Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Accelerated Wound Healing in Diabetic Rat by miRNA-185-5p and Its Anti-Inflammatory Activity
Authors Wang KX, Zhao LL, Zheng LT, Meng LB, Jin L, Zhang LJ, Kong FL, Liang F
Received 12 March 2023
Accepted for publication 12 May 2023
Published 7 June 2023 Volume 2023:16 Pages 1657—1667
DOI https://doi.org/10.2147/DMSO.S409596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Kui-Xiang Wang,1 Li-Li Zhao,1 Ling-Tao Zheng,2 Li-Bin Meng,1 Liang Jin,3 Long-Jun Zhang,4 Fan-Lei Kong,1 Fang Liang2
1Department of Orthopaedics, Xingtai People’s Hospital of Hebei Medical University, Xingtai, Hebei Province, 054000, People’s Republic of China; 2Department of Endocrinology, Xingtai People’s Hospital of Hebei Medical University, Xingtai, Hebei Province, 054000, People’s Republic of China; 3Department of Hand and Foot Surgery, Xingtai People’s Hospital of Hebei Medical University, Xingtai, Hebei Province, 054000, People’s Republic of China; 4Department of Plastic and Burn, Xingtai People’s Hospital of Hebei Medical University, Xingtai, Hebei Province, 054000, People’s Republic of China
Correspondence: Fang Liang, Email [email protected]
Aim: Addressing both inflammation and epithelialization during the treatment of diabetic foot ulcers is an important step, but current treatment options are limited. MiRNA has important prospects in the treatment of diabetic foot refractory wound ulcers. Previous studies have reported that miR-185-5p reduces hepatic glycogen production and fasting blood glucose levels. We herein hypothesized that miR-185-5p might play an important role in the field of diabetic foot wounds.
Materials and Methods: MiR-185-5p in skin tissue samples from patients with diabetic ulcers and diabetic rats were measured using quantitative real-time PCR (qRT-PCR). The streptozotocin-induced diabetes rat model (male Sprague-Dawley rats) for diabetic wound healing was conducted. The therapeutic potential was observed by subcutaneous injection of miR-185-5p mimic into diabetic rat wounds. The anti-inflammation roles of miR-185-5p on human dermal fibroblast cells were analyzed.
Results: We found that miR-185-5p is significantly downregulated in diabetic skin (people with DFU and diabetic rats) compared to controls. Further, in vitro upregulation of miR-185-5p decreased the inflammatory factors (IL-6, TNF-α) and intercellular adhesion molecule 1 (ICAM-1) of human skin fibroblasts under advanced glycation end products (AGEs). Meanwhile, the increase of miR-185-5p promoted cell migration. Our results also confirmed that the topical increase of miR-185-5p decreases diabetic wound p-nuclear factor-κB (p-NF-κB), ICAM-1, IL-6, TNF-α, and CD68 expression in diabetic wounds. MiR-185-5p overexpression boosted re-epithelization and expedited wound closure of diabetic rats.
Conclusion: MiR-185-5p accelerated wound healing of diabetic rats, reepithelization, and inhibited the inflammation of diabetic wounds in the healing process, a potentially new and valid treatment for refractory diabetic foot ulcers.
Keywords: diabetes, miRNA, wound healing, inflammation
Introduction
Diabetic foot ulcers (DFU) are one of the most debilitating complications of long-term diabetes. DFU is at least partly the result of infection of uncontrolled foot wounds caused by neuropathy, peripheral arterial disease1,2 and chronic low-grade inflammation.3 Long-term hyperglycemia promotes the activation of NF-κB,4 leading to chronic inflammation5 and impairing skin fibroblast activation.6 Difficult healing of foot ulcers in diabetes is a complex problem in clinical diseases. However, the treatment of refractory diabetic foot ulcers is insufficient, and better therapeutic interventions are urgently needed.
Chronic inflammation is involved in the onset and development of diabetic complications.7 One of the significant elements implicated in this inflammatory reaction is NF-κB, which is activated by many stimuli relevant to diabetic foot (including pro-inflammatory cytokines and AGEs).8,9 NF-κB is a crucial nexus in regulating many chemokines, cell adhesion proteins such as ICAM-1, and inflammatory cytokines (IL-6, TNF-α, and so on).10,11 Clinical observation reports that increased serum/plasma ICAM-1 level in patients with type 1 diabetes (T1DM) and type 2 diabetes is positively correlated with proteinuria.12 The chronic inflammatory state contributes to long-term complications of diabetes, including retinopathy, cardiovascular disease, DFU, and nephropathy.7 How to improve the low-grade inflammatory state of diabetes so as to reduce the difficulty of diabetes foot healing needs further discussion.
