Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Abnormalities of Gray Matter Volume and Its Correlation with Clinical Symptoms in Adolescents with High-Functioning Autism Spectrum Disorder
Authors Zhao X, Zhu S, Cao Y, Cheng P, Lin Y, Sun Z, Jiang W, Du Y
Received 13 November 2021
Accepted for publication 4 March 2022
Published 1 April 2022 Volume 2022:18 Pages 717—730
DOI https://doi.org/10.2147/NDT.S349247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiaoxin Zhao,1 Shuyi Zhu,1 Yang Cao,2 Peipei Cheng,1 Yuxiong Lin,1 Zhixin Sun,1 Wenqing Jiang,1 Yasong Du1
1Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Suzhou Guangji Hospital, Suzhou, People’s Republic of China
Correspondence: Yasong Du, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, No. 600 Wanping Nan Road, Shanghai, 200030, People’s Republic of China, Tel +86 18816935313, Email [email protected]
Background: Previous studies have indicated abnormal gray matter volume (GMV) in individuals with autism spectrum disorder (ASD); however, there is little consistency across the findings within these studies, partly due to small sample size and great heterogeneity among participants between studies. Additionally, few studies have explored the correlation between clinical symptoms and GMV abnormalities in individuals with ASD. Here, the current study examined GMV alterations in whole brain and their correlations with clinical symptoms in a relatively large and homogeneous sample of participants with ASD matched typically developing (TD) controls.
Methods: Forty-eight adolescents with high-functioning ASD and 29 group-matched TD controls underwent structural magnetic resonance images. Voxel-based morphometry was applied to investigate regional GMV alterations. The participants with ASD were examined for the severity of clinical symptoms with Autism Behavior Checklist (ABC). The relationship between GMV abnormalities and clinical symptoms was explored in ASD group using voxel-wise correlation analysis within brain regions that showed significant GMV alterations in individuals with ASD compared with TD controls.
Results: We found increased GMV in multiple brain regions, including the inferior frontal gyrus, medial frontal gyrus, superior frontal gyrus, superior temporal gyrus, occipital pole, anterior cingulate, cerebellum anterior lobe, cerebellum posterior lobe, and midbrain, as well as decreased GMV in cerebellum posterior lobe in individuals with ASD. The correlation analysis showed the GMV in the left fusiform was negatively associated with the scores of sensory factor, and the GMV in the right cerebellum anterior lobe was positively associated with the scores of social self-help factor.
Conclusion: Our results indicated that widespread GMV abnormalities of brain regions occurred in individuals with ASD, suggesting a potential neural basis for the pathogenesis and symptomatology of ASD.
Keywords: autism spectrum disorder, symptoms severity, Autism Behavior Checklist, structural magnetic resonance imaging, gray matter volume
Background
Autism spectrum disorder (ASD), which is characterized by persistent deficits in social interaction and communication, repetitive behaviors and restricted interests, is a severe neurodevelopMental disorder and a leading cause of mental disability among Children worldwide. In 2018, the prevalence of ASD is reported to be 1 in 44 children aged 8 years and is on the rise.1 Besides, low self-care ability and increased unemployment or underemployment are commonly present among patients with ASD when they reach adulthood.2 Consequently, high prevalence and disability bring about enormous social and economic burden.3
As early diagnosis of ASD is known to improve long-term functioning of affected children and enhance the quality of life of ASD individuals when they achieve adulthood, there is urgent requirement for timely and accurate diagnosis of ASD so as to provide efficient services and prevent the patient’s condition from worsening. However, early identification and diagnosis of ASD is difficult on account of the heterogeneity in severity and types of ASD symptoms, changes in diagnostic criteria, co-occurrence with other mental disorders, and the absence of biological diagnostic markers. Unfortunately, the pathophysiological mechanism of ASD remains poorly understood and controversial.
Over the last few decades, multiple studies have demonstrated that brain abnormalities are associated with the disease.4 There is one prominent theory that ASD involves the rapid enlargement of total brain volume in the first few years of life,5,6 while the picture in adolescents or adults is murkier.7 In contrast, several studies reported that the total brain volume of ASD was enlarged in adolescents.8,9 Increasing evidence from morphological studies emerged that this increase in total brain volume might prominently come from gray matter, but not white matter.10–12 The patterns of gray matter volume (GMV) abnormalities have become of significance and may provide further insights into understanding the neuropathology of ASD. Previous postmortem studies have revealed structural and morphometric changes of gray matter in ASD patients.13,14 During the past decade, magnetic resonance imaging (MRI) has made astounding advances and various MRI-based methods are applied to characterize morphological differences in ASD. Studies using region of interest (ROI) analysis have reported specific regions with a relatively greater enlargement of GMV, such as the temporal lobe,15 some regions with a combination of increases and reduction of GMV.16 However, Haar et al17 failed to find any volumetric differences in regional gray matter using an ROI approach. An earlier study18 in ASD children 3 to 4 years of age revealed cerebral enlargement in cerebellum and amygdala in children with ASD compared to TD. Voxel-based morphometry (VBM) studies, which, unlike the ROI approach, has the ability to detect differences in regional GMV throughout the whole brain without any a priori regional assumptions, have also produced variable findings. The most prevalent results were GMV abnormalities in the cerebellum. Cerebellar GMV reductions of ASD patients were detected widely in vermis, anterior and posterior lobules while increased cerebellar GMV were also revealed.16,19–21 In addition, the GMV abnormalities were reported in various brain regions located in frontal lobe, temporal lobe, parietal lobe, anterior cingulate cortex, insula, caudate nucleus, lingual gyrus and so on,19,22,23 which provided evidence supporting that atypical cerebello-thalamo-cortical network in ASD.24,25 However, there were more than a few studies reported no significant difference of GMV alterations between ASD individuals and TD controls.17,26–28 These inconsistent findings may be partially attributable to small sample sizes or differences in imaging methodology and demographic and clinical characteristics of the samples. A recent meta-analysis study used coordinate-based anatomical likelihood estimation (ALE) analysis of VBM studies examining high-functioning autism spectrum disorder (HFASD), showed that just 6 out of 21 studies recruited more than 25 individuals with ASD.29 Small sample size limits the statistical power for investigation of comparation during study data analysis, especially for VBM method, as it requires correction for multiple comparisons. Methodological differences may also have an effect on the measurement of GMV and lead to variable results, such as image acquisition and image analysis. As ASD is a heterogeneous developmental disorder,30 there is an inherent variability between affected individuals, with diverse clinical manifestations and different levels of intelligence, which is most likely associated with variations in neuroanatomical abnormalities.31,32 Therefore, the picture emerging is that GMV abnormalities deserve further examination in individuals with ASD through the recruitment of relatively large-size and homogeneous samples of patients, reducing the influence from potential confounding factors.
