Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Aberrant Thalamic-Centered Functional Connectivity in Patients with Persistent Somatoform Pain Disorder
Authors Sun X, Pan X, Ni K, Ji C, Wu J, Yan C, Luo Y
Received 18 September 2019
Accepted for publication 11 January 2020
Published 23 January 2020 Volume 2020:16 Pages 273—281
DOI https://doi.org/10.2147/NDT.S231555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Xia Sun,1,* Xiandi Pan,2,* Kaiji Ni,1 Chenfeng Ji,1 Jiaxin Wu,3 Chao Yan,4 Yanli Luo1
1Department of Psychological Medicine, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 2Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Department of Psychiatry, Tongji Hospital of Tongji University, Shanghai, People’s Republic of China; 4Key Laboratory of Brain Functional Genomics (MOE&STCSM), Shanghai Changning-ECNU Mental Health Center, School of Psychology and Cognitive Science, East China Normal University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanli Luo
Department of Psychological Medicine, Renji Hospital, Shanghai Jiaotong University School of Medicine, Pujian Road 160, Shanghai 200127, People’s Republic of China
Tel +86-21-68382998
Email [email protected]
Chao Yan
Key Laboratory of Brain Functional Genomics (MOE&STCSM), Shanghai Changning-ECNU Mental Health Center, School of Psychology and Cognitive Science, East China Normal University, North Zhongshan Road 3663, Shanghai 200062, People’s Republic of China
Tel +862162232963
Email [email protected]
Purpose: Recent task-based fMRI studies have shown that Persistent Somatoform Pain Disorder (PSPD) patients demonstrated aberrant activity in a wide range of brain regions associated with sensation, cognition and emotion. However, these specific task-based studies could not clearly uncover the alterations in the spontaneous brain networks that were associated with the general pain-related symptoms in PSPD.
Patients and Methods: In the present study, 13 PSPD patients and 23 matched healthy controls (HCs) were enrolled. Resting state and 3D structural imaging data were collected during magnetic resonance imaging (MRI) scans. Ninety regions of interest (ROIs) were selected from the automated anatomical labeling (AAL) template. The functional connectivity toolbox “CONN” was used to calculate the functional connectivity (FC) coefficients.
Results: Our results showed that PSPD patients exhibited increased FCs between the left thalamus and the right amygdala, the right hippocampus, and multiple sub-regions of the occipital lobe when compared to HCs. Correlation analysis revealed a negative correlation between the left thalamus-right amygdala FC and the level of anxiety in PSPD patients.
Conclusion: These findings suggest that the altered FC between thalamus and amygdala may be the neural mechanisms underlying the pain-related anxiety in PSPD.
Keywords: persistent somatoform pain disorder, functional magnetic resonance imaging, resting-state, functional connectivity
Introduction
Persistent somatoform pain disorder (PSPD), also called somatoform pain disorder or pain disorder, is known as a special type of somatoform disorders. It is characterized by the predominant complaint of persistent and distressing pain, which could not be sufficiently explained by a physiological process or a physical disorder (ICD-10, Version: 2016). Since the pathogenesis of this disease remains still unclear, there is nearly no effective treatment, which not only gravely affects the living conditions of the patients but also brings undue burdens to the medical system.1 Therefore, it is compelling to investigate the underlying psychopathology of PSPD symptoms.
In the past years, with the rise of brain imaging research, the neurological mechanism of PSPD was gradually revealed. Structural magnetic resonance imaging researcher exhibited that cortical thickness of patients with chronic pain disorder was thinner than healthy controls, where were localized to regions that correspond to sensory and affective dimensions of pain processing, including the left precentral, postcentral gyri, left inferior temporal sulcus, right middle frontal, inferior parietal gyri, and right anterior temporal pole.2 During the cognitive task, patients with somatoform pain disorder have decreased prefrontal brain activation compared with healthy controls.3 By using noxious or stress stimuli during the functional MRI (fMRI) scanning, researchers have found that patients with PSPD demonstrated dysfunctions in a variety of brain regions associated with sensation (such as the thalamus) and emotional processing (such as the insular cortex, the amygdala, the cingulate cortex, and the operculo-insular cortex).4–7 However, these task-based studies could not clearly uncover the basic pathogenesis of PSPD, because the pain symptoms usually appear without the influence of real stimuli from the outside environment.
