Back to Journals » Cancer Management and Research » Volume 12
Absolute Counts of Peripheral Lymphocyte Subsets Correlate with the Progression-Free Survival and Metastatic Status of Pancreatic Neuroendocrine Tumour Patients
Authors Gong Y, Fan Z, Luo G , Huang Q, Qian Y, Cheng H, Jin K, Ni Q, Yu X , Liu C
Received 21 April 2020
Accepted for publication 21 June 2020
Published 3 August 2020 Volume 2020:12 Pages 6727—6737
DOI https://doi.org/10.2147/CMAR.S257492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Yitao Gong,1– 4,* Zhiyao Fan,1– 4,* Guopei Luo,1– 4,* Qiuyi Huang,1– 4 Yunzhen Qian,1– 4 He Cheng,1– 4 Kaizhou Jin,1– 4 Quanxing Ni,1– 4 Xianjun Yu,1– 4 Chen Liu1– 4
1Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, People’s Republic of China; 3Shanghai Pancreatic Cancer Institute, Shanghai 200032, People’s Republic of China; 4Pancreatic Cancer Institute, Fudan University, Shanghai 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianjun Yu; Chen Liu
Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, No. 270 DongAn Road, Shanghai 200032, People’s Republic of China
Tel + 86 21-64175590 ext 1307
Fax + 86 21-64031446
Email [email protected]; [email protected]
Purpose: Pancreatic neuroendocrine tumours (panNETs) are rare tumours of pancreas. Lymphocyte subsets in the peripheral blood are reported to reflect tumour prognosis and progression. The objective of the study is to investigate the hypotheses that the levels of peripheral lymphocytes may reflect tumour progression and may predict the prognosis of pancreatic neuroendocrine tumours (panNETs).
Patients and Methods: A retrospective cohort study consisting of 73 patients diagnosed with panNETs was conducted. Kaplan–Meier methods and Log rank tests were used to compare the survival rates, and a Cox regression model was used to perform multivariate analyses.
Results: panNET patients with distant metastasis were associated with lower peripheral total T cell (p = 0.039) and CD4+ T cell (p = 0.006) counts. Lower peripheral B cells (p = 0.007) and higher peripheral NK cell (p = 0.001) counts indicated worse progression-free survival (PFS) in Log rank tests. In multivariate analyses, low B cell count (hazard ratio (HR): 6.769, 95% confidence interval (CI): 2.158 to 21.228, p = 0.001) and high NK cell count (HR: 3.715, 95% CI: 1.164 to 11.855, p = 0.027) were independent risk factors for progression. NK cells and B cells were also significantly associated with PFS following radical surgical resection.
Conclusion: Peripheral total T cell and CD4+ T cell counts may reflect the distant metastasis status in panNET patients. The absolute count of peripheral B cells and NK cells may independently predict the progression of panNET patients, making them promising prognostic indicators and potential targets for treatment of panNETs.
Keywords: pancreatic neuroendocrine tumours, panNETs, lymphocyte subsets, progression-free survival, B cells
Background
Pancreatic neuroendocrine tumours (panNETs) are rare neuroendocrine neoplasms that arise in the islet of Langerhans.1 PanNETs comprise less than 3% of all pancreatic neoplasms, and the annual incidence rate is less than 1 per 100,000 individuals, but the incidence has steadily increased over the last two decades, partly due to improved diagnostic imaging.1,2 Unlike other pancreatic malignancies, which are notorious for their aggressiveness and high mortality rates, panNETs are characterized by their indolent tumour biology and relatively better prognosis, with a 5-year survival rate of 42.1%.3 PanNETs are dichotomized into functional (F-panNETs) and non-functional (NF-panNETs) based on the hormonal secretion status of the tumour and the resulting clinical symptoms.4 According to the new classification of panNETs proposed by the World Health Organization (WHO) in 2017, panNETs can be subcategorized into well-differentiated (G1, G2, or G3) panNETs and poorly differentiated (G3) pancreatic neuroendocrine carcinomas (panNECs). In this study, we mainly focused on well-differentiated panNETs. It is reported that old age,5 presence of lymph node and distant metastasis,5,6 positive margins,7 high fluorodeoxyglucose (FDG) uptake,8 and high ki-67 index all negatively correlate with the prognosis of well-differentiated panNETs, and particularly, ki-67 index is a major proliferative factor in panNETs with a significant prognostic value.9 However, those traditional parameters for tumour staging do not always satisfactorily predict the prognosis of panNETs, which highlights the need for novel prognostic biomarkers for panNETs.
