Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Accuracy Rate of Methylene Blue Injection in Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer Patients: A Prospective Observational Study
Authors Aziz HK, Azhar Y, Widarda IR, Abdurahman M, Erdiansyah Z, Nugraha P, Lukman K
Received 9 September 2023
Accepted for publication 5 December 2023
Published 7 December 2023 Volume 2023:15 Pages 891—897
DOI https://doi.org/10.2147/BCTT.S439325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Harlan Kasyfil Aziz,1 Yohana Azhar,2 Irra Rubianti Widarda,2 Maman Abdurahman,2 Zuldi Erdiansyah,1 Prapanca Nugraha,1 Kiki Lukman1
1Department of Surgery, Faculty of Medicine, University of Padjadjaran, Bandung, West Java, Indonesia; 2Department of Surgery, Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia
Correspondence: Kiki Lukman; Yohana Azhar, Email [email protected]; [email protected]
Objective: Breast cancer is the most prevalent cancer in women and can spread to the lymph nodes in the axilla. The sentinel lymph node is the first lymph node to be targeted for the spread or metastasis of cancer cells involving the lymph nodes. The aim of this study is to compare the accuracy rate of methylene blue injection into sentinel lymph nodes in early-stage breast cancer patients who have undergone incisional and excisional biopsies.
Methods: This is a prospective observational study conducted in two general hospitals in West Java, Indonesia. The research subjects in this study were early-stage breast cancer patients with no lymph node metastasis (N0) who had undergone a biopsy. There were 83 study subjects included in this study. The sentinel lymph node biopsy was taken after injection of methylene blue into the peritumoral area. Blue nodes in the axilla were marked as positive lymph node biopsy results and sent for histopathology examination.
Results: Patients who underwent excisional biopsy surgery had a sensitivity rate of 85.3% and a specificity of 93.3%, while the accuracy rate in patients who underwent incisional biopsy surgery had a sensitivity rate of 79.2% and a specificity of 80%.
Conclusion: There was no difference in the accuracy of SLNB using methylene blue in patients with early-stage breast cancer with N0 who had a history of incisional and excisional biopsy.
Keywords: biopsy, breast cancer, methylene blue, sentinel lymph nodes
Introduction
The status of axillary nodes plays an important role in the management of patients with breast cancer, both for determining prognosis and subsequent therapy. Sentinel lymph node biopsy (SLNB) is proven to be one of the most accurate procedures for axillary staging in breast cancer patients. The sentinel lymph node (SLN) is the first lymph node to be targeted for the spread or metastasis of cancer cells involving the lymph nodes.1–3 The SLN can be detected using several methods, mainly by injecting a certain substance into the area around the tumor.4
Lymph nodes will perform phagocytosis of all foreign bodies that enter them through afferent vasa, including cancer cells and materials introduced from outside the body.4–6 Based on this fact, regional lymphatic flow may be useful to determine whether the first lymph nodes along the colored lymphatic flow are enlarged due to the phagocytosis process of cancer cells entering them. It can also be found that contrast enters the lymph nodes of the next station without entering the first station. Methylene blue itself is a dark blue contrast agent consisting of the chemical bond composition C16H18CIN3S, usually used for bacterial or other biological staining. Methylene blue contrast is affordable and does not cause hypersensitivity reactions or other severe complications except for a little skin necrosis that can be avoided with the correct stenting technique, especially in cases of breast conserving surgery. Side effects such as discoloration of urine, stool, and skin at the injection site are temporary and not severe.5,7–11
The accuracy of methylene blue injection is determined by the presence of blue lymph nodes in the axillary lymphatic ducts.12,13 The sentinel lymph node biopsy was initiated by infiltrating the skin around the tumor with around 5 cc of 1% methylene blue. The breast was then massaged for 5 min. After the tumor has been removed, the axillary lymph nodes will be identified. If there were less than three blue axillary lymph nodes, or sentinel nodes, a biopsy would be performed. However, if there were more than three, an axillary dissection would be performed.13 The accuracy assessed was whether the blue axillary lymph nodes with positive histological examination results were metastatic.14 The sensitivity of SLNB ranged from 77% to 94.5%, with false negativity ranging from 5.5% to 16.7%.5,15,16
The initial diagnosis of breast cancer is made by a biopsy, either an incisional, excisional, or core biopsy.3 Biopsies, especially large volumes of excisional biopsies, may disrupt the mapping of the lymphatic system. Patients with a history of excisional biopsy may have had their lymphatic system damaged; therefore, sentinel accumulation into the excised tumor cavity may occur. Based on this condition, patients with a history of excisional biopsy may not provide an accurate picture for lymphatic system mapping.6,17 A prospective study conducted by Wong, who examined the accuracy of sentinel lymph node biopsy (SLNB), stated that there was no difference in the level of accuracy in breast cancer patients who had performed incisional biopsy and core biopsy.4,18 Therefore, this study aims to investigate the comparison of the accuracy rate of methylene blue injection into sentinel lymph nodes in early-stage breast cancer patients who have undergone incisional biopsy and excisional biopsies.