Fibroblasts are primary proto-resident cells involved in diabetic wound healing, including the decomposition of fibrin clots, the formation of the extracellular matrix (ECM), and shrinkage of the wound.13,14 Inflammatory disorders of the skin are frequently associated with refractory diabetic foot ulcers.3 Studies have shown that the pathogenic phenotype leading to chronic inflammation in diabetic wounds is associated with the effects of the reactive glucose metabolite methylglyoxal,15 AGEs,16 and migration inhibitor (MIF) on dermal cells.6 Dermal fibroblasts from diabetic donors exhibited insulin-induced decreased glucose uptake and reduced insulin receptor expression.
In vitro culture of dermal fibroblasts from diabetic donors exhibited Increased secretion of IL-6 and MIF in fibroblasts from diabetic donors and decreased secretion of procollagen type I C peptide (PICP, a marker of collagen production).6 How to improve the low inflammatory state of diabetic skin fibroblasts to facilitate the closure of diabetic foot refractory ulcers deserves our consideration.
Several miRNAs (miRs) have been closely correlated with the progression and severity of diabetic foot refractory wounds.17 Some studies have reported that miR-31-5p has been shown to improve wound healing,18 and miR-26a could increase the severity of DFU.19 Moreover, topical application of miR-155 inhibitors on diabetic wounds can increase the expression of fibroblast growth factor 7 in diabetic wounds, decreases wound inflammation, increase reepithelialization, and thus accelerate wound closure.20 Yanhui et al have reported that miR-145a-5p blocked M1 macrophage polarization while promoting in vitro M2 phenotypic activation, an anti-inflammatory effect that makes miR-145a-5p mimics to accelerate diabetic wound closure.21 Previous studies have reported that miR-185-5p reduces hepatic glycogen production and fasting blood glucose levels.22 Li reported that miR-185-5p was steeply decreased in diabetic nephropathy mice models and high glucose (HG) state-induced HK-2 cells.2 The overexpression of MiR-185-5p significantly reduced the expression of pro-inflammatory cytokines in RAW264.7 macrophages and strongly inhibited phagocytosis.18 However, the role of miR-185-5p in the anti-inflammatory effect of the diabetic wound healing process remains elusive.
This work analyzed the differential expression of miR-185-5p and the secretion of several inflammatory cytokines in DFU and nondiabetic patients. In addition, to simulate the situation in the skin of diabetic patients, we analyzed the secretion of these inflammatory cytokines and the expression of miR-185-5p in cultured human skin fibroblasts exposed to glucose metabolites AGEs. Later, we further investigated the roles of miR-185-5p on wound healing in rat models of type 1 diabetes and human skin fibroblasts exposed to AGEs. This study may provide a new approach to treating diabetic chronic ulcers.
Methods
Animals Model for Diabetic Wound Healing
24 Male Sprague-Dawley (SD) rats (10–12 weeks old, 250–350g) were bought from Laboratory Animals of HeBei Medical University (HMU) (Shijiazhuang, Hebei, China). They were reared in a 22–24 degrees environment. The environment gave the rats free access to water and food in the pathogen-free facilities of the HMU experimental Animal Lab. The Institutional Animal Care Committee of HMU agreed upon all animal research.
After two weeks of adaptive feeding, the rats were accepted 40 mg/kg streptozotocin (STZ) (Sigma, USA) (100 mM citrate-buffered saline solution, pH 4.5) by intraperitoneal injection.23 After two injections of STZ on days 1 and 3, the blood glucose test (> 16.7 mmol/L) confirmed successful induction of diabetes on day 1st, day 3rd, and day 7th. The glucose level of all rats in wound healing remained at more than 16.7 mM within the experimental procedure. Rats were preserved for type 1 diabetes mellitus (T1DM) disease for 6 weeks before the wounding experiments.
The wound induction experiment was as described in previous experiments.23 Briefly, the normal and diabetic rats’ back hair was shaved off, and the rats were anaesthetized with isoflurane. A 1.0 cm diameter full-thickness circular wound of the rat was made on the dorsal skin by a round hole punch and surgical scissors. Then, the rats were randomly split into 3 groups, with 8 in each group, and the wound closure status was evaluated.
Diabetic Wound Healing Treatments
Diabetic rat wounds were treated with miR-185-5p mimics and mimic-negative control (mimic-NC); Normal age-matched rats wounds were treated with mimic-NC. All injuries were treated by topical injection at the wound edge, once every three days post wounding (on the 1, 4, 7, and 10 days), with miR-185-5p mimics (2.5 nmol/100 ul) or with a negative control oligo (2.5 nmol/100 ul). A seed miR-185-5p mimic was synthesized only to target the seed sequence of miR-185-5p, and a seed control oligo was also synthesized (Ribobio Co., Guangzhou, China). The skin specimens from wounds were harvested on day eight post-wounding for further assays.
