of acne is complex with multifactorial etiology. Abnormal follicular keratinization, increased sebum production secondary to hyperandrogenism, follicular colonization, inflammation, endocrine disorder, and hormone-based therapies have been implicated in the pathogenesis of acne.3,4 The contribution of genetic elements in the pathogenesis of acne has so far been identified. Inflammatory microenvironment and increased sebum production potentiate the risk of Cutibacterium acnes.1,4,5 Clinical features of acne include the formation of open and closed comedones, papules, pustules, nodules, and cysts.3
The selection of effective therapy is generally based on differential diagnosis and identification of the underlying causes of acne.3,6 Treatment modalities of acne are generally based on the grade and severity of lesions, drug’s efficacy, and safety. Patient-related factors (comorbidities and endocrine history) should be considered before therapy initiation to setting realistic treatment goals.7 Different treatment modalities are available for acne management.7,8 Topical retinoids (adapalene, tretinoin, and tazarotene) are the first-line therapies for acne treatment, and adapalene is well tolerated than other topical retinoids.7,9 Combined topical and systemic therapies are recommended for severe cases.9
Oral isotretinoin (OI) is a synthetic retinoid exhibiting superior efficacy for the treatment of severe papulopustular or nodular acne. It is also used to treat moderate acne if other treatment modalities have failed or acne relapses have developed.3,10,11 OI is commonly recommended, at doses 0.3–0.5 mg/kg/day, over approximately 6 months. The dose may be augmented to 1 mg/kg/day through longer treatment duration, if needed.12
Despite rare laboratory abnormalities after OI therapy, routine monitoring of lipid profile and liver functions remains a common practice.13 However, recent studies do not necessitate routine monitoring of such functions in healthy young individuals and suggest less frequent routine laboratory testing.14 Mucocutaneous side effects such as dry eyes, nose, and lips, and dermatitis had been reported in patients taking OI. Accordingly, artificial tears, nasal emollients, and skin moisturizers should be used to minimize skin dryness.15 OI side effects are dose-dependent, predictable, and manageable.11 OI is absolutely contraindicated during pregnancy because of its teratogenicity. Therefore, pregnancy test for women of childbearing age is highly recommended before and during therapy.3
Although OI is highly effective, acne relapse may occur after discontinuation of therapy. The rate of acne relapse varies between 10% and 60%. Various factors were postulated as potential risk factors for acne relapse. As reported in the literature, acne relapse was related to severity of acne, site of lesions, age, male sex, family history of acne, prior treatment with topical retinoids, and discontinuation of OI therapy.16,17 In addition, low cumulative doses of isotretinoin and short duration of treatment are strong predictors of acne relapse.17–19
Despite being the golden therapy for severe acne vulgaris, OI has been associated with acne relapse. This study was conducted to evaluate the risk factors associated with acne relapse in Sudanese patients using OI therapy.
Materials and Methods
Study Design and Setting
A descriptive, cross-sectional study was conducted at Awaad Center for Dermatology and Venereology in Khartoum city-Sudan, from November 2020 to February 2021. Awaad center for dermatology and venereology is one of the biggest dermatology centers in Khartoum city. The center consists of clinics, a theater, an aesthetic section, and a medical laboratory.
Participants and Sampling Technique
Patients with acne vulgaris, attending the dermatology center during the study period, were recruited to participate in the study. 225 patients using oral isotretinoin (OI) for acne treatment were enrolled in the study.
Inclusion and Exclusion Criteria
Patients with moderate nodular acne and severe acne (papulopustular, nodulocystic, and conglobate acne) were enrolled in the study. Patients with moderate acne who are either treatment-resistant or prone to significant scarring were also included in the study.
In moderate acne, erythematous papules and pustules are the predominant lesions. Moderately severe acne shows erythematous papules, pustules, painful and inflamed nodules. Severe acne is a cluster of numerous or extensive painful and inflamed nodules and pustules as well as scarring.20 Both genders (females and males) were included in the study.
Patients taking intermittent doses of OI as well as patients who refused to participate in the study were excluded.
