Back to Journals » OncoTargets and Therapy » Volume 12
Aberrantly High Expression Of NOK/STYK1 Is Tightly Associated With The Activation Of The AKT/GSK3β/N-Cadherin Pathway In Non-Small Cell Lung Cancer
Authors Huang Z , Ma N, Xiong YL, Wang L, Li WM, Lai YY, Zhang CX, Zhang ZP, Li XF, Zhao JB
Received 26 March 2019
Accepted for publication 31 October 2019
Published 27 November 2019 Volume 2019:12 Pages 10299—10309
DOI https://doi.org/10.2147/OTT.S210014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Zhao Huang,1,* Nan Ma,2,* Yan-Lu Xiong,1,* Lei Wang,1 Wei-Miao Li,1 Yuan-Yang Lai,1 Chen-Xi Zhang,1 Zhi-Pei Zhang,1 Xiao-Fei Li,1 Jin-Bo Zhao1
1Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, Xi’an, Shaanxi 710038, People’s Republic of China; 2Department of Ophthalmology, Tangdu Hospital, Fourth Military Medical University, Xi’an, Shaanxi 710038, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin-Bo Zhao; Xiao-Fei Li
Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, 1 Xinsi Road, Baqiao, Xi’an, Shaanxi 710038, People’s Republic of China
Tel +86 29 8471 7367
Fax +86 29 8471 7367
Email [email protected]; [email protected]
Purpose: High metastasis is a leading risk factor for the survival of non-small cell lung cancer (NSCLC) and epithelial-mesenchymal transition (EMT) is a vital step of metastasis. The expression of novel oncogene with kinase domain (NOK) has been observed in some human malignancies, including non-small cell lung cancer (NSCLC); however, the biological function of NOK in NSCLC remains unclear. In the study, we explored the function of NOK in NSCLC, with an aim to elucidate the relevant underlying mechanisms.
Patients and methods: We investigate the expression of NOK, p-Akt, p-GSK-3β, E-cadherin and N-cadherin expression by immunohistochemical analysis using tissue microarrays of 72 paired NSCLC samples of cancerous and adjacent normal tissues. The associations between NOK expression and clinicopathological factors, overall survival, other proteins were assessed. Immunofluorescence analysis of NSCLC tissues was performed to study the location of NOK, Akt and GSK-3β. Up or down-regulated of NOK were conducted in two NSCLC cell lines to analyze its impact on AKT/GSK3β pathway.
Results: Statistical analysis revealed NOK expression increased in NSCLC tissues compared with normal tissues (P<0.05). It also showed that low NOK expression were associated with a higher possibility of non-lymphatic metastasis, an early pN stage and clinical stage (P<0.05). Moreover, NOK expression was positively correlated with the expression of oncogene p-Akt (Thr308), p-GSK-3β (Ser9) and N-cadherin (P<0.05). Immunofluorescence analysis of NSCLC tissues revealed that NOK is co-located with Akt and GSK-3β. Further study in NSCLC cell lines revealed that NOK overexpression can activate the AKT/GSK3β pathway. Conversely, knockdown of NOK can suppress the AKT/GSK3β pathway.
Conclusion: Our results suggest that NOK overexpression correlated significantly with lymphatic metastasis, advanced pN and clinical stage in NSCLC. And NOK may promote EMT by activating the AKT/GSK3β/N-cadherin pathway in NSCLC.
Keywords: NOK/STYK1, epithelial-mesenchymal transition, Akt, GSK3β, N-cadherin, non-small cell lung cancer
Introduction
Lung cancer is the primary cause of cancer-related deaths globally worldwide. Non-small cell lung cancer (NSCLC) comprises a majority of lung cancer cases. After undergoing curative surgical resection, large numbers of NSCLC patients still die from relapse and metastases.1,2
Epithelial-mesenchymal transition (EMT) plays a critical role during tumor metastasis. EMT is associated with the loss of epithelial properties and gain of mesenchymal phenotypes, whereby a decreased expression of E-cadherin and an increased expression of N-cadherin is observed.3,4 It is well-known that EMT mediates a good deal of cancer malignant transformation.5–9 However, there is an urgent need to comprehensively explain the mechanisms that regulate EMT in NSCLC.