Estimation of Wound Healing Rate
The diabetic skin wound was photographed on the operation day and once every other day after the operation. On the 0th, 2nd, 4th, 6th, 8th, 10th, 12th, and 14th days of the experiment, the Canon G9 device was used to take pictures and record. Wound size (wound area) was depicted and measured with ImageJ version 1.46 (NIH Image, USA). According to the calculation formula of wound closure rate: diabetic wound healing rate (%) = (area of the wound on day 0 - wound area on day n) / area of the injury on day 0× 100%.23
Isolation, Culture, and Treatment of Human Skin Fibroblasts
The foreskin was collected from healthy young children (n = 3, age 6–8 years old, sex: males). The Medical Ethics Committee of Xingtai People’s Hospital (Xing Tai, Hebei, China) have approved the obtained protocol for human skin fibroblasts. After annular resection, the foreskin was thoroughly washed with PBS (Gibco, Grand Island, USA) containing 100 μg/mL penicillin-streptomycin (Sigma, USA), and the subcutaneous tissue was removed as much as possible. The obtained skin was cut into about 1 mm x 1 mm pieces. These pieces were digested at 37 °C for 40 minutes in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) containing 0.1% collagenase I (Sigma-Aldrich Co., USA; 100mg collagenase I: PBS 100 mL). The cell suspension obtained by centrifugation was filtered through a 75μm cell filter and then centrifuged at 1500 rpm for 10 min. Human skin fibroblasts were collected and cultured in a DMEM high-glucose medium containing 10% serum (Gibco, Grand Island, USA) and cultivated at a cell incubator (37 °C, 5% CO2, 100% humidity). At 70–80% confluence, cells were detached with 0.25% trypsin-EDTA (Beyotime, China) and passaged a 1:3 division ratio every 3 days. The Bioethics Committee of Xingtai People’s Hospital approved and agreed upon the research topic.
To induce cell models in diabetes states, human skin fibroblasts at 80% confluence were stimulated with different concentrations of AGEs (Bioss, bs-1158p, China) (100, 200, or 400 μg/mL) under a serum-free medium for 24 h. Treat human skin fibroblasts in the above six-well plates with different concentrations of glucose (sugar concentration: low glucose: 5.5 mM, HG: 33.0 mM) and hyperosmotic mannitol solutions (configuration method: 5.5 mM glucose and 27.5 mM mannitol) for 72 h. Prepare for subsequent testing of miR-185-5p by real-time quantitative PCR (qRT-PCR).
Cell Transfection
The following procedure is transfection. Human skin fibroblasts were cultivated in 6-well plates (3 × 104 cells/well) until achieving 30–50% confluence. Then the fibroblasts were transfected with miR-185-5p mimic or inhibitor together with their corresponding NC (Ribobio Co., Guangzhou, China) (concentration: 50 nM) by Lipofectamine 3000 (Invitrogen, Scientific, USA) according to the manufacturer’s guidance. Collect protein and supernatant at 72 h after cell transfection and RNA at 48 h after cell transfection.
Wound Healing Assays of Fibroblasts
Human skin fibroblasts are seeded in 6-well plates and transfected with miR-185-5p mimics and mimic-NC for approximately 48 h under AGEs conditions. When human skin fibroblasts reach 90–100% confluency, ie, transfected miR-185-5p mimic or inhibitor for about 48 hours, the wound was established by manually scraping the cell monolayer with a ten μL pipette tip. After rinsing with PBS three times to remove cell debris, human skin fibroblasts were cultured with a serum-free DMEM culture medium for 24 h. Cell migration results were observed under a microscope and taken a picture at different time points (0 h, 24 h) to quantify the migration area of wound healing using Image J software.
Diabetic Skin Tissue Collection and RNA Extraction
Foot ulcer tissues were collected from patients with diabetic foot (n = 4, age 42–60 years old, sex: males) and nondiabetic patients (n = 4, age: 42–65 years old, sex: male). The skin wound tissues of the diabetic rats’ wound models were also collected in a – 80°C refrigerator (the preparation method is as follows). Our paper was approved and agreed upon by the Bioethics Committee of Xingtai People’s Hospital; All patients and healthy people have obtained prior informed consent and approval.