Data Collection Tool
The data were collected using patients’ medical files and self-administered, well-designed questionnaire, with closed- and open-ended questions. The questionnaire was reviewed by experts in the academic field and clinical practice and translated into Arabic, and then back-translated to English by English-speaking translators. It was pre-tested in 30 patients who were excluded from the sample size of the study. The internal consistency of the items was evaluated by calculating Cronbach’s Alpha coefficient (Cronbach’s α= 0.75).
The questionnaire consists of two sections. The first section included socio-demographic characteristics (e.g, age, gender, marital status, and family history of acne). The second section covered patients’ practice toward acne treatment (use of OI with prescription, use of OI with fatty meals, use of OI before or after meal, awareness about potential acne relapse, contraception discussion, and skin-care ways during and after isotretinoin therapy).
The clinical data were gathered from the patient’s medical file in collaboration with doctors in the dermatology center. Medical information includes body weight, localization of acne, daily dose of OI, duration of treatment, maintenance therapy, early discontinuation of OI before completing the planned OI course, acne relapse within a 2-year follow-up after completion of OI therapy, and medications for acne relapse. The cumulative dose in mg/kg was calculated from OI daily dose in mg/kg and the duration of treatment in days [Cumulative dose (mg/kg) = daily dose (mg/kg) × duration of treatment (days)].
Outcome Measures
The primary outcome was rate of acne relapse following OI therapy. Acne relapse is defined as the reappearance of acne after apparent healing, necessitating a prescription medication after OI treatment or the need for an additional course of OI.19,21
Data Analysis Methods
Data was analyzed using Statistical Package for the Social Sciences (SPSS version 25). For numerical data, mean and standard deviation were calculated. For categorical data, descriptive statistics, including percentage and frequency, were carried out to describe the distribution of patients’ characteristics. Chi-square test and logistic regression analysis were used to determine the association between acne relapse and the potential risk factors of relapse. P-value <0.05 was considered statistically significant.
Ethical Clearance
Ethical approval to conduct the research was taken from Khartoum University, Faculty of Pharmacy, Research Board (Research Ethics Committee, No. 85-30-9-2020). In addition, ethical clearance was obtained from the Scientific Ethics Committee of the Health Service-Ministry of Health-Sudan. All research is in accordance with the Helsinki Declaration. Permission to conduct the research was granted by the general managers of the dermatology and venereology center. The aim of the study was clearly explained to the patients, and voluntary participation was guaranteed. Before initiating the study, informed consent was obtained from patients, and confidentiality of participants’ information was assured by using coding data stored in password-protected files.
Results
Socio-Demographic Characteristics of Patients Using Oral Isotretinoin
Out of 225 patients, the majority were single females (88.9%), with a mean age of 26.0±4.2 years. Approximately two-thirds of patients (65.8%) had a family history of acne vulgaris. About 52% of patients weighed 56–70 kg, and their acne localizes on the face and body (Table 1).
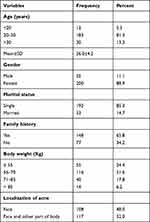 |
Table 1 Socio-Demographic Characteristics of Participants Using Oral Isotretinoin for Acne Vulgaris Treatment (N=225) |
Isotretinoin Dosage Regimen and Maintenance Therapy Among the Participants
Among patients using OI, the daily dose ranged from 0.25 to 1 mg/kg/d, with frequent daily dose of 40–49 mg (57.3%) over 3–6 months (81.8%). 45.8% of the patients received a cumulative dose of less than 100 mg/kg body weight, and 36.4% had a cumulative dose of 120–159 mg/kg. Only 36% of patients received maintenance therapy for 6 months after completion of the planned OI course, and 49.4% of them used topical adapalene as maintenance therapy (Table 2).
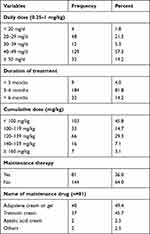 |
Table 2 Oral Isotretinoin Dosage Regimen and Maintenance Therapy in Patients with Acne Vulgaris |
Acne Relapses Among Patients Receiving Oral Isotretinoin Course
At 24-month follow-up, approximately 36% of patients experienced acne relapse that commonly occurred within 6–18 months after the last OI therapy. Unfortunately, 27.5% of patients, who developed acne relapse, did not receive any type of therapy. Isotretinoin (45%), with different dosage regimens, was frequently used for patients suffering from acne relapse. Sixteen percent of patients (16.3%) used 40 mg isotretinoin for 4–8 months, and 11.3% used tretinoin cream (Table 3).