Novel oncogene with kinase domain (NOK), also known as serine threonine tyrosine kinase 1 (STYK1), consists of a kinase domain, intracellular domain, and transmembrane domain. NOK, a member of the receptor-type protein tyrosine kinase-like family,10 is able to enhance tumor cell proliferation and tumor progression by acting as a growth factor membrane receptor. Aberrant expression of NOK has been found in a wide range of cancers, including lung, ovarian, breast, colorectal, prostate, and renal cell cancers.11–16 Recently, NOK was found to be capable of promoting the malignant transformation of tumors in some cancers by activating the PI3K/AKT pathway.17,18 All these studies indicate that NOK overexpression is associated with cancer progression, and NOK can induce EMT by activating the Akt pathway. However, the pathological function of NOK in the malignant transformation of NSCLC remains unknown. In this study, we estimated the NOK expression and its correlation with the Akt pathway and EMT marker expression in NSCLC. Furthermore, we investigated the possible signaling pathways by which NOK could contribute to EMT. These findings may provide a clue to investigate the oncogenic activity of NOK and identify the possible underlying mechanisms of EMT in NSCLC.
Materials And Methods
Patients And Tissue Specimens
Non-small cell lung cancer tissue specimens from 72 patients diagnosed with NSCLC were obtained from the Department of Thoracic Surgery, Tangdu Hospital, between 2009 and 2014. None of the patients had been treated with any preoperative chemotherapy or radiotherapy. Cancerous tissues and adjacent normal lung tissues were obtained from each individual after the resection of the tumors. All tissue specimens (n=144) were snap-frozen in liquid nitrogen immediately after the collection for subsequent analysis. The average age was 60.33 years (range, 30–80 years) with a mean survival time of 19.46 months. The clinical stages were determined according to the pathological tumor/node/metastasis (TNM) classification system (7th edition) for malignant tumors established by the International Union Against Cancer (UICC).19 The experimental protocols were approved by the Regional Ethics Committee for Clinical Research of the Fourth Military Medical University. Written informed consent was obtained from all patients. The study was performed in accordance with the Declaration of Helsinki.
Cell Culture
The human NSCLC cell lines A549 and SPC-A-1 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium (Hyclone, Logan, TX, USA) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin, and 100 μg/mL streptomycin in a humidified 5% CO2-enriched atmosphere at 37°C.
Lentivirus And Plasmids Transfection
For the overexpression of NOK, the A549 cells were transfected separately with 1×107 titration units of packaging lentiviruses (STYK1-OE lentivirus 42863–1, and negative control lentivirus con238) synthesized by Gene Chem (Shanghai, China). The SPC-A-1 cells were also transfected separately with 2×107 titration units of packaging lentiviruses. Fresh RPMI-1640 containing 2 μg/mL puromycin (Gene Chem) and 10% FBS was used to culture the A549 and SPC-A-1 cells after three days of transfection. After two weeks, the culture was diluted with fresh puromycin-containing medium and grown under puromycin selection pressure. Several clones from each transfection group were then selected and screened for NOK protein expression by Western blotting. Transfected cell clone mixtures from each transfection group were used for further experiments.
For the knockdown of NOK, the A549 cell lines were transfected with the plasmids with two human NOK-targeting shRNAs and one NC shRNA (Gene Chem) respectively. Stable A549 cell lines were screened for 7 days using 2 mg/mL puromycin (Gene Chem).
Tissue Microarray Construction
The 144 specimens were fixed in 4% paraformaldehyde for 24 h, and then dehydrated using a graded series of ethanol solutions and embedded in paraffin. A tissue-arraying instrument (Quant Center Pannoramic 250/MIDI, 3DHISTECH, Ltd., Budapest, Hungary) was used to select the typical area to construct a tissue microarray (TMA) based on the observation of hematoxylin and eosin (HE)-stained sections. Every TMA comprised 72 tumorous tissues and 72 corresponding adjacent normal tissues.