The obtained skin tissue (50–100 mg) was homogenized, purified, and extracted RNA using TRI reagent (Ambion, USA), including isopropanol, anhydrous alcohol, and chloroform (according to the instructions of the RNA extraction). Slightly dry and dissolve the extracted RNA particles in DEPC water (20 μL) in preparation for reverse transcription into cDNA. All sample specimens are kept at −80 °C pending further qRT-PCR analysis.
Measurement of miR-185-5p Levels by qRT-PCR
As previously described, the total RNA was extracted from the treated wound tissues and cells using the RNA extraction kits (ThermoFisher Scientific, USA). Reverse transcription of RNA from different samples into cDNA using PrimeScript™RT Master Mix (ThermoFisher Scientific, USA) and qPCR using the CFX RT-PCR system (BIO-RAD, Hercules, CA, USA) using SYBR Premix Ex Taq II (BIO-RAD, USA). The expression of miR-185-5p relative to U6 was calculated using the 2−ΔΔCt method. Repeat at least three times per experiment.
Enzyme-Linked Immunosorbent Assay (ELISA)
DFU/diabetic rats’ tissues were accurately weighed and ground into a paste. They were added 9 times PBS at the ratio of weight (mg): volume (μL) = 1:9. In an ice bath environment, 10% homogenate was prepared, 2500~3000 rpm/min, centrifuged for 10 min, and the supernatant was taken to determine the level of IL-6, TNF-a, ICAM-1 by following ELISA.
The expression of TNF-a, IL-6, ICAM-1 in the acquired supernatant (human skin fibroblasts and skin tissue) was detected by the ELISA kit (Beyotime, Shanghai, China) according to special instructions, including human ELISA Kit (IL-6, TNF-, and ICAM-1) and the ELISA Kit of rat species (ICAM-1, IL-6, and TNF-α). Each experiment was triplicated using an Enzyme labelling apparatus (Rayto, RT-6100, Shenzhen, China).
Western Blot
The extracted proteins (cells and tissues after intervention) were electrophoresed by 10% sodium lauryl sulfate polyacrylamide-gel electrophoresis (100V, 1h) and transferred to a polyvinylidene fluoride membrane (300 mA, 1h) (Bio-Rad, USA). The above membrane was placed in 5% skim milk for blockage for 1 h. The washed membrane was exposed to the anti-ICAM-1 (Santa Cruz, sc-18853, USA), anti-NF-κB (Abclonal, A10609, Wuhan, China), anti-p-NF-κB (CST, 3033S, USA), anti-GAPDH (Protein Technology, 60004-1-Ig, Wuhan, China) and anti-tubulin (Protein Technology, 10068-1-AP, Wuhan, China) and placed overnight at 4 °C. Then, the membranes were exposed to secondary antibodies (Proteintech, Wuhan, China) for 1 h. The blots showed up via an enhanced chemiluminescence kit (Advansta, California, USA). Each Western blot experimental result has at least three replicates.
Immunofluorescence Staining and Histological Analysis
On day 8 of wound induction, wound tissue, including healthy tissue (including epidermis and dermis), about 1 cm in diameter, is excised, fixed with 4% paraformaldehyde, and then these specimens are buried with paraffin. The samples were cut into slices. The slices are stained with hematoxylin, eosin (HE), and Masson staining. It is then photographed under a microscope, and finally, the epithelialization of these slices was analyzed by Image J. Incubate tissue sections in 3% H2O2 to reduce endogenous peroxidase activity. Then, these tissue sections were soaked in 10 mM sodium citrate buffer and microwaved for 20 min. We recover these specimen sections. After these specimens are cooled, the sections are blocked by 1% BSA and incubated overnight at 4 °C with CD3 and CD68 antibodies (Proteintech, Wuhan, China). These specimen sections were removed from the primary antibody overnight. Next day, they were washed three times with PBS at room temperature for 10 min each, and then infiltrated with Alexa Fluor 594-conjugated secondary antibody for 1 h at room temperature. We stain nuclei blue with DAPI and red with CD3 and CD68. The sections are then imaged using confocal microscopes. T cells (CD3+) and macrophages (CD68+) are quantified by measuring the fluorescence intensity of the independent microscope field of view with 200 x magnification. The CD3 and CD68 fluorescence signal densities were quantified using Image J software.
Statistics
The data collected for this experiment came from at least three independent experimental results and were statistically analyzed using GraphPad Prism 6 (Graph Pad Software, Inc., La Jolla, CA, USA). Data from these experimental results are shown as mean ± standard deviation (SD). When comparing the differences between the two groups, the Student’s t-test was used to confirm it; When comparing data differences between three or more groups, one-way ANOVA and Tukey were used. P < 0.05 is considered statistically significant.