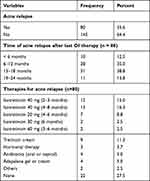 |
Table 3 Rate and Therapies of Acne Relapse |
Early Discontinuation of Therapy and Patients’ Practice Towards OI Use
About sixteen percent (16.4%) of participants discontinued OI therapy earlier due to side effects (51.4%) and non-affordability/unavailability of the drug (40.5%). Awareness about acne relapse had been observed in two-thirds of patients (62.7%), and 76.4% were advised to use contraceptive methods during isotretinoin therapy. More than 90% of patients had good practice regarding the use of OI with doctor’s prescription only, and administration of the drug after meals. About 69% of patients practiced different ways of skin care (Table 4).
 |
Table 4 Early Discontinuation of Therapy and Patients’ Practice Towards OI Use |
Association Between Acne Relapse and Patient’s Practice Towards Acne Treatment
There was no significant association between acne relapse and patients’ practice regarding the use of prescription OI (P=0.41), the use of OI with fatty meal (P=0.10), the use of OI after or before meal (P=0.55), and using skin care ways (P=0.21) (Supplementary File 1).
Factors Associated with Acne Relapse After Oral Isotretinoin Therapy (Logistic Regression Model)
Predictive factors of acne relapse were evaluated using logistic regression analysis. Early discontinuation of therapy was a positive predictor of acne relapse. The odds of acne relapse were 3.99 times more likely to occur in patients who have early discontinued OI (OR=3.99; 95% CI: 1.67–9.51; p=0.002) than those completing the planned OI course. On the contrary, patients receiving cumulative doses of 120–139 mg/kg (OR=0.23; 95% CI: 0.10–0.53; p=0.001) were less likely to experience acne relapse than those taking cumulative doses of less than 100 mg/kg. Moreover, a significant reduction in acne relapse was observed in patients taking cumulative doses of 140–159 mg/kg (OR=0.15; 95% CI: 0.03–0.77; p=0.02) vs patients taking cumulative doses less than 100 mg/kg (Table 5).
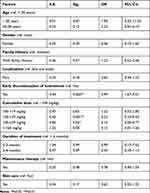 |
Table 5 Predictive Factors of Acne Relapses After Oral Isotretinoin Therapy (Logistic Regression Model) |
Discussion
Acne is a skin disease frequently encountered in adolescence, mostly in females, but it may occur in adults. It affects the self-image, quality of life, and productivity of teenagers and adults.3,22,23 Different treatment modalities are available for acne vulgaris, ranging from topical medications in mild cases to oral antibiotics, hormonal therapy, and oral isotretinoin (OI) as first-line therapy for moderately severe to severe cases.24 Acne relapse has become a serious concern, with increased relapse rate after using low OI doses. In Sudan, there are sparse data about acne relapse. This study aimed to assess the rate and risk factors of acne relapse among Sudanese patients using OI therapy.