Researchers have shown increasing interest in spontaneous and low-frequency neural activities in PSPD patients using resting-state fMRI or electroencephalography (EEG). For instance, it was found that somatoform pain disorder patients exhibited higher regional homogeneity (ReHo) in the left precentral gyrus, the prefrontal cortex and default-mode network, but decreased ReHo in the bilateral primary somatosensory cortex, the posterior cerebellum, and the occipital lobe comparing with healthy controls.8,9 Further, using independent component analysis to isolate intrinsic connectivity networks (ICNs) and to calculate the functional network connectivity (FNC) such as intra- and inter-ICNs, Zhao and his colleagues found altered FNCs between the sensorimotor network, the audio network, the visual network, the default-mode network, the executive control network, the salience network, the right-frontoparietal network, the left-frontoparietal network, and the cerebellum network in PSPD patients.10 A resting-state EEG study revealed that somatoform pain disorder patients exhibited hyperexcitability resting-state cortical oscillations at the parietal region.11 The evidence above suggests that PSPD patients manifest large-scale brain functional reorganization at different levels.
However, the whole-brain FC pattern of PSPD remains still largely unknown. Recently, researchers have recognized that brain regions are connected and that disturbances within whole-brain FC pattern could influence the onset, expression and course of diseases like Parkinson’s disease, schizophrenia, depressive disorder, and anxiety disorders.12–15 We believed that whole-brain FC pattern was altered in PSPD and this change would provide new evidence for exactly localizing the functionally abnormal brain areas. To confirm this hypothesis, the present study adopted 90 anatomically cerebrum regions from the automated anatomical labeling (AAL) template16 as regions of interest (ROIs), and a seed-based FC analysis was used to explore the whole-brain FC pattern in PSPD patients and HCs. Through comparisons between groups, we anticipate that the altered FC mainly related to pain sensation, cognition, emotion and memory brain regions such as the thalamus, the somatosensory cortex, the prefrontal cortex, the amygdala and the hippocampus.
Materials and Methods
Ethics Statement
This study was approved by the local Ethics Committee of Tongji Hospital of Tongji University (No.141). All participants were informed of the experimental procedures and written informed consent was obtained from every participant prior to the experiment. All procedures were conducted in accordance with principles expressed in the Declaration of Helsinki and each of the participants was paid 500 (Chinese Yuan) for completing the experiment.
Participants
Thirteen PSPD patients were enrolled from May 2012 to May 2016 through the inpatient and outpatient service in Tongji Hospital of Tongji University, and 23 HCs were enrolled via advertisements posted in nearby communities. All the patients were diagnosed by one psychiatrist and they all met the following inclusion criteria: (1) diagnosis of PSPD according to the criteria of 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10): F45.4; (2) the duration of disease was longer than 6 months; (3) between the age of 18 and 65; (4) right-hand dominance, and exclusion criteria: (1) presence of pain symptoms due to severe somatic diseases; (2) presence of severe somatic diseases, such as cardiovascular disease, cerebrovascular disease, epilepsy; (3) presence of other mental disorders; (4) substance abuse; (5) current pregnancy.