Immunohistochemistry
Paraformaldehyde-fixed NSCLC tissue blocks were cut into 5-μm TMA sections and embedded in paraffin. The sections of TMA were then dewaxed in xylene and rehydrated using a graded series of ethanol solutions. Endogenous peroxidase activity was blocked by immersing the sections in a solution of 3% hydrogen peroxide for 30 min. Then, the sections were microwaved in 10 mM citrate buffer (pH 6.0) at 95°C for 20 min to perform antigen retrieval. After being blocked with goat serum for 30 min, the sections were incubated with the primary antibody (anti-Styk1 ab97451,20 anti-AKT1 (phospho S473) ab81283,21 anti-pan-AKT (phospho T308) ab38449,22 anti-GSK3 beta (phospho Y216) ab75745,23 anti-GSK3 beta (phospho S9) ab75814,24 anti-E Cadherin ab4077225,26 from Abcam, Cambridge, UK; and N-cadherin (D4R1H)27 from Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. After being washed in PBS (phosphate-buffered saline), the sections were incubated with the appropriate horseradish peroxidase (HRP)-labeled goat anti-rabbit/mouse antibodies. Then, the samples were incubated with reagents of the DAB Elite kit (Dako, Denmark), and counterstained with hematoxylin.
Immunohistochemistry Evaluation
For the evaluation of protein levels, semi-quantitative immunohistochemistry (IHC) was performed according to the percentage of positive cells and the intensity of staining. H-score was assigned for each antigen; this score was derived from the equation: H = Σ(pi*i), where “pi” represents the percentage of positive cells and “i” represents the intensity.28 And The intensity of specific staining was characterized as negative (0), weak (1+), moderate (2+), and strong (3+). An H-score was calculated using the following formula:29

The cut-off value used to categorize the NOK expression levels as high and low was defined by using a median value of the scores. Samples with low NOK expression were those with scores below or equal to the cut-off value, while those with high NOK expression had scores above the cut-off value. Pannoramic 250/MIDI (3DHISTECH) was used to scan and digitalize the slides. Pannoramic Viewer v. 2.0 and NuclearQuant application for PV v.2.0.0.46136, both of which are manufactured by 3DHISTECH, were used to analyze the slides.30
HE Staining And Immunofluorescence
Paraformaldehyde-fixed blocks of cancerous tissues with high NOK expression from 20 patients (10 squamous cell carcinoma and 10 adenocarcinoma patients) were cut into 5-μm sections and embedded in paraffin. A continuous adjustment of slides was performed for the HE staining and immunofluorescence analysis. Histology of the NSCLC tissue cross-sections was observed by performing HE staining following the fixation of the sections in 4% paraformaldehyde for 24 h. The tissue sections were dewaxed in xylene and rehydrated using a graded series of ethanol solutions. To perform antigen retrieval, the sections were microwaved in EDTA buffer (pH 8.0). After being subjected to blocking for 30 min, the slides were incubated with the first primary antibody (Anti-pan-Akt, ab8805; Abcam) at 4°C overnight. Next, the slides were washed in PBS and incubated with the corresponding HRP-labeled secondary antibodies for 50 min, and then with the CY3 reagent for 10 min. After being washed in TBST, the slides were microwaved to remove the first primary antibodies and secondary antibodies that had been bound to the tissues. Next, according to the previous process, the slides were incubated with the other primary antibodies (anti-GSK3 beta [G8], ab2602, Abcam, Cambridge, UK; NOK, sc-81701, Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight, and then with the corresponding HRP-labeled secondary antibodies, FITC, and CY5 reagent. The nuclei were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI).
Immunofluorescence Analysis
Ten randomly selected fields from each section were viewed using Caseviewer2.0 (3DHISTECH) at a 900× magnification. The percentage of tumor cells with positive NOK expression, positive NOK & Akt expression, and positive NOK & Akt & GSK3β expression in each field was quantified. All slides were evaluated by three independent investigators, and the final outcome reported was the mean of the percentage from the three investigators.