Tumour progression is a complicated process that involves tumour-host immunological interactions – the host immune status profoundly influences tumour development and vice versa.10 Peripheral immune cells are believed to reflect the host immunity in tumour patients, and several reports have demonstrated that peripheral immune cell counts may have a remarkable impact on tumour prognosis.11–13
Lymphocyte subpopulations are immune cells that are vital to anti-tumour immunity and include T cells (that specialize in cellular immunity), B cells (that specialize in humoural immunity) and natural killer (NK) cells (that directly kill tumour cells and virus-infected cells).14–16 CD3+ T cells can be mainly subdivided into 2 subsets according to their immunological functions and cell surface markers: CD8+ T cells and CD4+ T cells. CD8+ T cells are also known as cytotoxic T cells (identified as CD3+CD8+ T cells), which kill tumour cells by releasing cytokines, cytotoxic granules, or via Fas/FasL interactions.17 CD4+ T cells (identified as CD3+CD4+ T cells) are commonly divided into conventional T helper (Th) cells, which play a pivotal role in regulating the immune responses of other immune cells, including cytotoxic T cells, B cells and macrophages, and regulatory T cells, which are characterized as immunosuppressive lymphocytes that inhibit the activation and proliferation of effector T cells.18 B cells and NK cells are identified as CD3−CD19+ cells and CD3−CD16+CD56+ cells, respectively.
Since these lymphocyte subsets strongly correlate with tumour immune surveillance and may thus influence tumourigenesis and tumour progression,19 alterations in the levels of peripheral lymphocyte subsets may reflect the state of tumour development and may predict tumour prognosis. The neuroendocrine system plays a critical role in regulating the immune system, which further suggests that the immunological status of the host, which can be reflected by the levels of peripheral lymphocyte subsets, strongly affects the prognosis of neuroendocrine tumour patients.20 However, thus far, the prognostic significance of the levels of peripheral lymphocyte subsets in neuroendocrine tumours has not been thoroughly investigated. In this study, we aim to investigate the hypothesis that peripheral lymphocytes may reflect tumour progression and may predict the prognosis of panNETs.
Materials and Methods
Design and Patients
A retrospective, follow-up, cohort study consisting of 78 panNET patients who met the inclusion criteria from May 2009 to January 2018 was conducted at Shanghai Cancer Center, Fudan University, China, and 5 of the participants were lost to follow-up. Ultimately, 73 patients were included in the cohort. The diagnosis of resected panNETs was based on post-operational pathology report, and the diagnosis of patients who did not undergo surgical resection was confirmed by cytological or histological pathology report obtained via endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA), or percutaneous liver biopsy (PLB) when liver metastasis was present. The inclusion criteria were as follows: (1) patients who were histologically or cytologically diagnosed with panNETs; (2) patients with complete data on peripheral lymphocyte subgroup counts; and (3) patients who received major treatment in our centre, which includes surgical resection and chemotherapy. The exclusion criteria were as follows: (1) patients without follow-up data; (2) patients with poorly differentiated panNECs according to the fifth edition of the WHO classification of tumours; (3) patients with secondary malignancies or multiple primary malignancies; (4) patients with diseases of the immune system or a history of viral or bacterial infections within 2 weeks before major treatments; and (5) patients with haematological disorders.
We collected baseline clinicopathological data, including sex, age, tumour location, tumour stage, tumour grade, tumour functional status, tumour size, and major treatment received, from the patients’ medical history from Shanghai Cancer Center. The tumour grade was determined based on the fifth edition of the WHO Classification of Tumour, and was reviewed by expert pathologists. Tumour sizes were measured according to the last enhanced computed tomography (CT) scans prior to major treatment.