Many of previous studies investigating GMV alterations in ASD have recruited only young children or adult cohorts, even mixed groups of participants at different age levels. However, relatively few attempts have been made to investigate adolescents with ASD. The results have also been inconsistent. For example, some studies have found increased GMV in the medial frontal gyrus (MFG) in adolescents with ASD,33–35 but other studies have not.36–38 Moreover, decreased GMV has been reported in the cerebellum in adolescents with ASD,33,38 However, some authors failed to replicate the finding,34,36,37 or even found increased GMV.35 A previous meta-analysis has explored GMV alterations in pediatric patients with ASD, but only five VBM studies of adolescent populations were included.38 Therefore, the central question of how the brain GMV alterations manifest in adolescent ASD remains unaddressed and deserves further examination.
Several pioneering studies have examined the relationships between GMV alterations and autistic symptom severity in individuals with ASD. For instance, altered GMV was reported to be negatively associated with symptom severity in several regions.39 Recently, Supekar et al40 reported GMV in the motor cortex, supplementary motor area, and cerebellum was related to repetitive/restricted behaviors in ASD girls whereas GMV in the right putamen was related to repetitive/restricted behaviors in ASD boys, with some sex-specific effects. A more recent study observed that GMV of the inferior frontal gyrus (IFG), rectus, and caudate were negatively correlated with the subscale scores or total score on the Scale for the Autism Behavior Checklist (ABC) in Chinese boys with low functioning ASD.41 The inconsistency of the studies above could be due, in part, to the relatively small sample size, or differences in sample characteristics and methodological approaches. In the current study, we used VBM approach42 to investigate alterations in regional GMV of individuals with ASD. We also tried to examine correlations between GMV alterations and the severity of autistic symptoms in individuals with ASD, using the subscales of the ABC. To address issues of sample variability and the inconsistency in neuroanatomical findings, we recruited a relatively larger sample and used strict inclusion criteria for individuals with ASD according to age, gender, intelligence quotient (IQ), and diagnosis, and age, gender, and IQ-matched TD controls.
Methods
Participants
We recruited 58 adolescents with HFASD and 66 typically developing (TD) controls at Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine from February, 2017 to May, 2020. Seven ASD participants and one control subject were excluded because their MRI images were of substandard quality such as excessive movements or technical issues. Additionally, three other ASD and two TD participants were excluded to restore group matching on age, gender, and full-scale intelligence quotient (FSIQ), resulting in a final sample of 48 ASD (39 males, 9 females) and 63 TD (43 males, 20 females) participants (Table 1). The ASD participants were diagnosed according to DSM-5,43 and met the criteria for ASD. ASD participants also completed a clinical diagnostic interview with a trained and board-certified psychiatrist experienced in evaluating ASD and comorbid psychiatric conditions. All ASD participants were medication naive and did not have any comorbid psychiatric disorders (eg, attention deficit hyperactivity disorder, oppositional defiant disorder, anxiety disorders, and mood disorders). Children with ASD-related disorders (eg, tuberous sclerosis, Fragile-X syndrome) or other neurological disorders (eg, epilepsy, Tourette’s syndrome) were excluded. TD controls were enrolled from Shanghai by advertisements posted in the local school and community. They were interviewed for recruitment and had no self-reported history of ASD, or any current or previous psychiatric or neurological condition. TD controls with first-degree family history of psychotic episodes were also excluded. IQ was assessed using the Wechsler intelligence Scale for Children 4th edition (WISC-IV).44 Every participant with ASD scored above the common cutoff for the distinction of “low” and “high” functioning ASD (FSIQ > 80).45 All participants were Han Chinese, and classified as right-handers according to the Annett Handedness Scale.46 The patients had a mean ± SD age of 13.0 ± 1.9 years (range, 10–18) and a mean ± SD FSIQ scores of 106.9 ± 19.2 (range, 80–145). The controls had a mean ± SD age of 12.9 ± 1.8 years (range, 10–18) and a mean ± SD FSIQ scores of 106.9 ± 19.2 (range, 80–143).
 |
Table 1 Demographic Data and Abnormal Behavioral Performance in Individuals with ASD and TD Controls |
General inclusion criteria for both groups included as follows: (1) aged 10–18 years, (2) right-handed, (3) an ability to possess a full comprehension of the survey instructions and contents. All research procedures employed in this study were in strict conformity with the guidelines of the Declaration of Helsinki. The study protocol was approved by the Medical Research Ethics Committee of Shanghai Mental Health Center. Written informed consents were obtained from each participant and his/her legally authorize guardians prior to inclusion in the study.