Western Blotting
Cells were lysed with RIPA buffer supplemented with a protease inhibitor cocktail and phosphatase. The lysates were boiled in sodium dodecyl sulfate (SDS) loading buffer and the proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). The resultant protein bands were transferred onto a nitrocellulose membrane. Then, the membrane was incubated with primary antibodies (anti-Styk1 ab97451, anti-AKT1 (phospho S473) ab81283, anti-pan-AKT (phospho T308) ab3844, anti-GSK3 beta (phospho Y216) ab75745, anti-GSK3 beta (phospho S9) ab75814, anti-E Cadherin ab40772 from Abcam; snail (C15D3) and N-cadherin (D4R1H) from Cell Signaling Technology), all of which were dissolved in a 5% non-fat milk-based solvent, followed by incubation with HRP-conjugated anti-rabbit/mouse secondary antibodies. The protein bands were visualized by using the ECL detection reagent (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
All statistical analyses were performed using the SPSS 24.0 software (SPSS, Inc., Chicago, IL, USA). Graphs were constructed using the GraphPad Prism5 software (GraphPad Software Inc., CA, USA). Paired t-test was used to compare the NOK expression between tumorous tissues and the corresponding adjacent normal tissues. K-M survival analysis was used to investigate the relationship between NOK expression and the clinical outcome (overall survival) of NSCLC patients. Pearson correlation analysis was used to evaluate the relationship between NOK expression and the levels of other proteins. A one-way ANOVA was used to compare the significance of differences among groups, and the numeric data were presented as the means ± standard deviations. P < 0.05 were considered statistically significant.
Results
The Upregulated Expression Of NOK In Human NSCLC Tissues Is Correlated With Metastasis In NSCLC
The expression of NOK was mainly localized in the cancer cell cytoplasm; NOK expression in the nuclei was sparse (Figure 1A). The protein levels of NOK in the samples of NSCLC were assessed by TMA-based IHC to analyze the expression pattern of NOK. According to the IHC results, NOK was observably upregulated in the cancerous tissues, compared to the case for the normal tissues. A paired t-test revealed that there was a significant difference between the cancerous and normal specimen groups (P<0.0001; Figure 1B). The K-M survival analysis revealed that patients with high NOK expression (median survival time: 12.0 months) showed a trend of shorter OS than those of low NOK expression (median survival time: 19.5 months, P=0.0790; Supplementary Figure 1), which was consistent with our previous study.12 The association between NOK expression and the clinicopathological variables was analyzed (Table 1). The NOK expression was significantly associated with sex (P=0.013), pN stage (N0 vs.N1, p<0.001; N0 vs.N2, p=0.017), lymphatic metastasis (P<0.001), and clinical stage (P=0.013). No significant relationship was found between the NOK expression and age (P=0.157), smoking history (P=0.594), pT stage (P=1.000), and histological type (P=1.000).
 |
Table 1 Correlation Between NOK Expression And Clinical Variables Of The NSCLC Cases |
NOK Expression Positively Correlates With The p-Akt (Thr308), p-GSK-3β (Ser9), And N-Cadherin Levels In NSCLC Tissues
The inter-relationship between NOK expression and some vital biomarkers of malignant proliferation, including the oncogene p-Akt (Thr308, Ser473), the important multifunctional protein kinase p-GSK3β (Tyr216, Ser9), and two EMT markers (E-cadherin and N-cadherin), was investigated. In all cases, it was seen that the expression of p-Akt (Ser473) and p-GSK-3β (Tyr216) was steady; however, in samples with high NOK expression, it was seen that the expression of p-Akt (Thr308), p-GSK-3β (Ser9), and N-cadherin was upregulated and the E-cadherin expression was downregulated (Figure 2A). As shown in Figure 2B, the NOK expression was positively correlated with the expression of p-Akt (Thr308) (P=0.0112, r=0.2974), p-GSK-3β (Ser9) (P=0.0003, r=0. 4135), and N-cadherin (P=0.0002, r=0.4212), but not with that of p-Akt (Ser473) (P=0.6526, r=0.0029), p-GSK-3β (Tyr216) (P=0.8547, r=0.0005), or E-cadherin (P=0.9614, r=0.0001).