In the current study, the majority of patients were females. Similarly, previous studies revealed a higher prevalence of acne in females than males, with male-to-female ratio of 1:1.8.1,2,25 This finding could be attributed to hormonal changes affecting females during menstruation, contraceptive use, and endocrine abnormalities (PCOS).3 The vast majority of participants were young adults, with a mean age of 26.0 years (SD: 4.2). Acne primarily affects adolescence; however, the prevalence of acne in adult patients is increasing.2
The study results demonstrated that most patients weighed 50–70 kg and had a family history of acne vulgaris. In previous studies, acne severity was associated with overweight/obese and family history of acne.1,26 According to the literature, genetic inheritance plays a role in the multifactorial pathogenesis of acne.4,27,28 In the current study, acne appeared on the faces and bodies of 51% of the patients. Likewise, an earlier study noted that acne lesions are most likely to occur on the face, neck, chest, and back where numerous sebaceous glands are present.29
The relapse rate in this study was observed in 36% of patients over the 2-year follow-up. A comparable relapse rate had been reported in the literature.19,30 In a Turkish study, the relapse rate was found to be 37.3%.30 Significant variability in the rates of acne relapse among patients using OI had been reported; the rate ranges between 10% and 60%.16 In a previous study, 69.5% of patients relapsed within 2 years after completing OI course.17 Acne relapses were reported in 44% of patients; 92.4% of them experienced several relapses with similar severity.22 In Brazil, the rate of acne relapse was 25.5% in patients taking the full course of isotretinoin, and it reached 81% in those receiving an irregular and incomplete course of treatment.31 These discrepancies in the rate of acne relapse could be attributed to differences in the relapse definitions, daily dose prescription, cumulative dose of isotretinoin, length of follow-up, selection criteria of the patients, and genetic elements.21
During the 2-year follow-up in this study, acne relapse commonly occurred within 6–18 months after OI therapy. According to a previous study, the average time to relapse was around 15 months.17 An earlier study revealed that 32.7% of patients relapsed within 12 months after completion of the planned therapy.19
The association between acne relapse and different treatment-specific or patient-specific factors has been featured in this study. Of all variables analyzed in this study, early discontinuation of OI therapy and cumulative doses of OI were the only predictive factors of acne relapse. Early discontinuation of OI therapy was positively associated with acne relapse. These findings are in line with previous studies. According to the literature, patients developing acne relapse discontinued their treatment earlier than those who completed the planned OI course. Therefore, extending OI therapy after complete acne clearance may help minimize acne relapse.17,30
In this study, high cumulative doses of OI (120–159 mg/kg) were negatively associated with acne relapse. As reported in the literature, a higher cumulative dose of OI (>220 mg/kg) decreased the risk of acne relapse if compared with a low cumulative dose (<220 mg/kg).19 In comparison with OI low-daily doses, the conventional doses (0.5–1 mg/kg/day and cumulative doses of 120–150 mg/kg) showed positive clinical outcomes regarding response and relapse rate.18 Previous findings also suggested that high cumulative doses of OI and continuation of therapy for more than 1 month after complete acne clearance can effectively treat acne with a lower relapse rate and better long-term outcomes if these doses are tolerable.17,19
Contrastingly, a previous study highlighted the non-advantageous effect of OI cumulative dose higher than 120 mg/kg on acne relapse when compared with a medium cumulative dosage (100–120 mg/kg). The high cumulative dose is adversely associated with an increased risk of OI side effects and poor medication adherence.32 Moreover, neither daily dosage nor cumulative dose influenced acne relapse as long as treatment continued for ≥2 months after the acne had completely resolved.33 These contradictory findings support the concept of extending OI (at least 1 month) after complete clearance of acne.
The study results showed an insignificant association between acne relapse and maintenance therapy after completion of isotretinoin course. However, patients receiving maintenance therapy were less likely to have acne relapse (OR=0.78) than those who did not receive maintenance therapy. Regular use of topical retinoids as maintenance therapy was a significant protective factor against acne relapse, even at low cumulative doses.16
The study results did not show any significant association between acne relapse and other factors such as gender, age, family history, acne localization, and duration of treatment. These findings are consistent with previous studies; gender, age, and family history were not associated with acne relapse.21,34 Conversely, a previous analysis concluded that male sex was a prognostic factor for relapse.16 Such discrepancy in findings could be due to differences in the characteristic of the populations under study, such as individual susceptibility and tolerability to treatment, medication compliance, skin care, and genetic elements.
Concerning patients’ practice towards acne treatment in this study, most patients were aware of the potential risk of acne relapse, using OI with a prescription, and receiving information about contraception during OI therapy. Isotretinoin is an oral prescription-only medication with a teratogenic effect (FDA pregnancy category X).35 Therefore, contraception should be discussed with females of childbearing potential before therapy initiation. Pregnancy prevention programs, such as iPLEDGE program in the USA, play a major role in selecting the appropriate contraception methods. Pregnancy tests are required before therapy, monthly during treatment, and 1 month after therapy completion.3 In Saudi Arabia, teratogenicity and contraception methods were discussed with 25% of women receiving OI.21
As reported in the literature, OI is a lipophilic drug that should be taken with a high-fat meal to enhance absorption. Unfortunately, most patients in this survey did not adhere to this recommendation, and accordingly, low bioavailability of OI and a high risk of relapse may occur.36
Regarding skin-care practice in this study, most patients used acne-specific cleansers and moisturizers. Although the association between skin care and acne relapse was insignificant, it seems that skin care was negatively associated with acne relapse (OR=0.62). OI causes mucocutaneous side effects such as irritation and dryness in the eyes, nose, lips, and skin. Therefore, acne-specific cleansers and moisturizers might be appropriate to minimize mucocutaneous side effects.15,20,37 Patient counseling is an effective method to encourage patients to use moisturizers and increase their medication-taking behaviors.