Assessment of Autistic Symptoms
The ABC is a checklist of non-adaptive behaviors47 that consists of 57 items in 5 areas of categories, including problems related to Sensory (sensation and perception; 9 items), Relating (relation and connection; 12 items), Body concept (physical activity and rigid use of objects; 12 items), Language (communication and interaction; 13 items) and Social self-help (adaptability and self-care; 11 items).48 ABC items were rated as “yes” (rated as 1, with symptom) or “no” (rated as 0, without symptom) for each question during the assessment. ABC was well-established instruments for the screening and diagnosis of childhood autism.49 It has been widely used in clinical and scientific research, now being one of the most mature rating scales for domestic use.50
Imaging Acquisition
All magnetic resonance imaging (MRI) data were collected at Shanghai Mental Health Center using a 3-Tesla Siemens Verio scanner. A standard head coil was padded with foam to reduce head motion and scanner noise. T1-weighted anatomical MRI were acquired using a 3D-MPRAGE sequence: repetition time (TR) = 2530 ms; echo time (TE) = 2980 ms; inversion time = 1100 ms; flip angle (FA) = 7°; field of view (FOV) = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1 mm, no gap; 192 sagittal slices; and acquisition time = 363 s. All brain scans were examined by an experienced neuroimaging physician and were found to be free from organic brain pathology (for example, tumors, cerebrovascular malformation, and hydrocephalus).
Data Preprocessing and Processing
GMV alterations were examined on T1 images using VBM toolbox in Statistical Parametric Mapping software (SPM, http://www.fil.ion.ucl.ac.uk/spm) and the diffeomorphic anatomical registration through an exponentiated Lie algebra (DARTEL) algorithm was taken to achieve more precise segmentation and normalization.51 Individual MR images were segmented to gray matter, white matter and cerebrospinal fluid sections. The mean images of the individual gray matter were then created. Individual gray matter images were aligned to the Montreal Neurological Institute (MNI) space. Finally, all GMV images were smoothed with a 8-mm full-width at half-maximum isotropic Gaussian kernel.
Statistical Analysis
The demographic characteristics were analyzed with SPSS software (PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc). Two-sample t-test was used for testing the differences in age and FSIQ between adolescents with HFASD and TD controls. Gender difference was tested with the Pearson Chi-Square test. The height threshold of statistical significance was set at p < 0.05.
Group-level analyses were carried out to examine brain regions with significant detectable GMV abnormalities in adolescents with HFASD. Group-level analyses were carried out to examine GMV abnormalities in adolescents with HFASD. The GMV over the whole-brain structures were compared between the ASD group and TD control group applying permutation-based statistical analysis with 5000 permutations, with age, gender, FSIQ, and total intracranial volume (TIV) as covariates. Statistical significance was defined as p < 0.001, correcting for family-wise error (FWE) correction using threshold-free cluster enhancement (TFCE).52 The altered GMV brain areas in ASD group were used as inclusion masks to perform the following voxel-wise correlation analysis.
Voxel-wise correlation analyses performed to explore the brain regions that correlated with the severity of autistic symptoms including the total score and each component of ABC (Sensory, Relating, Body concept, Language and Social self-help), respectively. The covariates age, gender, FSIQ, and the TIV were considered as covariates of no interest and were entered into the model to adjust for their potential confounding effects on relation between GMV and symptom characteristics. The above-mentioned clusters, survived thresholding at p < 0.001 with FWE-TFCE correction, were applied to include only those voxels that showed significant GMV differences between adolescents with HFASD and TD controls. Correlations were undertaken for significance using the randomized permutation-based nonparametric inference with 5000 permutations and TFCE multiple comparison-corrected. The correlations were considered significant at a p-value of 0.01 corrected for TFCE owing to an exploratory nature of this analysis.
Results
Participants’ Demographic Characteristics
Table 1 showed the demographic characteristics and abnormal behavioral performance of adolescents with HFASD and TD controls in the current study. There were no significant differences in age (p = 0.74), gender (p = 0.123), and FSIQ (p = 0.211) between adolescents with HFASD and TD controls.
Regional GMV Abnormalities in ASD Compared with TD Controls
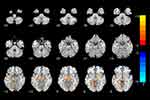
Compared with TD controls, increased GMV values were observed in adolescents with HFASD in the right IFG, right MFG, left superior frontal gyrus (SFG), left superior temporal gyrus (STG), right occipital pole, anterior cingulate, right cerebellum anterior lobe (CAL), right cerebellum posterior lobe (CPL), and right midbrain (P < 0.001 for all, FWE-TFCE-corrected; Figure 1 and Table 2). In contrast, decreased GMV values were found mainly in the left CPL in the HFASD group relative to TD (P < 0.001 for all, FWE-TFCE-corrected; Figure 1 and Table 2). Individual age, gender, FSIQ scores and TIV were used as covariates during the group comparisons. Subsequently, the correlation analysis in HFASD group was performed within the regions of GMV abnormalities.
 |
Table 2 Brain Regions with Significant GMV Alterations Between Individuals with ASD and TD Controls |
Relationship Between GMV Values and Autistic Symptoms
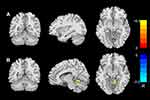
In the HFASD group, there were significantly negative correlations between the GMV value in the left fusiform and the scores of Sensory factor (r = −0.48, P < 0.01, TFCE-corrected; Figure 2A), and positive correlations between the GMV values in the right CAL and the Social self-help factor (r = 0.45, p <0.01; TFCE-corrected; Figure 2B). The ABC total score, Relating, Body concept, and Language showed no significant correlations with the GMV values within the regions that showed significant group differences. The results of above correlation analysis of the adolescents with HFASD are summarized in Table 3.
 |
Table 3 Significant Correlations Between ABC Scores and GMV in ASD |
Discussion
In the current neuromorphometric study, an automated and unbiased voxel-based approach was used to investigate the whole-brain GMV abnormalities in the adolescents with HFASD, as well as its relationship to the severity of their clinical symptoms. Based on the present study, the GMV in the adolescents with HFASD was widely increased in numerous regions (ie, the right IFG, left superior frontal gyrus, left STG, right occipital pole, left ventral anterior cingulate (VAC), right dorsal anterior cingulate (DAC), right CAL, right CPL, and right midbrain). In contrast, the GMV of left CPL in the HFASD group was decreased compared to the TD group. Moreover, the GMV in the left fusiform was negatively associated with the scores of Sensory factor, and the GMV in the right CAL was positively associated with the Social self-help factor. These findings demonstrated that the GMV alterations are present in individuals with ASD, suggesting that the abnormalities of GMV, as the primary neuropathology of ASD, might play a fundamental role in the nature of clinical symptoms of this disorder. It’s worth to mention that these significant correlations between altered GMV in these regions (left fusiform and right CAL) and autistic symptoms were corrected for multiple comparisons using TFCE, but unfortunately they did not pass FWE-correction in the multiple testing. We speculate that the small effect size of GMV abnormalities on psychopathology is likely a matter of location of the gray matter impairment rather than a matter of the intensity of the impairment.