Clinical Assessments
Multiple self-reported scales were used to assess the pain intensity and the emotional problems, including the Visual Analogue Scale (VAS), the Zung Self-Rating Anxiety Scale (SAS) and the Zung Self-Rating Depression Scale (SDS). The VAS is a one-dimensional method to measure the pain intensity by marking one score on the line where “0” stands for no pain and “10” for worst pain, respectively.17 The SAS, which contains 20 items, was applied to assess the anxiety severity.18 Each response was rated on a 4-point scale, from “none of the time” to “most of the time”. The Chinese version of SAS with acceptable reliability and validity was adopted in the present study.19,20 In addition, the SDS was employed to assess the level of depression.21 Twenty items reflect four groups of specific symptoms of depression: Psycho-emotional symptoms (like “I feel down-hearted and blue”), Somatic disorders (such as “I notice that I am losing weight”), Psychomotor disorders (eg, “I am restless and can’t keep still”) and Depressive mental disorder (eg “I am more irritable than usual”). The Chinese version of SDS with acceptable reliability and validity was used in the present study.19,20
MRI Data Acquisition
Functional neuroimaging data were acquired using a Siemens Trio 3.0 Tesla MRI scanner (Siemens, Erlangen, Germany) at the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University. At the beginning of the fMRI scan, participants were instructed to keep their eyes closed, not to think of anything in particular and not to fall asleep. Foam pads were used to tightly fix the participant’s head to reduce movement.22 Rs-fMRI data were collected axially using an echo-planar imaging (EPI) sequence: echo time = 30 ms, repetition time = 2000 ms, flip angle =90°, field of view = 192 mm×192 mm, axial slices = 33, slice thickness = 4 mm, no gap, matrix = 64×64. T1-weighted images covering the entire brain were obtained in a sagittal orientation employing a magnetization-prepared rapid gradient echo sequence before each resting image. 240 fMRI image volumes and 192 T1 image volumes were collected for each participant.
fMRI Data Pre-Processing
Prior to analysis, the first 10 volumes of the fMRI data of each participant were discarded to allow for magnetization equilibrium.9 The data were subsequently preprocessed and analyzed in the functional connectivity toolbox “CONN” v.16b (http://www.nitrc.org/projects/conn) running on Matlab R2014a and using the Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) default parameter choices. The pipeline procedures included functional slice timing correction, functional realignment and unwarping, functional center to (0,0,0) coordinates, structural center to (0,0,0) coordinates, structural segmentation & normalization, functional normalization, functional outlier detection (ART-based scrubbing), and smoothing (8-mm FWHM Gaussian filter). Then, the toolbox step to a denoising procedure: the confounding effects such as the white matter, cerebrospinal fluid, realignment results, scrubbing results, and the rest were regressed out of the fMRI time series, and after that, the data were bandpass-filtered (0.01 to 0.08 Hz)23 and linear detrended.
ROI Regions Selection
Regions of interest (ROIs) were selected from the automated anatomical labeling (AAL) template (www.fil.ion.ucl.ac.uk/spm/ext/), which was an atlas widely used for manual microanatomical parcellation of the single-subject MNI-space template brain.24 The entire cerebrum was divided into 90 regions which were considered as seeds and nodes in the later FC analysis. The index, abbreviations, and MNI coordinate of the 90 cerebrum regions used in this study are shown in Supplementary information Table S1.
ROI-to-ROI FC Analysis
Regional mean blood oxygen-level-dependent (BOLD) time series was estimated by averaging the time series of all voxels at each ROI.25,26 Bivariate correlation coefficient was used to measure the level of linear association of the BOLD time series between each pair of ROI:27
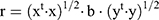
Before entering into a further statistical analysis, a Fisher’s r-to-z transformation was applied in order to improve the normality assumptions of the general linear model.27 To this end, we constructed a 90 × 90 matrix where each cell represented the FC between two brain regions. Then, multiple comparisons were adjusted by applying the correction of False discovery rate (p<0.05).
Correlation Analysis
To investigate the relationship between identified FCs and scores on the clinical assessments, we performed a Spearman’s rank correlation analysis to determine the relationships between the z-scores of the identified FCs and the VAS scores/SAS scores/SDS scores/duration of illness by using SPSS 20.0 (IBM, USA).
Results
Participants’ Characteristics
PSPD patients and HCs showed no significant difference in age and gender ratio. Table 1 presents the demographic characteristics of all participants and scores of the clinical assessment in the PSPD patients.
 |
Table 1 Demographic and Clinical Data of Patients with PSPD |
FC Within Groups
The whole-cerebrum FC results of the PSPD and HC groups are presented in Figure 1A and B, respectively. On the whole, most of the strong functional connectivities (large z-scores) were found between inter-hemispheric symmetric regions (the node near the diagonal) such as between the left rectus and the right rectus, the left insula and the right insula, the left-anterior cingulate cortex and the right-anterior cingulate cortex, the left calcarine and the right calcarine. In addition, strong connectivities were observed between the anatomically adjacent brain areas such as the calcarine fissure, the cuneus, the lingual gyrus, the superolateral occipital gyrus, the medial occipital gyrus, the inferior occipital gyrus, and the fusiform gyrus.