Materials and Methods
This is a prospective observational study conducted in two general hospitals in West Java, Indonesia, and follows the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.19,20 The research subjects in this study were early-stage breast cancer patients (cancer not spreading beyond the breast or lymphatic nodes including stage I, IIA, IIB, and IIIA)21,22 with N0 (no metastases to nearby lymph nodes) who had undergone a biopsy for the confirmation of their breast cancer diagnosis at Two General Hospital in West Java, Indonesia, during January–December 2022. Identification of cancer type and immunohistochemistry in the form of ER, PR, Ki67, and Her2Neu was done after the first biopsy.
The inclusion criteria in this study were patients with early-stage N0 breast cancer proven clinically through ultrasound and physical examination who had undergone incisional biopsy, core biopsy, or excisional biopsy but had not undergone mastectomy surgery or chemotherapy. The researchers decided that the incisional biopsy and core biopsy were grouped together as the tumor is still present with minimal disruption to the surrounding tumor’s tissue. We exclude patients who have a history of allergy to foreign agent injections; patients with impaired renal function; patients who have previous lymph disorders or disorders, whether caused by cancer or others; patients with a history of previous surgery in the axillary area; and patients with a history of non-malignant breast disease and requiring surgery in the breast area under study. The study sample size was calculated using Lemeshow et al’s formula for differences in proportion, and the minimum sample size needed was 36 patients.23,24
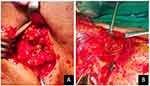
All patients are scheduled for either breast conserving surgery or mastectomy. When the patient is anesthetized and the operating field has been prepared, 2 mL of 1% methylene blue is injected in the peritumoral area at 3, 6, 9, and 12 o’clock. Breast massage was performed gently for 5 min. In breast conserving therapy surgery, after tumor removal, a separate incision is made at the axillary line to locate the sentinel lymph node. In mastectomy surgery, sentinel lymph nodes are identified after a flap is made superior to the junction of the tip of the pectoralis major with the latissimus dorsi. Macroscopic identification of blue-stained lymph nodes is performed and all sentinel nodes positive were defined as blue nodes or lymph nodes with blue lymphatic channels as shown in Figure 1, and then SLNB is performed. All procedures were performed on axillary lymph node dissection (ALND) levels I–II. The dissected tissue was then sent for histopathologic examination. After hematoxylin and eosin (H&E) staining of the preparations, histopathological data were obtained in the form of positive and negative metastatic nodes. The histopathology result was classified as pathological type classification from the College of American Pathologists version 1.2.0.0.25 The differences between variables were analyzed using chi-square analysis. Sensitivity and specificity measurements were performed to assess the accuracy of SLNB in incisional and excisional biopsy patients compared with the confirmation of the histopathologic examination. The statistical analysis was calculated using Statistical Package for the Social Sciences (SPSS) 26 software. A p value <0.05 indicates a significant relationship.
Results
There were 83 subjects included in this study; the characteristics of the research participant are shown in Table 1, with the majority of the tumor located at the right breast (65.1%), the group of incisional and excisional biopsies being balanced (51% and 49%), and the majority of the tumor type being non-special type (90.4%). The differences between intraoperative findings variables based on the histopathology result of SLNB are shown in Table 2. Significant differences were shown in cancer type with a p value of 0.016. The accuracy of SLNB according to the biopsy type is shown in Table 3.