Limitations
A single-centered, cross-sectional study limits the generalizability of the results. Additionally, the study was conducted during the COVID-19 pandemic, leading to a small sample size; therefore, the study may not be generalized to all patients with acne.
Conclusions
The daily dose of OI therapy ranged from 0.25 to 1 mg/kg/d, with frequent daily doses of 40–49 mg over 3–6 months. The majority of patients received cumulative doses of less than 100 mg/kg. The rate of acne relapse is relatively high (36%), but it is within the reported range of acne relapse. Low cumulative doses and early discontinuation of therapy were substantial risk factors for acne relapse. Most participants received prescription isotretinoin, and females of childbearing potential had received counseling about contraception methods.
Recommendations
- Encouragement of patients to complete oral isotretinoin course and adhere to their medications to avoid acne relapse.
- High cumulative doses or extended OI therapy (≥2 months) after complete acne clearance are recommended to minimize the risk of acne relapse.
- Counseling patients about isotretinoin side effects and the use of contraception measures before, during, and 1 month after therapy is urgently needed.
Acknowledgments
The authors acknowledged all members of Awaad center for dermatology and venereology, Khartoum, Sudan, for their support and assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):5754.
2. Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: a retrospective study of 1167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
3. Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2018;4(2):56–71. doi:10.1016/j.ijwd.2017.10.006
4. Kurokawa I, Nakase K. Recent advances in understanding and managing acne. F1000Res. 2020;9:792. doi:10.12688/f1000research.25588.1
5. Podgórska A, Puścion-Jakubik A, Markiewicz-żukowska R, Gromkowska-Kępka KJ, Socha K. Acne vulgaris and intake of selected dietary nutrients-a summary of information. Healthcare. 2021;9(6):668. doi:10.3390/healthcare9060668
6. Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100(8):475–484.
7. Fox L, Csongradi C, Aucamp M, du Plessis J, Gerber M. Treatment Modalities for Acne. Molecules. 2016;21(8):1063. doi:10.3390/molecules21081063
8. Scopelliti MG, Kothare A, Karavitis M. A novel 1726-nm laser system for safe and effective treatment of acne vulgaris. Lasers Med Sci. 2022;37(9):3639–3647. doi:10.1007/s10103-022-03645-6
9. Eichenfield DZ, Sprague J, Eichenfield LF. Management of acne vulgaris: a review. JAMA. 2021;326(20):2055–2067. doi:10.1001/jama.2021.17633
10. Bettoli V, Guerra-Tapia A, Herane MI, Piquero-Martín J. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943–951. doi:10.2147/CCID.S234231
11. Bagatin E, Costa CS, Rocha M, et al. Consensus on the use of oral isotretinoin in dermatology - Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(Suppl1):19–38.
12. Leung AK, Barankin B, Lam JM, Leong KF, Hon KL. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10(12). doi:10.7573/dic.2021-8-6
13. Barbieri JS, Shin DB, Wang S, Margolis DJ, Takeshita J. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82(1):72–79. doi:10.1016/j.jaad.2019.06.025
14. Affleck A, Jackson D, Williams HC, Chavez P, Albrecht J. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187(6):857–865.
15. Sevimli Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992. doi:10.1177/0300060519847367
16. Morales-Cardona CA, Sánchez-Vanegas G. Acne relapse rate and predictors of relapse following treatment with oral isotretinoin. Actas Dermosifiliogr. 2013;104(1):61–66. doi:10.1016/j.ad.2012.05.004
17. Tran PT, Berman HS, Leavitt E, Hogeling M, Cheng CE. Analysis of factors associated with relapse in patients on their second course of isotretinoin for acne vulgaris. J Am Acad Dermatol. 2021;84(3):856–859.