Group Differences in FC Intensity
PSPD patients showed increased intensity of FCs between the left thalamus and the right amygdala, the left thalamus and the right hippocampus, the left thalamus and multiple sub-regions of the occipital lobe including bilateral calcarine fissure and surrounding cortex, bilateral lingual gyrus, bilateral fusiform gyrus, and left Inferior occipital gyrus (p FDR-corrected <0.05). However, the intensity of FC was observed decreased between the left amygdala and the right putamen in the PSPD patients (Table 2 and Figure 2).
 |
Table 2 Group Difference in the Intensity of ORI-to-ORI FC Between Patients with PSPD and HCs |
Relationship Between FC Intensity and Clinical Symptoms in PSPD Patients
A strong negative correlation (r = −0.61, p = 0.03) between the standardized intensity of the left thalamus–the right amygdala FC and the scores on the SAS (Figure 3) was observed. However, no any other significant correlations were found between the intensity of identified FCs and VAS scores, SDS scores or duration of illness.
Discussion
In this study, we investigated the functional connectivity across the whole cerebrum in PSPD patients using rs-fMRI. Similar to a newly study,28 we found considerable functional connectivity altered in PSPD patients. By Degree centrality analysis and functional connectivity analysis, Liu et al found six functional hubs (the bilateral inferior occipital gyrus, bilateral calcarine fissure, left paracentral lobule) and multiple decreased FC values between these six regions. Differently, the present study only found one functional hub (the left thalamus) and exhibited a thalamic-centered whole-brain FC altered.
Increased Thalamic-Centered Functional Connectivity in PSPD Patients
Through a seed-based whole-cerebrum FC analysis, for the first time, we found an aberrant thalamic-centered FC pattern in PSPD patients. Consistent to our findings, previous animal studies found that a lesion made to the thalamic nuclei could help to alleviate the symptoms of neuropathic pain,29,30 and previous human brain imaging studies revealed that the thalamus was one of the crucial brain areas which involved in the pain processing.31–33 For instance, one task-related fMRI study on the somatoform pain disorder found that patients showed increased activation of thalamus under the stimulation of pin-prick pain and cognitive stress.4 Nevertheless, other resting-state fMRI researches reported comparable levels of ReHo or FNC of the thalamus in PSPD patients, which was not in line with our findings.8–10 This difference may be derived from the diverse spatial scale of the statistical subjects and diverse statistical method of the correlation coefficient.
It is generally known that the thalamus is the destination of ascending sensory pathway where the sensory information is relayed to the cerebral cortex and limbic system via the thalamocortical radiations and the thalamus-limbic pathway, respectively.34 Hence, the thalamus is regarded as the essential organ of the affective side of sensation, especially pain.32 Previous studies have found that the thalamus showed greater spontaneous firing and excessive burst firing in patients with chronic pain.35–37 In the animal models of peripheral neuropathic pain, thalamus also showed spontaneous hyperexcitability, evoked hyperactivities, expansion of receptive fields, and bursting firing.38,39 It may be suggested that the persistent chronic pain and associated symptoms of PSPD were caused by spontaneous hyperexcitability or evoked hyperactivities in the thalamus, from which the spontaneous pain-related impulses transmitted to other brain regions such as the occipital lobe, the amygdala, the hippocampus via the thalamocortical radiations and the thalamus-limbic pathway.