Results
MiR-185-5p Expression Was Downregulated and Inflammation Factors Were Increased in Wound Tissues from Diabetes
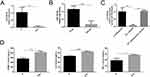
We investigated the miR-185-5p expression in wound tissues from diabetic foot patients and STZ-induced-diabetic rats to study whether miR-185-5p is related to poor wound healing. The results provided that in the DFU and the diabetic rats’ wound tissues, the miR-185-5p expression was significantly down-regulated (Figure 1A and B). Meanwhile, the decreased level of miR-185-5p was found in human skin fibroblasts at an HG concentration of 33.0 mM compared with 5.5 mM glucose (Figure 1C). We also found the levels of IL-6, TNF-α, and ICAM-1 expression were increased in wound tissues from DFU (Figure 1D). Those results presented that the decrease of miR-185-5p might be closely associated with the low-grade inflammation state of diabetic foot ulcers.
MiR-185-5p Inhibited Inflammation Factors of Human Skin Fibroblast Under AGEs Conditions
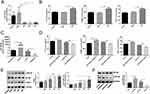
The abundant evidence has elucidated the roles of miR-185-5p on cell inflammation and oxidative stress, such as macrophages,24 high glucose-induced human kidney tubular cells,2 and mouse mesangial cells.25 We examined whether miR-185-5p affects inflammation-related factors expression in human skin fibroblasts under AGEs conditions. First, we investigated the expression of miR-185-5p, ICAM-1, and p-NF-κB/NF-κB in human skin fibroblast under AGEs with different concentrations (100ug/mL, 200ug/mL, 400ug/mL). It was found that the expression of miR-185-5p was declined, and ICAM-1 and p-NF-κB/NF-κB proteins were increased under AGEs with different concentrations (100 ug/mL, 200 ug/mL, 400 ug/mL) (Figure 2A), and then we also found inflammation-related factors IL-6, ICAM-1 and TNF-α expression levels were elevated in human skin fibroblast under AGEs (concentration 200 ug/mL, 24h) by ELISA assays (Figure 2B).
Whether the function of human skin fibroblasts was influenced by miR-185-5p, human skin fibroblasts were transfected with miR-185-5p inhibitor/mimic (Figure 2C). IL-6, ICAM-1, and TNF-α expression levels were found to be increased in human skin fibroblast transfected with miR-185-5p inhibitor under AGEs (concentration 200 ug/mL, 24h) by ELISA assays, and vice versa (Figure 2D). It was shown that the p-NF-κB/NF-κB was elevated in human skin fibroblast transfected with miR-185-5p inhibitor under AGEs (concentration 200 ug/mL, 24h) by Western blot assays, and the opposite results were found in human skin fibroblast transfected with miR-185-5p mimic (Figure 2E and F). In brief, these experiment results provided that the miR-185-5p upregulation could improve inflammation in skin fibroblasts induced by AGEs.
MiR-185-5p Mimics Accelerated Diabetic Wound Healing Through Increasing Reepithelization
Whether miR-185-5p affects wound healing of diabetes deserves more attention. First, The NC or miR-185-5p mimic was locally injected on the back of diabetic rats after a 1 cm diameter wound on the 1st, 4th, 7th, and 10th day. The healing speed of diabetic wounds treated with miR-185-5p mimic was faster than that of the NC (Figure 3A and B). The wound tissue harvested on day 8 was used for HE and Masson. Histologic analysis displayed the epidermis and dermis became thicker in the miR-185-5p mimic-treated wounds compared with the NC-treated wounds within 8th days after the treatment (Figure 3C). We also observed that wounds treated with miR-185-5p mimic exhibited better granulation formation, collagen deposition, and denser alignment (Figure 3F and G). We also found the increase of miR-185-5p significantly repressed the migration of human skin fibroblasts under AGEs (Figure 3D and E). These results suggested that miR-185-5p promoted diabetic wound healing through increasing reepithelization.
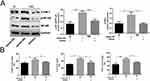
MiR-185-5p Mimic Inhibited NF-κB and ICAM-1 Expression in Diabetic Rat Wound Tissue
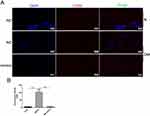
Because miR-185-5p was involved in the regulation of inflammation, meanwhile, miR-185-5p promoted diabetic wound healing. To further evaluate whether miR-185-5p inhibited inflammation factor in diabetic rat wound tissue, our results showed that the p-NF-κB/NF-κB, ICAM-1, IL-6, and TNF-α protein expression was decreased in the miR-185-5p mimic-treated diabetic wounds (Figure 4). We also further evaluate immune cell infiltration at day 8 post-wounding, we performed fluorescent immunohistochemical staining for CD3, to identify T-cells and CD68 to identify macrophages (Figure 5). Macrophage infiltration in diabetes wounds treated with miR-185-5p mimic was lower than in the wounds treated with negative control (Figure 5). However, T cell infiltration has not been changed in the diabetic wounds treated with an increased miR-185-5p compared with the wounds treated with negative control (Supplemental Figure 1). These results all prompted that miR-185-5p overexpression suppressed inflammation in diabetic wound healing.