In the current study, the results of widespread GMV alterations in several regions in individuals with ASD compared with that of TD controls are consistent with most of the studies examining GMV values in patients with ASD,19,39,53–55 suggesting that the disruption of GMV might contribute to the psychopathology of ASD. However, some other studies failed to report any difference in GMV values between patients with ASD and TD controls,17,26–28 or even most of the brain regions with decreased GMV in Chinese boys with low functioning ASD.41 The discrepancy of the studies above could be partly due to the relatively small sample size, different sample characteristics, limited brain regions investigated, and differences in GMV analysis (voxel-based approach vs ROI-based approach) in these previous studies.
Our current results showed a general pattern of increased GMV in numerous brain regions in the ASD group, compared with TD group, except in the part of the cerebellum. A few studies have emerged accounting for the patterns of neuroanatomical abnormalities in ASD across development, and shed light on alterations in GMV reported in our research. These studies has describe a distinct neurodevelopmental trajectories in ASD individuals across the lifespan, which is characterized by an early brain overgrowth,56,57 followed by developmental arrest in late childhood and early adolescence,58 and accelerated neuroatrophy in adulthood.59,60 According to the theory, the brains of individuals with ASD should be larger than those of TD controls during adolescence, even though there is a lag in brain development in this period. Our results showed both increases and decreases in cerebellar GMV in adolescents with ASD with VBM analysis, which is consistent with some previous studies.16,20,21 However, some previous studies failed to find any significant difference in cerebellar GMV between patients with ASD and TD controls,28,61 even only increased39,62 or decreased41,63,64 cerebellar GMV in individuals with ASD. There may be several reasons for this discrepancy, such as the small sample size, differences in sample characteristics (eg, different developmental ages, gender, and IQ), differences in scanner techniques, including field strengths and sequence parameters, and differences in GMV analysis (postmortem autopsy vs region of interest analysis vs voxel-based approach) in these previous studies.
ASD is a complex disorder that is characterized by multiple symptoms ranging from low-level (eg, sensory processing) to high-level (eg, language, socio-emotional processing, self-referential processing) functions. Consequently, it is not surprising that the extent of neuroanatomical changes reported in our research and in previous studies, involves regions and neural networks throughout the whole brain. Sensory processing is a primary concern for ASD patients. Sensory processing relies on several brain regions, including STG, postcentral gyrus, precuneus, superior parietal cortex, prefrontal cortex, occipital pole, fusiform gyrus, basal ganglia and cerebellum.65–69 A recent study has reported that the larger GMV in early sensory regions (eg, lingual gyrus, STG, postcentral gyrus, rolandic operculum, temporal pole, IFG, middle frontal gyrus) significantly correlate with atypical sensory processing of visual, auditory, tactile, and taste/smell modalities.70 In our study we found GMV alterations in the IFG, STG, occipital pole and cerebellum. We also found significant correlations between GMV in the fusiform and the scores of Sensory factor on the ABC. Therefore, these neuroanatomical abnormalities may be related to the commonly seen sensory processing deficits in individuals with ASD.
A defining feature of ASD is abnormal social cognition, including deficits in facial emotion perception, theory of mind and empathy.71–75 Neural underpinnings of the social behavioral abilities have been investigated extensively, and consists of the amygdala, inferior frontal cortex, medial prefrontal cortex, anterior cingulate cortex, insula, STG, and fusiform gyrus, known collectively as “social brain”.71,72,76,77 Indeed, a previous VBM study also demonstrated the GMV in male adolescents with ASD was increased in those regions associated with social cognition, such as fusiform gyrus, anterior cingulate cortex, superior temporal sulcus, and STG.36 Brain imaging studies have observed hypoactivation in the fusiform face area during the perception of emotional facial expressions in patients with ASD.78–81 Studies that investigated the neural basis of theory of mind in ASD found that the deficits of these processes were associated with atypicalities in a set of regions,39,82–84 commonly termed theory of mind network, including the medial prefrontal cortex, precuneus, lateral orbitofrontal cortex, middle frontal gyrus, STG, and temporoparietal junction. Impairment in empathy has been implicated in ASD.85,86 Indeed, a previous lesion study has indicated that the IFG may be critical for emotional empathy.87 Additionally, in an anatomical MRI study investigating the associations between brain regions and empathy for emotion, Eilam-Stock et al revealed that GMV alterations in the IFG may be associated with deficits of emotional empathy.39 The results of our study also revealed GMV abnormalities in the IFG, fusiform gyrus, STG, and anterior cingulate cortex (VAC and DAC). These neuroanatomical abnormalities may be associated, therefore, with social cognition deficits commonly seen in ASD patients. The general picture may reflect an abnormal social cognitive neuroanatomic network.
Our results also suggest GMV abnormalities in several brain regions in ASD that may be connected with altered self-referential processing88,89 and autobiographical memory90,91 in the disease. The neural basis of self-referential processing has been explored in typically developing samples92 and point to a network of cortical midline structures including MFG, anterior cingulate cortex, posterior cingulate cortex, middle cingulate cortex and precuneus,89,92,93 and it’s worth noting that the MFG is regarded as an important hub of the network.94 Koush et al95 revealed that the SFG play a crucial role in the modulation of self-referential processing in the temporal parietal junction. Other study has also indicated that the IFG is involved in the self-related judgments.96 Our results of increased GMV in the IFG, SFG and MFG may be connected, therefore, with abnormal self-referential processing in individuals with ASD.