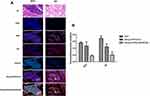
NOK Is Co-Located With Akt And GSK-3β In NSCLC Tissues
In order to assess the spatial location of NOK with regards to that of Akt and GSK-3β, the HE and immunofluorescence staining of NOK, Akt, and GSK-3β was performed in cancerous tissue sections from 10 squamous cell carcinoma and 10 adenocarcinoma patients with high NOK expression. The immunofluorescence staining showed that NOK was co-located with Akt and GSK-3β in 16.70±2.97% of tumor cells in squamous cell carcinoma cases and 20.53±11.38% of tumor cells in adenocarcinoma cases (Figure 3).
NOK Enhances The Activation Of The Akt/Gsk3β/N-Cadherin Signaling Pathway In NSCLC
Two NSCLC cell lines (A549 and SPC-A-1) were obtained and assessed to further examine the expression of NOK, p-Akt (Thr308, Ser473), p-GSK3β (Tyr216, Ser9), E-cadherin, and N-cadherin. In A549 cells, NOK, p-Akt (Ser473, Thr308), p-GSK-3β (Ser9), snail, E-cadherin, and N-cadherin were expressed at higher levels, compared to the case in SPC-A-1 cells (Figure 4A). There was a significant difference between the NOK, p-Akt (Thr308), p-GSK-3β (Ser9), snail, and E-cadherin expression levels in A549 and SPC-A-1 cells (Figure 4B).
To explore the mechanism underlying the involvement of NOK in NSCLC, two NSCLC cell lines (A549 and SPC-A-1) were transfected with NOK-overexpressing lentiviral constructs. It was found that the expression of p-Akt (Thr308), which was critical for Akt activation, increased, while the expression of p-Akt (Ser473), which was a surrogate marker of Akt activity, decreased. Moreover, the expression of p-GSK-3β (Ser9), snail, and N-cadherin increased, and the expression of p-GSK-3β (Tyr216) and E-cadherin decreased (Figure 4C).
Furthermore, a NSCLC cell line (A549) was transfected with the NOK-knockdown plasmids (NOK-shRNA). It was found that the expression of p-Akt (Thr308) and p-Akt (Ser473), p-GSK-3β (Ser9), snail, and N-cadherin decreased, and the expression of p-GSK-3β (Tyr216) and E-cadherin increased (Figure 4D).
Discussion
Our own previous studies have demonstrated that the overexpression of NOK is significantly associated with lymphatic metastasis in NSCLC.12 However, the relationship between NOK and EMT has not yet been elucidated and fully defined mechanistically. In this study, we verify that NOK has a tumorigenic activity and may correlate with tumor metastasis. Further studies have shown that NOK was co-located with Akt and GSK-3β, and that the overexpression of NOK can enhance the AKT/GSK3β/N-cadherin signaling pathway, which could contribute to EMT in NSCLC.
A growing body of evidence has indicated that NOK may be associated with the activation of Akt and inactivation of GSK3β. Some experimental efforts have shown that NOK could promote the EMT by activating the PI3K/Akt signaling pathway in gallbladder carcinoma and hepatocellular carcinoma.17,18 In breast cancer, NOK can form a tripartite complex with Akt and GSK3β, and promote the phosphorylation of GSK3β at Ser9, mediated by the phosphorylation of Akt at Thr308.31 With the phosphorylation at Ser9, the enzymatic activity of GSK3β is inhibited, which results in the upregulation of snail and downregulation of E-cadherin.32 However, the roles of NOK in the activation of the PI3K/Akt/GSK3β pathways in NSCLC is still ambiguous.