18. Al-Muqarrab F, Almohssen A. Low-dose oral isotretinoin for the treatment of adult patients with mild-to-moderate acne vulgaris: systematic review and meta-analysis. Dermatol Ther. 2022;35(4):e15311. doi:10.1111/dth.15311
19. Blasiak RC, Stamey CR, Burkhart CN, Lugo-Somolinos A, Morrell DS. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149(12):1392–1398. doi:10.1001/jamadermatol.2013.6746
20. Kraft J, Freiman A. Management of acne. Cmaj. 2011;183(7):E430–E435. doi:10.1503/cmaj.090374
21. Alshammari SA, Alamri Y, Alanazi AM., Almuhanna SA, Pinjabi L, Alsnaidi NA. Prevalence and associated risk factors of acne relapse among Saudi acne vulgaris patients using isotretinoin. Saudi Pharm J. 2020;28(3):374–379. doi:10.1016/j.jsps.2020.01.019
22. Dreno B, Bordet C, Seite S, Taieb C. Acne relapses: impact on quality of life and productivity. J Eur Acad Dermatol Venereol. 2019;33(5):937–943. doi:10.1111/jdv.15419
23. Kaikati J, Zoghaib S, Kechichian E, et al. The impact of acne treatment on quality of life and self-esteem: a prospective cohort study from Lebanon. Int J Womens Dermatol. 2021;7(4):415–421. doi:10.1016/j.ijwd.2021.03.005
24. Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 Suppl 1):S1–S23.e1. doi:10.1016/j.jaad.2017.09.078
25. Tayel K, Attia M, Agamia N, Fadl N. Acne vulgaris: prevalence, severity, and impact on quality of life and self-esteem among Egyptian adolescents. J Egypt Public Health Assoc. 2020;95(1):30. doi:10.1186/s42506-020-00056-9
26. Heng AHS, Say Y-H, Sio YY, Ng YT, Chew FT. Epidemiological risk factors associated with acne vulgaris presentation, severity, and scarring in a Singapore Chinese population: a cross-sectional study. Dermatology. 2022;238(2):226–235. doi:10.1159/000516232
27. Heng AHS, Say YH, Sio YY, Ng YT, Chew FT. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14(1):103. doi:10.1186/s12920-021-00953-8
28. Common JEA, Barker JN, Steensel M. What does acne genetics teach us about disease pathogenesis? Br J Dermatol. 2019;181(4):665–676. doi:10.1111/bjd.17721
29. Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician. 2012;86(8):734–740.
30. Demirci Saadet E. Investigation of relapse rate and factors affecting relapse after oral isotretinoin treatment in patients with acne vulgaris. Dermatol Ther. 2021;34(6):e15109. doi:10.1111/dth.15109
31. Nascimento C, Martins ALG, Milagres S, Jr I. Acne recurrence after treatment with oral isotretinoin: 5-year follow-up. Surg Cosmet Dermatol. 2011;3:188–191.
32. Skroza N, Tolino E, Balduzzi V, et al. Advantages of tailored isotretinoin treatment in moderate to severe acne: real-life data. Front Pharmacol. 2021;12:733526. doi:10.3389/fphar.2021.733526
33. Rademaker M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int J Dermatol. 2016;55(5):518–523. doi:10.1111/ijd.12942
34. Šijak D, Horvat I, Sonicki Z, et al. Correlation between family history and the age of onset of childhood acne in relation to sex and type of acne. Acta Dermatovenerol Croat. 2019;27(2):86–89.
35. Draghici -C-C, Miulescu R-G, Petca R-C, Petca A, Dumitrașcu MC, Șandr F. Teratogenic effect of isotretinoin in both fertile females and males (Review). Exp Ther Med. 2021;21(5):534. doi:10.3892/etm.2021.9966
36. Jones M, Armstrong AW, Baldwin H, Stein Gold L, Kircik LH. ARTICLE: advances in oral isotretinoin therapy. J Drugs Dermatol. 2021;20(5):s5–s11. doi:10.36849/JDD.s072A
37. Oon HH, Wong SN, Aw DCW, Cheong WK, Goh CL, Tan HH. Acne management guidelines by the Dermatological Society of Singapore. J Clin Aesthet Dermatol. 2019;12(7):34–50.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