The increased GMV in the right midbrain in the ASD group in the current study may be involved in the structural aberrations in the mesolimbic reward pathway which was reported to be related to social interaction impairments in children with ASD, especially in the ventral tegmental area.97 The mesolimbic reward pathway, which consists of the ventral tegmental area of the midbrain, the nucleus accumbens of the striatum, and the white matter tracts that reciprocally connects them, is a core brain circuit for processing reward value.98 A well-known ASD theory assumes that individuals with ASD find social stimuli less rewarding than their typical developmental peers, resulting in impaired social interaction.99 Preclinical animal models of ASD also showed a significant link between abnormal mysophobic reward pathway and aberrancies in reciprocal social interactions.100,101 Additionally, Rodier and Arndt revealed an anatomical dissociation between autistic behaviors, such as limited expressive movements of the face, eyes and vocal productions, and the malformation in the midbrain in the embryo.102 In our study, the midbrain was identified as an area of increased GMV in ASD, which may be the neuroanatomical basis of abnormal reward processing and social information processing in individuals with ASD.
The cerebellum is one of the most common sites of aberrance in ASD, which have been associated with ASD for more than two decades. The concept that the cerebellum is only concerned with fine motor function has been outdated; There are a lot of evidences that the cerebellum is related to higher cognitive functions such as language. Although a recent consensus paper emphasizes the neuroanatomical changes of the cerebellum in individuals with ASD,103 previous studies have yielded conflicting results. Indeed increased,9,18 decreased104,105 and preserved GMV106 have been reported in studies on cerebellar structures in individuals with ASD compared to TD controls. Consistent with previous studies, our results indicated both increased and decreased GMV in various brain regions of cerebellum. We found altered GMV located in the CPL, a region associated with cognitive and language tasks.107 Additionally, we observed increased GMV in the CAL and a significant positive correlation between the GMV in the right CAL and abnormal self-care ability in social life in individuals with ASD. As is well known, accumulated evidence has shown that deficits in sensorimotor integration and control in ASD are closely related to self-care performance in social life.108,109 Our results of GMV aberrance in the cerebellum anterior and posterior lobes may be related to abnormal sensorimotor function and cognitive function, respectively. Therefore, the topography of sensorimotor and cognitive subregions of the cerebellum emerge because the cerebellum forms closed-loop circuits with sensorimotor and cognitive areas of the cerebral cortex.110
Limitations
Several limitations should be noted when interpreting the results of our study. First, the sample size in our study was still relatively small on account of recruitment difficulties of adolescents with HFASD. The current study strictly limited the inclusion criteria (ie, adolescents with HFASD) to increase statistical power and reduce variability. However, as ASD is a heterogeneous condition with multiple risk factors and etiologies, our sample may not be sufficiently homogeneous. For that reason, future studies with larger samples of individuals falling on different sub-groups of the ASD spectrum are desirable to promote a better description of neuroanatomical changes that contribute to the symptomatology of ASD. On the other hand, our study used strict inclusion criteria and control for different variables, such as age and IQ, and hence, the generalizability of our findings is inevitably reduced. Moreover, one limitation to classification of ASD in current study is the use of only DSM-5 criteria, rather than combining with the systematic method such as Autism Diagnostic Observation Schedule Second Edition (ADOS-2), which is widely used instrument in the diagnosis of ASD. Therefore, the ASD subjects included in current study may have an overestimated risk of ASD diagnosis. Furthermore, owing to the cross-sectional design, we cannot affirm valid conclusions by virtue of the process of disease. In other words, it was impossible to tell exactly whether the autistic symptoms were present as the result of the altered GMV of the brain regions in ASD. Future longitudinal studies could quite likely provide further insight into a fundamental role of the aberrations of regional GMV in the neurobehavioral manifestations of ASD. Lastly, we measured regional GMV of brain using VBM, which is one of the most commonly used approaches in the study of neuroanatomical aberrations in clinical populations. However, other neuroanatomical methods, such as cortical folding and sulcal depth, cortical surface area, cortical thickness, as well as tract-based spatial statistics for white-matter tract, would be valuable to produce more results about other structural measures as well.
Conclusions
In summary, with fewer confounders, our findings indicate that individuals with ASD have altered GMV values in the numerous regions, suggesting the involvement of GMV abnormalities of ASD. Moreover, the GMV abnormalities of the fusiform and CAL were associated with the clinical symptoms, suggesting that GMV abnormalities may be associated with the psychopathology of ASD. The findings from this study seem to provide an empirical and theoretical basis for exploration for biological markers and further targeted therapy of ASD. While we speculated that the GMV abnormalities of brain regions may be the neuropathological mechanism underlying the symptomatology of ASD, the mechanisms still remain unknown. Thus, the picture that emerges is that GMV alterations require replication in larger samples in individuals with ASD.
Acknowledgments
This work is partly supported by the National Key Development Program (2017YFC1309903), the Three-Year Action Plan for the Construction of Shanghai Public Health System (GWV-10.1-XK19), and the Scientific Research Project of Shanghai Municipal Health Commission (20194Y0071). The authors thank the participants and their families recruited in this project for their participation and the research personnel for data collection, making this research possible.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Xiaoxin Zhao, Shuyi Zhu, Yang Cao, Peipei Cheng, Yuxiong Lin, Zhixin Sun, Wenqing Jiang, and Yasong Du declare that they have no conflicts of interest for this work.