 |
Table 1 Research Participant’s Characteristics |
 |
Table 2 Association Between the Variables and Histopathology of SLNB Results |
 |
Table 3 Accuracy of the Sentinel Lymph Nodes Biopsy |
Discussion
The most common tumor type is the non-special type (90.4%) in this study, which is more prevalent than in the literature, and which accounts for the non-special type at 70–75%.26,27 The presence of tumor cells in the lymph nodes is still a matter of debate, whether it is just a regional progression or an indicator of the systemic spread of malignancy. However, the presence of lymph node involvement is an indicator of poor prognosis in breast cancer, with a 5-year survival rate of approximately 28–40%. Axillary lymph node dissection is often performed to stage the cancer and also aid in malignancy control, thereby improving survival. However, in axillary lymph node dissection performed in patients without metastatic lymph nodes (cN0), 70–80% of these patients have pathologically cancer cell-free lymph nodes (pN0), increasing the risk of unnecessary procedures such as arm lymphedema, axillary sensory impairment, and shoulder adduction deficits.28–32
This study found that the accuracy of SNLB compared to the histological examination from the axillary lymph node dissection showed a sensitivity of 82.8% and a specificity of 88%. This value is slightly different for patients who have had different types of biopsies. The accuracy of SNLB in patients who had undergone an incisional biopsy was lower, with a sensitivity of 79.2% and a specificity value of 80%. Meanwhile, the accuracy of SNLB in patients who had undergone an excisional biopsy was higher, with a sensitivity of 85.3% and a specificity value of 93.3%. This finding is in line with the Wong et al study, which found that there was no significant difference in the identification of sentinel lymph nodes or false-negative rates in patients with various biopsy methods.4 The findings of Haigh et al are also in line with the findings in this study, namely that the study by Haigh et al showed that SLNB that was successfully performed in breast cancer patients was not fixated on the biopsy method, whether it was a core-needle biopsy, an incisional biopsy, or an excisional biopsy.33,34 The results of a study by Estourgie et al suggest that excisional biopsy may alter lymphatic drainage. They conducted a study on 25 female patients who would have undergone an excisional biopsy and had axillary procedures for further management. A lymphoscintigraphy procedure was performed prior to surgery. After the procedure, 17 out of 25 lymphoscintigraphy scans showed disruption of the lymphatic drainage pattern.35
This study shows that there is no significant relationship between SLNB and the type of biopsy performed. SLNB status is a prognostic factor that affects the overall survival rate of patients with breast cancer. Based on research conducted by Forghani et al, there is no significant difference in lymphatic mapping during SLNB in breast cancer patients who have undergone excisional biopsy first. This is in accordance with the results of this study, where there is no significant difference between SLNB results and the type of biopsy performed.18 A study by Alavifard et al also discussed the relationship between previous excisional biopsies and SLNB. They conducted the study because of the possibility of lymphatic mapping disorders after an excisional biopsy. However, after a prospective study, they stated that the sensitivity and predictive value did not experience a significant difference when SLNB was performed using a pre-operative lymphoscintigram.14 Similar results were also obtained in another study conducted by Maaskant-Braat et al to explain lymphatic mapping in patients undergoing breast conserving therapy surgery accompanied by axillary staging using SLNB or ALND. Their study states that lymphatic mapping can still be performed after BCT surgery, although the identification rate is relatively low.1 Alavifard et al also reported a case of a 24-year-old woman who had undergone excisional biopsy surgery on the left breast as a result of invasive ductal carcinoma. She developed a left axillary lump 3 weeks later and underwent SLNB. When the procedure was performed, it was seen that there was no disturbance of lymphatic mapping in breast cancer cases that had undergone an excisional biopsy.14 The findings in this study are contradictory to the theory that SLNB in breast cancer will be less accurate after an excisional biopsy of the primary tumor. This is related to the lymphatic disruption caused by the excisional biopsy itself and the inflammatory changes that cause this lymphatic mapping failure. In addition, this is also mentioned in the study of Krag et al, who found a 7-fold increase in the identification of lymph nodes after excisional biopsy.36 Feldman et al’s study also showed false-negative rates were only present in patients with a previous history of excisional biopsy. However, Feldman et al’s study also showed an increase in accuracy with minor adjustments to the biopsy protocol, such as changes in the number and volume of injections.37
The limitation of this study is that no lymphoscintigram was performed, so the lymphatic mapping pathway could not be clearly described. Further research is required to investigate lymphatic mapping in patients with previous biopsy histories. Sentinel lymph node biopsies have been extensively performed in various medical facilities for early-stage breast cancer patients due to their favorable diagnostic and prognostic value. Although this method has been initiated at Our General Hospital using methylene blue, it has not yet become routine due to equipment limitations and the predominance of advanced-stage patients at the hospital. Additionally, the utilization of radiotracer-guided SLNB, which aids injection in breast cancer patients, is still unfeasible. This limitation hampers the research due to inadequate facilities for implementing such a method. Further research using a prospective design is required where population factors can be controlled and confounding factors can be taken into account. A larger sample size for such variables as tumor size, tumor diameter, type of surgery, history of surgery, and duration of time after biopsy should be considered in the future. It is also necessary to conduct research for SLNB identification using dual-tracer guidance to help assess lymphatic mapping in patients. The use of SLNB for intraoperative diagnosis still needs to be developed by considering the various factors involved to obtain more favorable prognosis results.