Discussion
A low-grade inflammatory state observed in the study is an important factor that damages the difficult healing of diabetes ulcer. The main reason is that tissue damage, such as granzyme and perforin, is caused by the release of various protein levels involved in bacterial control, thus impairing the progress of epithelization in wound healing.26 Many scholars have confirmed that the chronic inflammatory state of diabetes is partially controlled by miRNA. At present, miR-185-5p,24 miR-497,27 miR-155 inhibitors28 have been found. They directly regulate inflammatory factors and exert anti-inflammatory effects under different conditions. Although previous studies have reported that miRNAs were valid in diabetic wound healing, only one is currently available in clinical trials.29 Therefore, further research is needed to advance the therapeutic role of miRNAs in refractory healing in diabetic wounds. This study explored the above issues and found that miR-185-5p was closely related to DFU and that the level of miR-185-5p in STZ-induced type 1 diabetes rats was higher than that in normal rats.
The expression of miR-185-5p was downregulated in wound tissue of diabetes; Studies consistent with our study are: Zheng et al found that miR-185-5p was downregulated in diabetes liver tissue;22 Li et al found miR-185-5p is poorly expressed in diabetes nephropathy tissue;2 Chen et al reported that miR-185-5p level was low-expression in palmitate-treated insulin resistance hepatocytes and high-fat diet mice.30 These studies all suggested that miR-185-5p might be involved in the initiation and progression of diabetes and diabetic complications.
This paper also explores the role of miR-185-5p in diabetic wound tissue. The anti-inflammation effect of miR-185-5p was observed through decreasing IL-6, TNF-α, ICAM-1 and p-NF-κB/ NF-κB levels in miRNA-185-5p mimic treated diabetic wounds. Notably, CD68 (to identify macrophages) was significantly decreased by miRNA-185-5p treatment in vivo. In summary, inflammatory responses in diabetic wound tissue were restained by miRNA-185-5p mimics therapy in wound healing.
In order to further clarify the internal mechanism of miRNA-185-5p in the wound-healing process of diabetes, we selected the most important cell in the wound-healing process - human skin fibroblasts. We analyzed the expression of miRNA-185-5p and inflammatory factors in human skin fibroblasts under AGEs-induced inflammation conditions. The results showed that miRNA-185-5p expression was reduced in human fibroblasts under AGEs conditions, and inflammation factors expression (ICAM-1, IL-6 and TNF-α) was increased, indicating that miRNA-185-5p may be associated with inflammation. A significant decrease in miR-185-5p in human skin fibroblasts under the action of AGEs (100, 200, 400 ug/mL) was consistent with a reduction in miR-185-5p expression in rat diabetic wound tissue and DFU skin. In addition, we found that under AGEs conditions, adding miRNA-185-5p mimics could inhibit ICAM-1, IL-6, and TNF-α high expression. Especially a remarkable reduction of p-NF-κB/ NF-κB was observed following the increase of miRNA-185-5p. Based on the prediction of the anti-inflammatory effect of miRNA-185-5p mimic in vitro, we confirmed its therapeutic effect on diabetic wound healing by accelerating wound healing after miRNA-185-5p mimic injection treatment in diabetic rat wounds.
Previous studies have shown that miR-185-5p is associated with chronic inflammatory diseases primarily because of its capacity to regulate inflammatory networks in macrophages.24 A recent study showed that the high expression of miR-185-5p reduces hypoxia/reoxygenation-induced pyrosis and inflammatory factor expression (LDH, IL-1β, and IL-18) in human cardiomyocytes.31 Ma et al found that the high expression of miR-185-5p effectively reduced the expression of pro-inflammatory factors, overturned the phagocytosis of LPS-induced RAW264.7 macrophages, and in turn, miR-185-5p inhibitors enhanced the inflammatory effect of LPS in macrophages. Its mechanism of action may be partially induced by regulating the CDC42/JNK pathway.24 It has been reported that high expression of miR-185-5p can reduce mechanical pain and thermal hyperalgesia in rats with contractile injury, inhibit the aggregation of microglia and astrocytes, and reduce the content of IL-1β, IL-6 and TNF-α, thereby reducing neuropathic pain induced by chronic contractile injury.32 The mechanism may be exerted by targeting MyD88 and CXCR4.32 However, miRNA-185-5p might reduce the inflammation of diabetic wound and human skin fibroblasts under AGEs (200 ug/mL) by inhibiting MyD88 and CXCR4.