References
1. Maenner MJ, Shaw KA, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70(11):1–16. doi:10.15585/mmwr.ss7011a1
2. Strickland D. Virtual reality for the treatment of autism. Stud Health Technol Inform. 1997;44:81–86.
3. Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161(4):343–349. doi:10.1001/archpedi.161.4.343
4. Hashem S, Nisar S, Bhat AA, et al. Genetics of structural and functional brain changes in autism spectrum disorder. Transl Psychiatry. 2020;10(1):229. doi:10.1038/s41398-020-00921-3
5. Kamdar MR, Gomez RA, Ascherman JA. Intracranial volumes in a large series of healthy children. Plast Reconstr Surg. 2009;124(6):2072. doi:10.1097/PRS.0b013e3181bcefc4
6. Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi:10.1016/j.neuroimage.2007.03.053
7. Aylward EH, Minshew NJ, Field K, et al. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi:10.1212/wnl.59.2.175
8. Freitag CM, Luders E, Hulst HE, et al. Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biol Psychiatry. 2009;66(4):316–319. doi:10.1016/j.biopsych.2009.03.011
9. Stanfield AC, McIntosh AM, Spencer MD, et al. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23(4):289–299. doi:10.1016/j.eurpsy.2007.05.006
10. Zhou Y, Yu F, Duong T. Multiparametric MRI characterization and prediction in autism spectrum disorder using graph theory and machine learning. PLoS One. 2014;9(6):e904055. doi:10.1371/journal.pone.0090405
11. Voineskos AN, Lett TAP, Lerch JP, et al. Neurexin-1 and frontal lobe white matter: an overlapping intermediate phenotype for schizophrenia and autism spectrum disorders. PLoS One. 2011;6(6):e20982. doi:10.1371/journal.pone.0020982
12. Palmen SJ, Hulshoff Pol HE, Kemner C, et al. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol Med. 2005;35(4):561–570. doi:10.1017/s0033291704003496
13. Bailey A, Luthert P, Dean A, et al. A clinicopathological study of autism. Brain. 1998;121(5):889–905. doi:10.1093/brain/121.5.889
14. Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi:10.1001/jama.2011.1638
15. Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi:10.1016/j.neuron.2007.10.016
16. Duerden EG, Mak-Fan KM, Taylor MJ, et al. Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012;5(1):49–66. doi:10.1002/aur.235
17. Haar S, Berman S, Behrmann M, et al. Anatomical abnormalities in autism? Cereb Cortex. 2016;26(4):1440–1452. doi:10.1093/cercor/bhu242
18. Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi:10.1212/wnl.59.2.184
19. Cauda F, Geda E, Sacco K, et al. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry. 2011;82(12):1304–1313. doi:10.1136/jnnp.2010.239111
20. Yu KK, Cheung C, Chua SE, et al. Can Asperger syndrome be distinguished from autism? An anatomic likelihood meta-analysis of MRI studies. J Psychiatry Neurosci. 2011;36(6):412–421. doi:10.1503/jpn.100138
21. Nickl-Jockschat T, Habel U, Michel TM, et al. Brain structure anomalies in autism spectrum disorder–a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33(6):1470–1489. doi:10.1002/hbm.21299
22. Chen R, Jiao Y, Herskovits EH. Structural MRI in autism spectrum disorder. Pediatr Res. 2011;69(5Pt 2):p63R–8R. doi:10.1203/PDR.0b013e318212c2b3
23. Mueller S, Keeser D, Samson AC, et al. Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS One. 2013;8(6):e67329. doi:10.1371/journal.pone.0067329
24. Fu Z, Tu Y, Di X, et al. Transient increased thalamic-sensory connectivity and decreased whole-brain dynamism in autism. NeuroImage. 2019;190:191–204. doi:10.1016/j.neuroimage.2018.06.003
25. Hull JV, Dokovna LB, Jacokes ZJ, et al. Resting-state functional connectivity in autism spectrum disorders: a review. Front Psychiatry. 2017;7:205. doi:10.3389/fpsyt.2016.00205
26. Chien YL, Chen YC, Gau SS. Altered cingulate structures and the associations with social awareness deficits and CNTNAP2 gene in autism spectrum disorder. Neuroimage Clin. 2021;31:102729. doi:10.1016/j.nicl.2021.102729
27. Riedel A, Maier S, Ulbrich M, et al. No significant brain volume decreases or increases in adults with high-functioning autism spectrum disorder and above average intelligence: a voxel-based morphometric study. Psychiatry Res. 2014;223(2):67–74. doi:10.1016/j.pscychresns.2014.05.013
28. Riddle K, Cascio CJ, Woodward ND. Brain structure in autism: a voxel-based morphometry analysis of the Autism Brain Imaging Database Exchange (ABIDE). Brain Imaging Behav. 2017;11(2):541–551. doi:10.1007/s11682-016-9534-5
29. DeRamus TP, Kana RK. Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. Neuroimage (Amst). 2014;7:525–536. doi:10.1016/j.nicl.2014.11.004
30. Chen JA, Peñagarikano O, Belgard TG, et al. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 2015;10:111–144. doi:10.1146/annurev-pathol-012414-040405
31. Loth E, Murphy DG, Spooren W. Defining precision medicine approaches to autism spectrum disorders: concepts and challenges. Front Psychiatry. 2016;7:188. doi:10.3389/fpsyt.2016.00188
32. Lombardo MV, Lai MC, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019;24(10):1435–1450. doi:10.1038/s41380-018-0321-0
33. Foster NE, Doyle-Thomas KA, Tryfon A, et al. Structural gray matter differences during childhood development in autism spectrum disorder: a multimetric approach. Pediatr Neurol. 2015;53(4):350–359. doi:10.1016/j.pediatrneurol.2015.06.013
34. Kaufmann L, Zotter S, Pixner S, et al. Brief report: CANTAB performance and brain structure in pediatric patients with Asperger syndrome. J Autism Dev Disord. 2013;43(6):1483–1490. doi:10.1007/s10803-012-1686-6
35. Cheng Y, Chou KH, Fan YT, et al. ANS: aberrant neurodevelopment of the social cognition network in adolescents with autism spectrum disorders. PLoS One. 2011;6(4):e18905. doi:10.1371/journal.pone.0018905
36. Waiter GD, Williams JH, Murray AD, et al. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22(2):619–625. doi:10.1016/j.neuroimage.2004.02.029
37. Brieber S, Neufang S, Bruning N, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48(12):1251–1258. doi:10.1111/j.1469-7610.2007.01799.x
38. Liu J, Yao L, Zhang W, et al. Gray matter abnormalities in pediatric autism spectrum disorder: a meta-analysis with signed differential mapping. Eur Child Adolesc Psychiatry. 2017;26(8):933–945. doi:10.1007/s00787-017-0964-4
39. Eilam-Stock T, Wu T, Spagna A, et al. Neuroanatomical alterations in high-functioning adults with autism spectrum disorder. Front Neurosci. 2016;10:237. doi:10.3389/fnins.2016.00237
40. Supekar K, Menon V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism. 2015;6:50. doi:10.1186/s13229-015-0042-z
41. Li G, Rossbach K, Jiang W, et al. Reduction in grey matter volume and its correlation with clinical symptoms in Chinese boys with low functioning autism spectrum disorder. J Intellect Disabil Res. 2019;63(2):113–123. doi:10.1111/jir.12552
42. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi:10.1006/nimg.2000.0582
43. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
44. Grizzle R. Wechsler intelligence scale for children. In: Goldstein S, Naglieri JA, editors. Encyclopedia of Child Behavior and Development.