Due to the low number of patient deaths in our cohort, instead of overall survival (OS), PFS was the primary endpoint, which was defined as the length of time from the beginning of treatment to the detection of disease progression or to death from any cause. Disease progression was defined as increase in number/size if pre-existing lesions and/or recurrence after previous radical surgery, and was based on the Response Evaluation Criteria in Solid Tumours (RECIST) criteria (version 1.1). Progression was assessed by imaging (including CT, magnetic resonance imaging (MRI), and 68-Gallium and/or FDG positron emission tomography (PET)/CT). The frequency of imaging re-examinations for each patient varied according to the different tumour stages and treatments. For patients who underwent radical surgery and did not require further treatment, the frequency of follow-up examinations gradually decreased. For patients who needed further treatment, imaging re-examinations were regularly carried out every 3 months.
The objective of this study was to assess the clinical significance of the absolute counts of lymphocyte subsets in the peripheral blood for predicting the prognosis of panNET patients. The additional objectives were to investigate the correlation between the levels of peripheral lymphocyte subsets and sex, age, tumour grade, tumour function, distant metastasis and nodal metastasis.
This study was approved by the Ethics Board of Shanghai Cancer Center, Fudan University, and all patients involved in this study provided informed consent for the use of their personal data for research purposes.
Blood Samples and Flow Cytometry
We determined the baseline value of the peripheral lymphocyte subset count by the flow cytometric analysis of patient blood samples. Before receiving major treatments, the peripheral blood samples of patients were collected and instantly processed for flow cytometric analysis using a MultiSET IMK Kit (BD Bioscience, USA). Antibodies (eBioscience, USA) against CD8, CD4, CD3, CD19, CD56 and CD16 were used, and we evaluated the absolute count of each subset. Data analysis was performed using FlowJo. We used CD3+ to represent total T cells; CD3+CD4+ to represent CD4+ T cells; CD3+CD8+ to represent CD8+ T cells; CD3-CD19+ to represent B cells; and CD3-CD16+CD56+ to represent NK cells.
Statistical Analyses
We used the Kaplan–Meier method with a 95% confidence interval (CI) to estimate PFS. Log rank tests were conducted to compare the survival curves of the two groups. Receiver operating characteristic (ROC) curve analyses were performed to set the cut-off value for each lymphocyte subset. The results of multivariate analyses were evaluated with the Log rank test and Cox regression hazard models. Spearman’s rank correlation tests were used to evaluate the correlation between the lymphocyte subset count and clinicopathologic characteristics. All the statistical analyses were performed using SPSS 23.0 software (SPSS, Inc., Chicago, IL). A p value less than 0.05 was considered statistically significant, and all the p values are two-sided.
Results
Patient Baseline Characteristics
Initially, 78 patients with panNETs were included in this study. Among these patients, 5 were lost to follow-up, and the lost-to-follow-up rate was 6.41%. All participants lost to follow-up were removed from the analysis, and 73 patients were ultimately included in the final investigation. The baseline characteristics of the patients are shown in Table 1. The median age of the patients was 50, ranging from 16 to 77. Of the 73 participants, 65 (89.0%) had undergone surgical resection, and 8 (11.0%) had received non-surgical treatment, which included somatostatin analogue treatment (Octreotide etc.), molecular targeted therapy (Sunitinib etc.), hepatic arterial infusion chemotherapy (HAIC) and transarterial chemoembolization (TACE). Seventeen patients had distant metastases, 14 of which were liver lesions, 1 had pulmonary lesion and 1 had peritoneal lesion; 9 patients with liver metastases had undergone surgical resection. There were 7 patients with functioning panNETs, 1 of them was gastrinoma, and 6 were insulinoma.
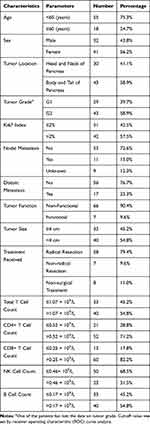 |
Table 1 Baseline Characteristics of the Patients |
Correlation of the Baseline Levels of Peripheral Lymphocyte Subsets with Clinicopathologic Parameters
The statistical correlations between the baseline absolute counts of total T cells, CD4+ T cells, CD8+ T cells, B cells and NK cells and several clinicopathologic parameters were evaluated with Spearman’s rank correlation tests and are shown in Supplement Table A1. Distant metastasis negatively correlated with the total T cell count (Spearman’s rho = −0.242, p = 0.039) and the CD4+ T cell count (Spearman’s rho = −0.317, p = 0.006). To further confirm this correlation, we used Mann–Whitney U-tests (see Figure 1) to compare the mean absolute counts of the lymphocyte subsets in patients with different distant metastatic statuses. The total T cell count (p = 0.039) and the CD4+ T count (p = 0.006) were significantly lower in patients with distant metastasis than those in patients without distant metastasis. However, the absolute counts of peripheral CD8+ T cells, NK cells and B cells did not show significant correlations with clinicopathologic parameters.