In addition, this study focuses on the promoting effect of miRNA-185-5p on the wound of diabetic rats. The diabetic wound healing process is the result of the involvement of a variety of cells, including skin fibroblasts, endothelial cells, stem cells, etc. Among them, endothelial dysfunction is also one of the characteristics of diabetic wounds that are difficult to heal. Previous studies have found that the elevation of miR-497 induces apoptosis of HUVECs by targeting VEGFR2 and downstream PI3K/AKT signalling pathways,33 so whether miRNA-185-5p regulates endothelial function damage in high glucose states is also a topic for future research.
Conclusion
MiR-185-5p accelerated wound healing of diabetic rats, reepithelization, and inhibited the inflammation of diabetic wounds in the healing process, a potentially new and valid treatment for refractory diabetic foot ulcers.
Data Sharing Statement
The raw data of these experimental results can be obtained from the corresponding authors according to the relevant requirements of the journal.
Ethics Approval and Consent
The Medical Ethics Committee of Xingtai People’s Hospital approved this study (approval number: 2022{014}), and it was conducted according to the Declaration of Helsinki.
Funding
The Medical Science Research Project of Hebei Province supported this article (No.20210053).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419. doi:10.3390/ijms18071419
2. Li G, Qin Y, Qin S, Zhou X, Zhao W, Zhang D. Circ_WBSCR17 aggravates inflammatory responses and fibrosis by targeting miR-185-5p/SOX6 regulatory axis in high glucose-induced human kidney tubular cells. Life Sci. 2020;259:118269. doi:10.1016/j.lfs.2020.118269
3. Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109–116. doi:10.1016/j.trsl.2021.05.006
4. Ko KI, Syverson AL, Kralik RM, et al. Diabetes-induced NF-κB dysregulation in skeletal stem cells prevents resolution of inflammation. Diabetes. 2019;68(11):2095–2106. doi:10.2337/db19-0496
5. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol. 2017;199(1):17–24. doi:10.4049/jimmunol.1700223
6. Nickel K, Wensorra U, Wenck H, Peters N, Genth H. Evaluation of immunomodulatory responses and changed wound healing in type 2 diabetes-A study exploiting dermal fibroblasts from diabetic and nondiabetic human donors. Cells. 2021;10(11):2931. doi:10.3390/cells10112931
7. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. doi:10.1016/j.immuni.2021.12.013
8. Atic R, Deveci E. Endothelin 1, NF-kappaB, and ADAM-15 expression in diabetic foot wounds. Bratisl Lek Listy. 2019;120(1):58–64. doi:10.4149/BLL_2019_009
9. Duda-Sobczak A, Falkowski B, Araszkiewicz A, Zozulinska-Ziolkiewicz D. Association between self-reported physical activity and skin autofluorescence, a marker of tissue accumulation of advanced glycation end products in adults with type 1 diabetes: a cross-sectional study. Clin Ther. 2018;40(6):872–880. doi:10.1016/j.clinthera.2018.02.016
10. Nonaka K, Kajiura Y, Bando M, et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-kappaB pathways in human gingival fibroblasts. J Periodontal Res. 2018;53(3):334–344. doi:10.1111/jre.12518
11. Clausen PJ, S. Jensen J, Jensen JS, Parving HH, Feldt-Rasmussen B, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17(9):644–649. doi:10.1046/j.1464-5491.2000.00347.x
12. Ciobanu DM, Mircea PA, Bala C, Rusu A, Vesa S, Roman G. Intercellular adhesion molecule-1 (ICAM-1) associates with 24-hour ambulatory blood pressure variability in type 2 diabetes and controls. Cytokine. 2019;116:134–138. doi:10.1016/j.cyto.2019.01.006
13. Wu M, Huang J, Shi J, Shi L, Zeng Q, Wang H. Ruyi jinhuang powder accelerated diabetic ulcer wound healing by regulating Wnt/β-catenin signaling pathway of fibroblasts in vivo and in vitro. J Ethnopharmacol. 2022;293:115321. doi:10.1016/j.jep.2022.115321