In conclusion, there was no difference in the accuracy of SLNB using methylene blue in patients with early-stage breast cancer with N0 who had a history of incisional or excisional biopsy. Patients who underwent excisional biopsy surgery had a slightly higher sensitivity rate and higher specificity rate than those who underwent incisional biopsy.
Data Sharing Statement
All data and tables used to support the findings of this study are included within the article and available upon request to the corresponding author.
Ethical Declaration
Dr. Hasan Sadikin Hospital's ethics committee approved the study with Ethical Approval No. LB.02.01/X.6.4/272/2021 and this study was conducted in accordance with the Declaration of Helsinki. The lead researcher was presenting the purpose of the study, the benefit, and the risk of the procedure taken by the research participant to the ethical committee board prior to approval. As sentinel lymph node biopsy with methylene blue injection is not a standard practice in West Java, Indonesia, especially in Dr. Hasan Sadikin Bandung, the implementation of this study became important. Each research participant was given detailed information regarding the purpose, benefit, and risk of the study. The procedure taken by every research participant was done by the same experienced breast surgery expert, a surgical oncologist, assisted by one surgical oncologist trainee and one surgical resident. Each research participant signed an informed consent form before participating in the study.
Acknowledgments
We thank all the patients who agreed to participate in this study and the trainees and surgical residents who helped carry out this study.
Funding
The authors received no funding for the preparation of this study.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Maaskant-Braat AJ, de Bruijn SZ, Woensdregt K, Pijpers H, Voogd AC, Nieuwenhuijzen GA. Lymphatic mapping after previous breast surgery. Breast. 2012;21(4):444–448. doi:10.1016/j.breast.2011.10.007
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Shim VC. How much is enough? The continuing debate on the axillary lymph node dissection in breast cancer. Perm J. 2007;11(2):77. doi:10.7812/TPP/06-085
4. Wong SL, Edwards MJ, Chao C, et al. The effect of prior breast biopsy method and concurrent definitive breast procedure on success and accuracy of sentinel lymph node biopsy. Ann Surg Oncol. 2002;9:272–277. doi:10.1007/BF02573065
5. Zahoor S, Haji A, Battoo A, Qurieshi M, Mir W, Shah M. Sentinel lymph node biopsy in breast cancer: a clinical review and update. J Breast Cancer. 2017;20(3):217–227. doi:10.4048/jbc.2017.20.3.217
6. Rahman M, Mohammed S. Breast cancer metastasis and the lymphatic system. Oncol Lett. 2015;10(3):1233–1239. doi:10.3892/ol.2015.3486
7. Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol. 2010;23(2):S26–32. doi:10.1038/modpathol.2010.36
8. Li J, Chen X, Qi M, Li Y. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: a systematic review and meta-analysis. PLoS One. 2018;13(9):e0204364. doi:10.1371/journal.pone.0204364
9. East JM, Valentine CS, Kanchev E, Blake GO. Sentinel lymph node biopsy for breast cancer using methylene blue dye manifests a short learning curve among experienced surgeons: a prospective tabular cumulative sum (CUSUM) analysis. BMC Surg. 2009;9:1–8. doi:10.1186/1471-2482-9-2
10. Filippakis GM, Zografos G. Contraindications of sentinel lymph node biopsy: are there any really? World Journal of Surgical Oncology. 2007;5(1):1. doi:10.1186/1477-7819-5-10
11. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi:10.1056/NEJMoa1600249
12. Mansel R, Webster D, Sweetland H. Breast anatomy and physiology. In: Hughes, Mansel & Webster’s Benign Disorders and Diseases of the Breast.
13. Williams NS, Bullstrode CJK, O’Connell PR. Bailey & Love’s Short Practice of Surgery. Annals of The Royal College of Surgeons of England; 2010:178.