Peripheral B Cell and NK Cell Absolute Counts Correlated with Progression-Free Survival in Patients with panNETs
At the end of the follow-up period, 28 (38.4%) of the participants (N=73) had progression. The median (range) duration of follow-up after panNETs diagnosis in all patients was 19 months (1.6 to 83 months), and the median (95% CI) PFS of all patients was 60.0 months (41.6 to 78.4 months). The fraction of progression, median progression-free time of each group and p value derived from Log rank tests are shown in Table 2. Kaplan–Meier curves of PFS are shown in Figure 2. Log rank tests indicated that the absolute counts of peripheral Total T cell (p = 0.036), CD4+ T cells (p = 0.006) and B cells (p = 0.007) positively correlated with the PFS of patients with panNETs – a higher count indicated a better prognosis; while peripheral NK cell count (p = 0.001) was found negatively associated with the PFS. The absolute counts of peripheral CD8+ T cells did not significantly correlate with PFS.
 |
Table 2 Progression Fraction and Median Progression-Free Survival of Each Group |
Absolute Counts of Peripheral NK Cells and B Cells May Independently Predict the Progression of panNETs
The results of univariate and multivariate analyses for the PFS of panNET patients based on a Cox regression model are shown in Table 3. In the multivariable-adjusted Cox regression model that included tumour grade, nodal metastasis, distant metastasis and treatment received, the adjusted hazard ratio (HR) for progression comparing those with low versus high peripheral B cell count and those with low versus high peripheral NK cell count were 6.769 (95% confidence interval (CI): 2.158 to 21.228) and 3.715 (95% CI: 1.164 to 11.855), respectively, suggesting that a low peripheral B cell count and a high peripheral NK cell count may independently predict progression of panNETs.
 |
Table 3 Univariate and Multivariate Analyses for PFS |
Absolute Counts of Peripheral B Cells and NK Cells Were Associated with Progression-Free Survival in panNETs Patients Following Radical Surgical Resection
To further investigate the prognostic value of peripheral lymphocyte subset counts in patients who had no distant metastatic sites and underwent radical surgical resection, we studied the subgroup of 56 patients without distant metastasis, and all of them underwent surgical resection. Kaplan–Meier curves of PFS are shown in Figure 3. Log rank tests indicated that high absolute counts of peripheral B cells (p = 0.011) correlated with better PFS of patients with panNETs, while the high peripheral NK cell count (p = 0.018) indicated worse PFS of patients with panNETs. The absolute counts of peripheral total T cells, CD8+ T cells and CD4+ cells did not significantly correlate with PFS. The results of univariate and multivariate analyses (Supplement Table A2) for the PFS of panNET patients based on a Cox regression model revealed that high peripheral NK cell counts (HR = 11.189, 95% CI: 2.334 to 53.645, p = 0.003) and low peripheral B cell counts (HR = 10.811, 95% CI: 2.202 to 53.075, p = 0.003) were independent risk factors for PFS in panNET patients who have undergone radical surgical resection.
Discussion
In this study, we examined the progression-free survival (PFS) of 73 panNET patients and their absolute counts of lymphocyte subsets in the peripheral blood in a retrospective cohort to evaluate the correlation of the prognosis and clinicopathological parameters of panNET patients and the levels of peripheral lymphocyte subsets, thus providing a novel prognostic biomarker for panNETs.
Our study revealed that the absolute counts of total T cells and CD4+ T cells strongly correlate with the metastatic status. Patients with distant metastasis tended to have lower total T cell and CD4+ T cell counts in the peripheral blood. Our study also demonstrated that a high NK cell count and a low B cell count may predict poor PFS and were independent prognostic factors for PFS after adjusting for tumour grade, nodal metastasis, distant metastasis, and treatment received in both the 73-patient cohort and in the subgroup of patients who had no distant metastases and underwent radical surgical resection; the rest of the subsets did not display significant correlations with PFS in multivariate analyses.