14. Huang C, Ogawa R. Role of inflammasomes in keloids and hypertrophic scars-lessons learned from chronic diabetic wounds and skin fibrosis. Int J Mol Sci. 2022;23(12). doi:10.3390/ijms23126820
15. Yang CT, Meng FH, Chen L, et al. Inhibition of methylglyoxal-induced AGEs/RAGE expression contributes to dermal protection by N-Acetyl-L-cysteine. Cell Physiol Biochem. 2017;41(2):742–754. doi:10.1159/000458734
16. Serban AI, Stanca L, Geicu OI, Munteanu MC, Dinischiotu A. RAGE and TGF-β1 cross-talk regulate extracellular matrix turnover and cytokine synthesis in AGEs exposed fibroblast cells. PLoS One. 2016;11(3):e0152376. doi:10.1371/journal.pone.0152376
17. Goodarzi G, Maniati M, Qujeq D. The role of microRNAs in the healing of diabetic ulcers. Int Wound J. 2019;16(3):621–633. doi:10.1111/iwj.13070
18. Yan C, Chen J, Wang C, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214–228. doi:10.1080/10717544.2021.2023699
19. Icli B, Nabzdyk CS, Lujan-Hernandez J, et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol. 2016;91:151–159. doi:10.1016/j.yjmcc.2016.01.007
20. Moura J, Sørensen A, Leal EC, et al. microRNA-155 inhibition restores Fibroblast Growth Factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep. 2019;9(1):5836. doi:10.1038/s41598-019-42309-4
21. Hao Y, Yang L, Liu Y, et al. mmu-miR-145a-5p accelerates diabetic wound healing by promoting macrophage polarization toward the M2 phenotype. Front Med. 2021;8:775523. doi:10.3389/fmed.2021.775523
22. Zheng H, Wan J, Shan Y, et al. MicroRNA-185-5p inhibits hepatic gluconeogenesis and reduces fasting blood glucose levels by suppressing G6Pase. Theranostics. 2021;11(16):7829–7843. doi:10.7150/thno.46882
23. Wang K, Chen Z, Jin L, et al. LPS-pretreatment adipose-derived mesenchymal stromal cells promote wound healing in diabetic rats by improving angiogenesis. Injury. 2022;53(12):3920–3929. doi:10.1016/j.injury.2022.09.041
24. Ma X, Liu H, Zhu J, et al. miR-185-5p regulates inflammation and phagocytosis through CDC42/JNK pathway in macrophages. Genes. 2022;13(3):468. doi:10.3390/genes13030468
25. Zhao D, Guo J, Liu L, Huang Y. Rosiglitazone attenuates high glucose-induced proliferation, inflammation, oxidative stress and extracellular matrix accumulation in mouse mesangial cells through the Gm26917/miR-185-5p pathway. Endocr J. 2021;68(7):751–762. doi:10.1507/endocrj.EJ20-0783
26. Gardner SE, Frantz RA. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs. 2008;10(1):44–53. doi:10.1177/1099800408319056
27. Zhang M, Yang D, Yu H, Li Q. MicroRNA-497 inhibits inflammation in DSS-induced IBD model mice and lipopolysaccharide-induced RAW264.7 cells via Wnt/β-catenin pathway. Int Immunopharmacol. 2021;101(Pt B):108318. doi:10.1016/j.intimp.2021.108318
28. Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64(6):1378–1387. doi:10.1016/j.jhep.2016.01.035
29. Pichu S, Vimalraj S, Viswanathan V. Impact of microRNA-210 on wound healing among the patients with diabetic foot ulcer. PLoS One. 2021;16(7):e0254921. doi:10.1371/journal.pone.0254921
30. Chen DL, Shen DY, Han CK, Tian Y. LncRNA MEG3 aggravates palmitate-induced insulin resistance by regulating miR-185-5p/Egr2 axis in hepatic cells. Eur Rev Med Pharmacol Sci. 2019;23(12):5456–5467. doi:10.26355/eurrev_201906_18215
31. Sun J, Zhu YM, Liu Q, et al. LncRNA ROR modulates myocardial ischemia-reperfusion injury mediated by the miR-185-5p/CDK6 axis. Lab Invest. 2022;102(5):505–514. doi:10.1038/s41374-021-00722-2
32. Huang A, Ji L, Huang Y, Yu Q, Li Y. miR-185-5p alleviates CCI-induced neuropathic pain by repressing NLRP3 inflammasome through dual targeting MyD88 and CXCR4. Int Immunopharmacol. 2022;104:108508. doi:10.1016/j.intimp.2021.108508
33. Tu Y, Liu L, Zhao D, et al. Overexpression of miRNA-497 inhibits tumor angiogenesis by targeting VEGFR2. Sci Rep. 2015;5:13827. doi:10.1038/srep13827
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.