According to the immunohistochemical evaluation in this study, it was seen that there is a significantly positive correlation between high NOK levels and the expression of p-Akt (Thr308), p-GSK3β (Ser9), and N-cadherin in NSCLC tissues, which has, to the best of our knowledge, not been mentioned in other studies. IHC of TMA was performed using a computer and with an objective standard; this made our result more reliable and precise. Furthermore, slides of the samples with high NOK expression (n=20) were selected for three-color immunofluorescence analysis, to study the potential co-location of NOK, Akt, and GSK3β. The results revealed that the co-location of NOK, Akt, and GSK3β was ubiquitous in the NSCLC tissues.
These results suggest that NOK could interact with Akt and GSK3β and might promote the activation of Akt and inactivation of GSK3β in NSCLC, which needs to be further proved in vitro. However, the antibodies against Akt and GSK3β that we used for our immunofluorescence analysis were not phosphorylated antibodies; this may have a negative impact on our results.
Our further in vitro exprements confirmed that NOK may contribute to EMT particularly by activating the p-Akt (Thr308)/p-GSK3β(Ser9)/N-cadherin signaling pathway in NSCLC. Two NSCLC cell lines with a significant difference of NOK expression were chosen to perform further studies. The differences between the protein levels in these two normal cell lines also indicate that high levels of NOK may be positively related with the expression of p-Akt (Thr308), p-GSK3β (Ser9), and N-cadherin; this is consistent with the results obtained in case of NSCLC tissues. In case of NOK overexpression, the expression of p-Akt (Thr308), p-GSK3β (Ser9), snail, and N-cadherin increased, while the expression of p-GSK3β (Tyr216) (activated form of GSK3β) and E-cadherin decreased; Then, the A549 cell line was chosen to perform the knockdown studies of NOK. In case of NOK knockdown, the expression of p-GSK3β (Tyr216) and E-cadherin increased, while the expression of p-Akt (Thr308), p-GSK3β (Ser9), snail, and N-cadherin decreased. The results of NOK-OE and NOK-KD in the cell lines revealed that NOK may play an important role in EMT progress of NSCLC via the activation of the p-Akt (Thr308)/p-GSK3β (Ser9)/N-cadherin signaling pathway. However, these results were limited, as currently, we cannot identify the effects of NOK on the EMT phenotype and its importance in the Akt/GSK3β signaling pathway.
These in vitro results also suggest an ambiguous role of NOK in the phosphorylation of Akt at Ser473 in NSCLC. The activation of Akt is regulated by a dual mechanism that requires its translocation to the plasma membrane and phosphorylation.33 The phosphorylation at Thr308 is essential and sufficient for the activation of Akt, but with the phosphorylation at Ser473, the activation of Akt could be maximized.33–35 In the A549 cells, under conditions of NOK overexpression, the expression of both p-Akt (Thr308) and p-Akt (Ser473) was upregulated. On the other hand, in SPC-A-1 cells, under conditions of NOK overexpression, only the expression of p-Akt (Thr308) was upregulated. These results reveal that NOK may not increase Akt phosphorylation in a manner completely identical to that in case of Akt activation in NSCLC cell lines from different backgrounds; the function of NOK in breast cancer is thus, different.31
In summary, our findings revealed that the overexpression of NOK may promote EMT by activating the AKT/GSK3β/N-cadherin pathway in NSCLC. Conversely, the knockdown of NOK may inhibit EMT by suppressing the AKT/GSK3β/N-cadherin pathway in NSCLC. The above results indicate that the mechanism whereby NOK modulates the Akt/GSK3β/N-cadherin pathway could be utilized as a target for investigating EMT in NSCLC. In the near future, we plan to investigate the function of NOK in EMT in more specific and comprehensive ways and further elucidate the relationship between NOK and the Akt/GSK3β signaling pathway.
Abbreviations
NOK, novel oncogene with kinase domain; STYK1, Serine threonine tyrosine kinase 1; EMT, epithelial-mesenchymal transition; NSCLC, non-small cell lung cancer; AKT, protein kinase B; GSK3β, glycogen synthase kinase 3β; SPSS, statistical package for the social science; HRP, horseradish peroxidase; PI3K, phosphatidylinositol 3 kinase; TMA, tissue microarray.