T cells mediate cellular immunity and play a prominent role in anti-tumour immune responses. T cells can be generally divided into CD4+ T cells, CD8+ T cells, memory T cells, and natural killer T cells. CD4+ T cells play a pivotal role in anti-tumour immunity by regulating most antigen-specific immune responses and thus orchestrating the complex anti-tumour immune process that suppresses tumour progression and development.21 Most remarkably, CD4+ helper T cells are responsible for providing regulatory cytokines that are required for the priming of CD8+ cytolytic T lymphocytes, which are believed to function as the vital effector cells that execute tumour cell killing.22 Moreover, CD4+ helper T cells may also boost humoural immunity by activating B cells, thus stimulating antibody production.23 Several studies have reported that there are decreases in total T cells and CD4+ T cells in patients with metastasis,13,23,24 and total T cells and CD4+ T cells were found to be lower in patients with cancer than in healthy people and to be lower in individuals with only primary tumours than in patients with one or more distant metastasis.25 We speculate that the decreases in total T cells and CD4+ cells were probably due to T cell exhaustion. T cell exhaustion is a state of T cell dysfunction that was originally reported in chronic viral infections but has recently been described in many types of tumours.26,27 T cell exhaustion is characterized by the gradual and progressive impairment of T cell function and can ultimately result in the physical loss of T cells.28 T cell exhaustion was reported in both CD4+ and CD8+ T cells, and notably, the exhaustion of tumour-specific CD8+ T cells was reported in metastasis from melanoma patients.26 A vicious cycle is thus formed, where an impaired immune status may lead to metastasis, and in patients with metastasis, the immune status is further compromised.
B cells predominantly function in humoural immunity, whose role in anti-tumour immunity, though less investigated than that of T cells, has attracted increasing attention.29 In addition to antibody secretion, B cells may also release cytokines that regulate other immune cells, orchestrating and regulating T cell and innate immune responses. Remarkably, B cells may enhance the activity of cytotoxic T cells and may have direct tumour-killing effects by secreting granzyme B and indirect tumoricidal effects that occur in an antibody-dependent manner.29 Therefore, B cells may play a crucial role in restricting tumour progression and development. Here, we demonstrated that peripheral B cells independently predict the progression of panNETs, which indicates that peripheral B cell levels may be a promising independent prognostic indicator in panNET patients and a potential target for panNET treatment.
We also noticed that, in contrast to B cells, a high absolute count of peripheral NK cells was associated with a shorter PFS and was an independent prognostic factor in patients that underwent radical surgical resection. This result was completely different from our hypothesis regarding NK cells, which are known to directly kill tumor cells through the release of toxic granules and to play a key role in suppressing tumor metastasis.30,31 In fact, multiple studies have reported similar results in various cancer types. Tietze et al32 reported that a low level of total peripheral NK cells may predict a better prognosis in melanoma patients than normal or high peripheral NK cell levels. Jonge et al11 reported similar results in melanoma patients and indicated that a certain type of NK cells may have an adverse effect on the anti-tumor responses by suppressing T cell activities via CD38, perforin, CD11a and IFNγ. Yang et al33 reported that a high absolute count of NK cells was associated with poor prognosis in pancreatic cancer patients. Though the anti-tumor activities of NK cells have been demonstrated and corroborated, mounting evidence on the pro-tumor and pro-metastatic role of NK cells in tumors is also emerging. First, the expression of NK cell receptors can be modified by tumor cells, which allows the tumor cells to evade the anti-tumor activity of NK cells.31 The levels of multiple receptors that may activate NK cells, including NKG2D, NKp30, NKp46, and perforin-positive NK cells, were found to be substantially downregulated in patients with pancreatic