14. Alavifard R, Kadkhodayan S, Shandiz FH, Dabbagh VR, Sadeghi R. Is sentinel node mapping possible in surgically removed ectopic axillary breast cancer? A case report. Nucl Med Rev. 2016;19(B):29–30. doi:10.5603/NMR.2016.0036
15. Dogan NU, Dogan S, Favero G, Köhler C, Dursun P. The basics of sentinel lymph node biopsy: anatomical and pathophysiological considerations and clinical aspects. J Oncol. 2019;2019:1–10. doi:10.1155/2019/3415630
16. Özdemir A, Mayir B, Demirbakan K, Oygür N. Efficacy of methylene blue in sentinel lymph node biopsy for early breast cancer. Eur J Breast Health. 2014;10(2):88. doi:10.5152/tjbh.2014.1914
17. Bandyopadhyay S, Bluth MH, Ali-Fehmi R. Breast carcinoma: updates in molecular profiling 2018. Clinics Lab Med. 2018;38(2):401–420. doi:10.1016/j.cll.2018.02.006
18. Forghani MN, Memar B, Jangjoo A, et al. The effect of excisional biopsy on the accuracy of sentinel lymph node mapping in early stage breast cancer: comparison with core needle biopsy. Am Surg. 2010;76(11):1232–1235. doi:10.1177/00031348100760112
19. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31. doi:10.4103/sja.SJA_543_18
20. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
21. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi:10.1093/annonc/mdz173
22. Azhar Y, Dewayani BM, Lukman K. Methylene blue sentinel lymph node biopsy for breast cancer learning curve in the COVID-19 era: how many cases are enough? F1000Res. 2023;11:740. doi:10.12688/f1000research.122408.2
23. Levy PS, Lemeshow S. Sampling of Populations: Methods and Applications. John Wiley & Sons; 2013:21–27.
24. Lwanga SK, Lemeshow S; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual. World Health Organization; 1991:7–9.
25. Fitzgibbons PL, Connolly J. Protocol for the Examination of Biopsy Specimens from Patients with Invasive Carcinoma of the Breast. College of American Pathologists (CAP); 2019:1–6.
26. Surabhi DM, Wilson JC, Singh M, Green L. Recognizing invasive breast carcinoma of no special type with medullary pattern. Radiol Case Rep. 2023;18(5):1788–1792. doi:10.1016/j.radcr.2023.01.052
27. Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica. 2020;112(1):25. doi:10.32074/1591-951X-1-20
28. Winchester. DJ:. Evaluation and Surgical Management of Stage I and II Breast Cancer. In: ‘Breast Cancer Atlas of Clinical Oncology’. Chicago: American Cancer Society; 2000:146.
29. Garcia-Tejedor A, Ortega-Exposito C, Salinas S, et al. Axillary lymph node dissection versus radiotherapy in breast cancer with positive sentinel nodes after neoadjuvant therapy (ADARNAT trial). Front Oncol. 2023;13:1184021. doi:10.3389/fonc.2023.1184021
30. Hara Y, Otsubo R, Shinohara S, et al. Lymphedema after axillary lymph node dissection in breast cancer: prevalence and risk factors—a single-center retrospective study. Lymphat Res Biol. 2022;20(6):600–606. doi:10.1089/lrb.2021.0033
31. Wibisana IG. Sentinel lymph node biopsy for breast cancer using methylene blue: a new anatomical landmark involving intercostobrachial and medial pectoral nodes. Medical J Indones. 2020;29(3):298–304. doi:10.13181/mji.oa.204008
32. Wariss BR, Costa RM, Pereira AC, Koifman RJ, Bergmann A. Axillary web syndrome is not a risk factor for lymphoedema after 10 years of follow-up. Support Care Cancer. 2017;25:465–470. doi:10.1007/s00520-016-3424-7
33. Haigh PI, Hansen NM, Qi K, Giuliano AE. Biopsy method and excision volume do not affect success rate of subsequent sentinel lymph node dissection in breast cancer. Ann Surg Oncol. 2000;7:21–27. doi:10.1007/s10434-000-0021-1
34. Suami H, Pan WR, Taylor GI. Historical review of breast lymphatic studies. Clin Anat. 2009;22(5):531–536. doi:10.1002/ca.20812
35. Estourgie SH, Valdes Olmos RA, Nieweg OE, Hoefnagel CA, Rutgers EJ, Kroon BB. Excision biopsy of breast lesions changes the pattern of lymphatic drainage. Br J Surg. 2007;94(9):1088–1091. doi:10.1002/bjs.5763
36. Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi:10.1056/NEJM199810013391401
37. Feldman SM, Krag DN, McNally RK, et al. Limitation in gamma probe localization of the sentinel node in breast cancer patients with large excisional biopsy. J Am Coll Surg. 1999;188(3):248–254. doi:10.1016/s1072-7515(98)00306-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.