Acknowledgments
We would like to thank all the members of the Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, Xi’an, China. This work was supported by the National Natural Science Foundation of China (81572252).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Higgins KA, Chino JP, Ready N, et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol. 2012;7(7):1141–1147. doi:10.1097/JTO.0b013e3182519a42
2. Matsumura Y, Hishida T, Shimada Y, et al. Impact of extratumoral lymphatic permeation on postoperative survival of non-small-cell lung cancer patients. J Thorac Oncol. 2014;9(3):337–344. doi:10.1097/JTO.0000000000000073
3. Wrighton KH. Cell migration: EMT promotes contact inhibition of locomotion. Nat Rev Mol Cell Biol. 2015;16(9):518. doi:10.1038/nrm4045
4. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi:10.1038/s41580-018-0080-4
5. Caramel J, Papadogeorgakis E, Hill L, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24(4):466–480. doi:10.1016/j.ccr.2013.08.018
6. Lee CH, Chang JS, Syu SH, et al. IL-1beta promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230(4):875–884. doi:10.1002/jcp.24816
7. Chen J, Yuan W, Wu L, et al. PDGF-D promotes cell growth, aggressiveness, angiogenesis and EMT transformation of colorectal cancer by activation of Notch1/Twist1 pathway. Oncotarget. 2017;8(6):9961–9973. doi:10.18632/oncotarget.14283
8. Huang Q, Pu M, Zhao G, et al. Tg737 regulates epithelial-mesenchymal transition and cancer stem cell properties via a negative feedback circuit between snail and HNF4alpha during liver stem cell malignant transformation. Cancer Lett. 2017;402:52–60. doi:10.1016/j.canlet.2017.05.005
9. Frixa T, Sacconi A, Cioce M, et al. MicroRNA-128-3p-mediated depletion of drosha promotes lung cancer cell migration. Carcinogenesis. 2018;39(2):293–304. doi:10.1093/carcin/bgx134
10. Liu L, Yu XZ, Li TS, et al. A novel protein tyrosine kinase NOK that shares homology with platelet- derived growth factor/fibroblast growth factor receptors induces tumorigenesis and metastasis in nude mice. Cancer Res. 2004;64(10):3491–3499. doi:10.1158/0008-5472.CAN-03-2106
11. Cao Q, Chen M, Li Z, et al. High Novel Oncogene with Kinase-Domain (NOK) gene expression is associated with the progression of renal cell carcinoma. Clin Lab. 2016;62(1–2):179–186. doi:10.7754/Clin.Lab.2015.150626
12. Chen P, Li WM, Lu Q, et al. Clinicopathologic features and prognostic implications of NOK/STYK1 protein expression in non-small cell lung cancer. BMC Cancer. 2014;14:402. doi:10.1186/1471-2407-14-402
13. Jackson KA, Oprea G, Handy J, Kimbro KS. Aberrant STYK1 expression in ovarian cancer tissues and cell lines. J Ovarian Res. 2009;2(1):15. doi:10.1186/1757-2215-2-15
14. Moriai R, Kobayashi D, Amachika T, Tsuji N, Watanabe N. Diagnostic relevance of overexpressed NOK mRNA in breast cancer. Anticancer Res. 2006;26(6c):4969–4973.