cancer, gastric cancer, and colorectal cancer, and these decreases were associated with tumor progression.34 Moreover, NK cells can also directly generate a pro-tumor microenvironment and may facilitate tumor metastasis. For instance, IFN-γ and TNF-α, two distinct anti-tumor cytokines released by NK cells, were reported to trigger the adaptive immune evasion of tumor cells and epithelial-to-mesenchymal transition (EMT),35 which is a phenotype-switching process that allows proliferative tumor cells to shift into a migratory and invasive state.36 NK cells may also cause tumor cells to acquire an EMT phenotype via the alteration of HLA-I, PD-L1, or NKG2D-L in tumor cells.35,37 Further, NK cells may promote tumor metastasis via the expression of matrix metalloproteinases (MMPs), which facilitate the degradation of the extracellular matrix and may, in turn, contribute to tumor cell migration and invasion, or via the tumor-related persistent inflammation that is induced by NK cell-secreted IL-6 and IL-32.31
A major limitation of our study was that it was a retrospective cohort study that was conducted at a single centre with a limited sample size, a relatively short follow-up time and a relatively low progression rate. The lack of a mechanistic investigation leads to another weakness. Therefore, prospective cohort studies at multiple centres need to be further conducted. More studies are needed to examine the specific mechanisms of these phenomena. Additionally, we mainly investigated peripheral lymphocyte subsets instead of locally infiltrating lymphocytes in tumour tissue, principally because peripheral lymphocyte subset levels are easier to obtain. However, though the local immune environment may be partially reflected by systemic immune status, the relationship between locally infiltrating lymphocytes and peripheral lymphocytes warrants further corroboration. Moreover, important information such as post-surgical complications, pre-existing diabetes or pancreatitis, and the baseline nutritional conditions of patients were not included in our study, which may threaten the validity of our results. Prospective studies in larger cohorts with clear records of clinicopathological characteristics should be performed to validate our findings.
Conclusion
In conclusion, our study revealed that the distant metastasis of panNET patients is associated with lower numbers of peripheral total T cells and CD4+ T cells. A low baseline absolute count of peripheral B cells and a high baseline absolute count of peripheral NK cells are associated with a shorter PFS in panNET patients. These results indicated the prognostic significance of peripheral B cells and NK cells for the progression of panNETs, making B cells and NK cells promising clinical parameters for predicting the prognosis of panNET patients and potential targets for panNET treatment.
Abbreviations
panNETs, pancreatic neuroendocrine tumors; panNECs, pancreatic neuroendocrine carcinomas; FDG, fludeoxyglucose; NK cells, natural killer cells; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration biopsy; PLB, percutaneous liver biopsy; PFS, progression-free survival; American Joint Committee on Cancer, AJCC; OS, overall survival; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; RECIST, response evaluation criteria in solid tumors; ROC, receiver operating characteristic; HR, hazard ratio; HAIC, hepatic arterial infusion chemotherapy; TACE, transarterial chemoembolization; EMT, epithelial-to-mesenchymal transition; MMPs, matrix metalloproteinases.
Ethics Approval and Informed Consent
This study was approved by the Ethics Board of Shanghai Cancer Center, Fudan University, and all patients involved in this study provided informed consent for the use of their personal data for research purposes.
Acknowledgments
The abstract of this paper was presented at the European Society of Medical Oncology as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Annals of Oncology (2019) 30 (suppl_5): v194-v197 (10.1093/annonc/mdz245).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68:471–487. doi:10.3322/caac.21493
2. Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15(2):409–427. doi:10.1677/ERC-07-0221
3. Ries LAG, Keel GE, Horner MD. SEER survival monograph cancer survival among adults: U.S. SEER program, 1988-2001 patient and tumor characteristics Edited by. 2007;(07):1988–2001.