45. Itahashi T, Yamada T, Nakamura M, et al. Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: a multimodal brain imaging study. Neuroimage Clin. 2015;7:155–169. doi:10.1016/j.nicl.2014.11.019
46. Annett M. The binomial distribution of right, mixed and left handedness. Q J Exp Psychol. 1967;19(4):327–333. doi:10.1080/14640746708400109
47. Marteleto MR, Pedromonico MR. Validity of Autism Behavior Checklist (ABC): preliminary study. Braz J Psychiatry. 2005;27(4):295–301. doi:10.1590/s1516-44462005000400008
48. Szatmari P, Bryson SE, Boyle MH, et al. Predictors of outcome among high functioning children with autism and Asperger syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44(4):520–528. doi:10.1111/1469-7610.00141
49. Rellini E, Tortolani D, Trillo S, et al. Childhood Autism Rating Scale (CARS) and Autism Behavior Checklist (ABC) correspondence and conflicts with DSM-IV criteria in diagnosis of autism. J Autism Dev Disord. 2004;34(6):703–708. doi:10.1007/s10803-004-5290-2
50. Li G, Rossbach K, Jiang W, et al. Resting-state brain activity in Chinese boys with low functioning autism spectrum disorder. Ann Gen Psychiatry. 2018;17:47. doi:10.1186/s12991-018-0217-z
51. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
52. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi:10.1016/j.neuroimage.2008.03.061
53. Anteraper SA, Guell X, Hollinshead MO, et al. Functional alterations associated with structural abnormalities in adults with high-functioning autism spectrum disorder. Brain Connect. 2020;10(7):368–376. doi:10.1089/brain.2020.0746
54. Cai J, Hu X, Guo K, et al. Increased left inferior temporal gyrus was found in both low function autism and high function autism. Front Psychiatry. 2018;9(542). doi:10.3389/fpsyt.2018.00542
55. Wang J, Fu K, Chen L, et al. Increased gray matter volume and resting-state functional connectivity in somatosensory cortex and their relationship with autistic symptoms in young boys with autism spectrum disorder. Front Physiol. 2017;8:588. doi:10.3389/fphys.2017.00588
56. Zwaigenbaum L, Young GS, Stone WL, et al. Early head growth in infants at risk of autism: a baby siblings research consortium study. J Am Acad Child Adolesc Psychiatry. 2014;53(10):1053–1062. doi:10.1016/j.jaac.2014.07.007
57. Zielinski BA, Prigge MB, Nielsen JA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137(Pt 6):1799–1812. doi:10.1093/brain/awu083
58. Mak-Fan KM, Taylor MJ, Roberts W, et al. Measures of cortical grey matter structure and development in children with autism spectrum disorder. J Autism Dev Disord. 2012;42(3):419–427. doi:10.1007/s10803-011-1261-6
59. Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi:10.1016/j.brainres.2010.09.101
60. Lange N, Travers BG, Bigler ED, et al. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Res. 2015;8(1):82–93. doi:10.1002/aur.1427
61. Allely CS, Gillberg C, Wilson P. Neurobiological abnormalities in the first few years of life in individuals later diagnosed with autism spectrum disorder: a review of recent data. Behav Neurol. 2014;2014:210780. doi:10.1155/2014/210780
62. Saha S, Chatterjee M, Sinha S, et al. A pioneering study indicate role of GABRQ rs3810651 in ASD severity of Indo-Caucasoid female probands. Sci Rep. 2021;11(1):7010. doi:10.1038/s41598-021-86496-5
63. Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci. 2014;8:92. doi:10.3389/fnsys.2014.00092
64. Riva D, Annunziata S, Contarino V, et al. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL study. Cerebellum. 2013;12(5):676–685. doi:10.1007/s12311-013-0469-8
65. Avanzino L, Tinazzi M, Ionta S, et al. Sensory-motor integration in focal dystonia. Neuropsychologia. 2015;79:288–300. doi:10.1016/j.neuropsychologia.2015.07.008
66. Yoder KJ, Porges EC, Decety J. Amygdala subnuclei connectivity in response to violence reveals unique influences of individual differences in psychopathic traits in a nonforensic sample. Hum Brain Mapp. 2015;36(4):1417–1428. doi:10.1002/hbm.22712
67. Panahi R, Jarollahi F, Akbari M, et al. The relationship between auditory sensory gating and cognitive functions on auditory and visual modalities in primary school children. Iran J Child Neurol. 2019;13(4):53–65.
68. Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32(23):7917–7925. doi:10.1523/JNEUROSCI.0307-12.2012
69. Zhang J, Su J, Wang M, et al. The posterior insula shows disrupted brain functional connectivity in female migraineurs without aura based on brainnetome atlas. Sci Rep. 2017;7(1):16868. doi:10.1038/s41598-017-17069-8
70. Yoshimura S, Sato W, Kochiyama T, et al. Gray matter volumes of early sensory regions are associated with individual differences in sensory processing. Hum Brain Mapp. 2017;38(12):6206–6217. doi:10.1002/hbm.23822
71. Bookheimer SY, Wang AT, Scott A, et al. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14(6):922–932. doi:10.1017/S135561770808140X
72. Libero LE, DeRamus TP, Lahti AC, et al. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex. 2015;66(p):46–59. doi:10.1016/j.cortex.2015.02.008
73. Eack SM, Mazefsky CA, Minshew NJ. Misinterpretation of facial expressions of emotion in verbal adults with autism spectrum disorder. Autism. 2015;19(3):308–315. doi:10.1177/1362361314520755
74. Berenguer C, Roselló B, Colomer C, et al. Children with autism and attention deficit hyperactivity disorder. Relationships between symptoms and executive function, theory of mind, and behavioral problems. Res Dev Disabil. 2018;83:260–269. doi:10.1016/j.ridd.2018.10.001
75. Stroth S, Paye L, Kamp-Becker I, et al. Empathy in females with autism spectrum disorder. Front Psychiatry. 2019;10:428. doi:10.3389/fpsyt.2019.00428
76. Corden B, Critchley HD, Skuse D, et al. Fear recognition ability predicts differences in social cognitive and neural functioning in men. J Cogn Neurosci. 2006;18(6):889–897. doi:10.1162/jocn.2006.18.6.889
77. Murai T. Social cognition and psychiatry. Seishin Shinkeigaku Zasshi. 2013;115(9):1004–1008.
78. Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–340. doi:10.1001/archpsyc.57.4.331
79. Pierce K, Müller R-A, Ambrose J, et al. Face processing occurs outside the fusiform `face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi:10.1093/brain/124.10.2059
80. Piggot J, Kwon H, Mobbs D, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43(4):473–480. doi:10.1097/00004583-200404000-00014
81. Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(4):481–490. doi:10.1097/00004583-200404000-00015
82. Morita T, Kosaka H, Saito DN, et al. Emotional responses associated with self-face processing in individuals with autism spectrum disorders: an fMRI study. Soc Neurosci. 2012;7(3):223–239. doi:10.1080/17470919.2011.598945
83. Mizuno A, Liu Y, Williams DL, et al. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134(Pt 8):2422–2435. doi:10.1093/brain/awr151
84. Mason RA, Williams DL, Kana RK, et al. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi:10.1016/j.neuropsychologia.2007.07.018
85. Greimel E, Schulte-Rüther M, Kircher T, et al. Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. Neuroimage. 2010;49(1):1055–1065. doi:10.1016/j.neuroimage.2009.07.057
86. Pepper KL, Demetriou EA, Park SH, et al. Self-reported empathy in adults with autism, early psychosis, and social anxiety disorder. Psychiatry Res. 2019;281:112604. doi:10.1016/j.psychres.2019.112604
87. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(Pt 3):617–627. doi:10.1093/brain/awn279
88. Uddin LQ. The self in autism: an emerging view from neuroimaging. Neurocase. 2011;17(3):201–208. doi:10.1080/13554794.2010.509320
89. Lombardo MV, Chakrabarti B, Bullmore ET, et al. Atypical neural self-representation in autism. Brain. 2010;133(Pt 2):611–624. doi:10.1093/brain/awp306
90. Crane L, Goddard L. Episodic and semantic autobiographical memory in adults with autism spectrum disorders. J Autism Dev Disord. 2008;38(3):498–506. doi:10.1007/s10803-007-0420-2
91. Lind SE, Bowler DM. Episodic memory and episodic future thinking in adults with autism. J Abnorm Psychol. 2010;119(4):896–905. doi:10.1037/a0020631
92. Northoff G, Heinzel A, de Greck M, et al. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi:10.1016/j.neuroimage.2005.12.002
93. Li Q, Xiang G, Song S, et al. Trait self-control mediates the association between resting-state neural correlates and emotional well-being in late adolescence. Soc Cogn Affect Neurosci. 2021;16(6):632–641. doi:10.1093/scan/nsab046
94. Lemogne C, le Bastard G, Mayberg H, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4(3):305–312. doi:10.1093/scan/nsp008
95. Koush Y, Pichon S, Eickhoff SB, et al. Brain networks for engaging oneself in positive-social emotion regulation. NeuroImage. 2019;189:106–115. doi:10.1016/j.neuroimage.2018.12.049
96. Kelley WM, Macrae CN, Wyland CL, et al. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi:10.1162/08989290260138672
97. Supekar K, Kochalka J, Schaer M, et al. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain. 2018;141(9):2795–2805. doi:10.1093/brain/awy191
98. O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519(18):3599–3639. doi:10.1002/cne.22735
99. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–239. doi:10.1016/j.tics.2012.02.007
100. Bariselli S, Tzanoulinou S, Glangetas C, et al. SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci. 2016;19(7):926–934. doi:10.1038/nn.4319
101. Krishnan V, Stoppel DC, Nong Y, et al. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature. 2017;543(7646):507–512. doi:10.1038/nature21678
102. Rodier PM, Arndt TL. The brainstem in autism. In: Bauman ML, Kemper TL, editors. The Neurobiology of Autism.
103. Fatemi SH, Aldinger KA, Ashwood P, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807. doi:10.1007/s12311-012-0355-9
104. Webb SJ, Sparks BF, Friedman SD, et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172(1):61–67. doi:10.1016/j.pscychresns.2008.06.001
105. Rojas DC, Peterson E, Winterrowd E, et al. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi:10.1186/1471-244X-6-56
106. Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62(12):1366–1376. doi:10.1001/archpsyc.62.12.1366
107. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi:10.1016/j.neuroimage.2008.08.039
108. Shafer RL, Wang Z, Bartolotti J, et al. Visual and somatosensory feedback mechanisms of precision manual motor control in autism spectrum disorder. J Neurodev Disord. 2021;13(1):32. doi:10.1186/s11689-021-09381-2
109. Chi IJ, Lin LY. Relationship between the performance of self-care and visual perception among young children with autism spectrum disorder and typical developing children. Autism Res. 2021;14(2):315–323. doi:10.1002/aur.2367
110. D’Mello AM, Stoodley CJ. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408. doi:10.3389/fnins.2015.00408
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.