15. Orang AV, Safaralizadeh R, Hosseinpour Feizi MA, Somi MH. Diagnostic relevance of overexpressed serine threonine tyrosine kinase/novel oncogene with kinase domain (STYK1/NOK) mRNA in colorectal cancer. Asian Pac J Cancer Prev. 2014;15(16):6685–6689. doi:10.7314/APJCP.2014.15.16.6685
16. Chung S, Tamura K, Furihata M, et al. Overexpression of the potential kinase serine/threonine/tyrosine kinase 1 (STYK 1) in castration-resistant prostate cancer. Cancer Sci. 2009;100(11):2109–2114. doi:10.1111/j.1349-7006.2009.01277.x
17. Hu YP, Wu ZB, Jiang L, et al. STYK1 promotes cancer cell proliferation and malignant transformation by activating PI3K-AKT pathway in gallbladder carcinoma. Int J Biochem Cell Biol. 2018;97(undefined):16–27. doi:10.1016/j.biocel.2018.01.016
18. Wang Z, Qu L, Deng B, et al. STYK1 promotes epithelial-mesenchymal transition and tumor metastasis in human hepatocellular carcinoma through MEK/ERK and PI3K/AKT signaling. Sci Rep. 2016;6(undefined):33205. doi:10.1038/srep33205
19. Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi:10.1097/JTO.0b013e31812f3c1a
20. Hu L, Chen HY, Cai J, et al. Serine threonine tyrosine kinase 1 is a potential prognostic marker in colorectal cancer. BMC Cancer. 2015;15(undefined):246. doi:10.1186/s12885-015-1285-y
21. Tankiewicz-Kwedlo A, Hermanowicz JM, Domaniewski T, et al. Simultaneous use of erythropoietin and LFM-A13 as a new therapeutic approach for colorectal cancer. Br J Pharmacol. 2018;175(5):743–762. doi:10.1111/bph.v175.5
22. Feng FB, Qiu HY. Effects of artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomed Pharmacother. 2018;102:1209–1220. doi:10.1016/j.biopha.2018.03.142
23. Jorge-Torres OC, Szczesna K, Roa L, et al. Inhibition of Gsk3b reduces Nfkb1 signaling and rescues synaptic activity to improve the rett syndrome phenotype in Mecp2-Knockout mice. Cell Rep. 2018;23(6):1665–1677. doi:10.1016/j.celrep.2018.04.010
24. Guinot A, Oeztuerk-Winder F, Ventura JJ. miR-17-92/p38alpha dysregulation enhances Wnt signaling and selects Lgr6+ cancer stem-like cells during lung adenocarcinoma progression. Cancer Res. 2016;76(13):4012–4022. doi:10.1158/0008-5472.CAN-15-3302
25. Xiong D, Zhu SQ, Wu YB, et al. Ring finger protein 38 promote non-small cell lung cancer progression by endowing cell EMT phenotype. J Cancer. 2018;9(5):841–850. doi:10.7150/jca.23138
26. Ramos-Gomes F, Bode J, Sukhanova A, et al. Single- and two-photon imaging of human micrometastases and disseminated tumour cells with conjugates of nanobodies and quantum dots. Sci Rep. 2018;8(1):4595. doi:10.1038/s41598-018-22973-8
27. Zhao B, Huang Z, Qin Z, et al. Enhancement of histone deacetylase inhibitor sensitivity in combination with cyclin-dependent kinase inhibition for the treatment of oral squamous cell carcinoma. Cell Physiol Biochem. 2019;53(1):141–156.
28. Budwit-Novotny DA, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425.
29. Azim HA, Peccatori FA, Brohée S, et al. RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res. 2015;17(undefined):24. doi:10.1186/s13058-015-0538-7
30. Xiong Y, Wang M, Zhao J, et al. SIRT3 is correlated with the malignancy of non-small cell lung cancer. Int J Oncol. 2017;50(3):903–910. doi:10.3892/ijo.2017.3868
31. Li J, Wu F, Sheng F, et al. NOK/STYK1 interacts with GSK-3beta and mediates Ser9 phosphorylation through activated Akt. FEBS Lett. 2012;586(21):3787–3792. doi:10.1016/j.febslet.2012.09.011
32. Zhou BP, Deng J, Xia W, et al. Dual regulation of snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940. doi:10.1038/ncb1173
33. Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8(1):55–62. doi:10.1016/S0959-437X(98)80062-2
34. Dangelmaier C, Manne BK, Liverani E, Jin J, Bray P, Kunapuli SP. PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb Haemost. 2014;111(3):508–517. doi:10.1160/TH13-06-0484
35. Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–871. doi:10.1016/j.devcel.2006.10.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.