4. Falconi M, Bartsch DK, Eriksson B, Taal BG, Vullierme MP. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning. Neuroendocrinology. 2012;95(2):98–119. doi:10.1159/000335587
5. Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2158 patients. J Gastrointest Surg. 2010;14(3):541–548. doi:10.1007/s11605-009-1115-0
6. Madeira I, Terris B, Voss M, et al. Prognostic factors in patients with endocrine tumors of the duodenopancreatic area. Gut. 1998;43(3):422–427. doi:10.1136/gut.43.3.422
7. Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumors. Ann Oncol. 2008;19(5):903–908. doi:10.1093/annonc/mdm552
8. Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur J Nucl Med Mol Imaging. 2017;44(3):490–499. doi:10.1007/s00259-016-3533-z
9. Hamilton NA, Liu T-C, Cavatiao A, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152(1):107–113. doi:10.1016/j.surg.2012.02.011
10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
11. De Jonge K, Ebering A, Nassiri S, et al. Circulating CD56 bright NK cells inversely correlate with survival of melanoma patients. Sci Rep. 2019;9(1):4487. doi:10.1038/s41598-019-40933-8
12. Yu Q, Yu C, Ling Z. Elevated circulating CD19 + lymphocytes predict survival advantage in patients with gastric cancer. APJCP. 2012;13(5):2219–2224. doi:10.7314/apjcp.2012.13.5.2219
13. Aliustaoglu M, Bilici A. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27(4):1060–1065. doi:10.1007/s12032-009-9335-4
14. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
15. von Andrian UH, Mackay CR. T-cell function and migration—two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi:10.1056/NEJM200010053431407
16. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi:10.1016/S1471-4906(01)02060-9
17. Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. doi:10.1038/sj.jid.5700001
18. Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70(4):326–336. doi:10.1111/j.1365-3083.2009.02308.x
19. Dunn GP, Old LJ, Schreiber RD. THE three es of cancer immunoediting. Annu Rev Immunol. 2004;22(1):329–360. doi:10.1146/annurev.immunol.22.012703.104803
20. Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what’s new? Neuroendocrinology. 2012;95(4):267–276. doi:10.1159/000334612
21. Dilillo DJ, Yanaba K, Tedder TF, Alerts E. B cells are required for optimal CD4 + and CD8 + T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–4016. doi:10.4049/jimmunol.0903009
22. Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368. doi:10.1084/jem.188.12.2357
23. Abbas A, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi:10.1038/383787a0
24. Robinson E, Segal R, Sc B, et al. Lymphocyte subpopulations in patients with multiple primary tumors. Cancer. 1999;85(9):2073–2076. doi:10.1002/(SICI)1097-0142(19990501)85:9<2073::AID-CNCR26>3.0.CO;2-J
25. Liu H, Deng W, Li J, Tang Y, Zhang L, Cui Y. Peripheral blood lymphocyte subset levels differ in patients with hepatocellular carcinoma. Oncotarget. 2016;7(47):77558–77564. doi:10.18632/oncotarget.13041
26. Baitsch L, Rufer N, Speiser DE, et al. Exhaustion of tumor-specific CD8 + T cells in metastasis from melanoma patients find the latest version: exhaustion of tumor-specific CD8 + T cells in metastasis from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi:10.1172/JCI46102
27. Riches JC, Davies JK, Mcclanahan F, et al. LYMPHOID NEOPLASIA T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2019;121(9):1612–1622. doi:10.1182/blood-2012-09-457531
28. Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–481. doi:10.1111/j.1365-2567.2010.03255.x
29. Tsou P, Katayama H, Ostrin EJ, Hanash SM. The emerging role of B cells in tumor immunity. Cancer Res. 2016;76(19):5597–5601. doi:10.1158/0008-5472.CAN-16-0431
30. López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32(2):135–154. doi:10.1016/j.ccell.2017.06.009
31. Lee HH, Kang H, Cho H. Natural killer cells and tumor metastasis. Arch Pharm Res. 2017;40(9):
32. Tietze JK, Angelova D, Heppt MV, Ruzicka T, Berking C. Low baseline levels of NK cells may predict a positive response to ipilimumab in melanoma therapy. Exp Dermatol. 2017;26(7):
33. Yang C, Cheng H, Zhang Y, Fan K, Luo G, Fan Z. Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Cancer Immunol Immunother. 2018;67(12):1815–1823. doi:10.1007/s00262-018-2235-8
34. Peng Y, Zhu Y, Zhang J, et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med. 2013;11(1):1. doi:10.1186/1479-5876-11-262
35. Lv N, Gao Y, Guan H, et al. Inflammatory mediators, tumor necrosis factor-α and interferon-γ, induce EMT in human PTC cell lines. Oncol Lett. 2015;10(4):
36. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):
37. Dondero A, Pastorino F, Della Chiesa M, et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology. 2015;5(1):e1064578–e1064578. doi:10.1080/2162402X.2015.1064578
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