In line with our findings, previous imaging studies have also reported altered FCs of the occipital areas in patients with chronic pain.40–42 The altered FCs were found between the occipital areas and the anterior cingulate cortex, the medial prefrontal cortex, the inferior frontal gyrus, and the insula. The altered connectivity between thalamus and visual cortex might be related to the impaired visual attention functioning in chronic pain condition.43,44 One of these studies also found that participants with chronic pain fixated significantly more frequently on pain words than pain-free participants, supporting the hypothesis that individuals with chronic pain displayed specific attentional biases toward pain-related stimuli.43
Additionally, studies have found altered FC between the hippocampus and multiple subareas of the cingulate cortex,45–47 and between the amygdala and multiple subareas of the cingulate cortex45,46,48 in chronic pain patients. Correspond with previous researches, the present study also showed altered FC between left thalamus and the hippocampus and the amygdala, and found a strong negative correlation between the altered left thalamus-right amygdala FC and the SAS scores. The amygdala and the hippocampus were related to the enhanced memory for pleasant and aversive stimuli.49,50 While the thalamus-amygdala pathway was shown to be involved in the affective behaviors such as stress/anxiety.34 We thus draw a relatively safe conclusion that the altered FC between thalamus and amygdala may be the neural mechanisms underlying the pain-related anxiety in PSPD.
Decreased Functional Connectivity in PSPD Patients
There was only one decreased FC that was found between the left amygdala and the right putamen in PSPD patients. As mentioned above, the amygdala was related to enhanced memory for pleasant and aversive stimuli.49,50 The putamen was mainly to regulate movements,51 and affect various kinds of learning.52 And a recent review recruiting 266 cutaneous pain fMRI studies found that the left putamen is the brain area which was consistently activated under chronic pain condition.53 A possible explanation for the decreased FC between the two brain regions might be derived from the potential neural alteration in the putamen associated with the impairments in learning of patients’ past negative experience caused by pain, which subsequently leads to reduced connectivity and activities in the amygdala while processing negative emotional signals.
Limitation
There were still some limitations in the current study. Firstly, the simple size of PSPD patients was very small and the amount of subjects in the two groups is quite different. It is inevitable that there is somewhat bias in the present study, though the statistical results were FDR corrected. In reality, there are quite a small proportion of PSPD patients who are willing to see the psychiatrist or psychologist in the mental health hospitals. In addition, for ethical reasons, the PSPD patients did not suspend any of their medication during the fMRI scan. So the influence of drugs such as antidepressant and antianxiety drug, and especially sedatives and hypnotics cannot be excluded. For example, midazolam and zolpidem can increase fluctuation and synchrony of the resting brain BOLD signal.54,55 These may restrict the relational explanatory capability of the present study in clinical.
Conclusion
In summary, the functional connectivity between the thalamus and other pain-related regions were altered in the PSPD patients, implying that the thalamus might play an important role in the psychopathological mechanism of PSPD. A strong negative correlation between the altered left thalamus-right amygdala FC and the SAS scores suggests that the altered FC may be the neural mechanisms underlying the pain-related anxiety in PSPD. Finally, we hope that our findings will be helpful in improving the understanding of PSPD.
Acknowledgments
The authors would like to thank all the patients and volunteers for their participation in this study, and thank the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University for their support. Thank Jacques Bradwejn for his comments on the manuscript. This research was supported by Innovative Research Team of High-Level Local Universities in Shanghai, and the grants from Shanghai Municipal Health Commission (2019ZB0201), and Key Specialist Projects of Shanghai Municipal Commission of Health and Family Planning (ZK2015B01), and the Programs Foundation of Shanghai Municipal Commission of Health and Family Planning (201540114), and the Fundamental Research Funds for the Central Universities (2018ECNU-QKT015, 2019ECNU-HWFW021).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Luo YL, Heeramun-Aubeeluck A, Huang X, et al. Factors influencing quality of life in Chinese patients with persistent somatoform pain disorder. Psychol Health Med. 2014;19(6):744–752. doi:10.1080/13548506.2013.878804
2. Magon S, Sprenger T, Otti A, Papadopoulou A, Gundel H, Noll-Hussong M. Cortical thickness alterations in chronic pain disorder: an exploratory MRI study. Psychosom Med. 2018;80(7):592–598. doi:10.1097/PSY.0000000000000605
3. Ren X, Lu J, Liu X, et al. Decreased prefrontal brain activation during verbal fluency task in patients with somatoform pain disorder: an exploratory multi-channel near-infrared spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:153–160. doi:10.1016/j.pnpbp.2017.05.006
4. Stoeter P, Bauermann T, Nickel R, et al. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: an fMRI study. NeuroImage. 2007;36(2):418–430. doi:10.1016/j.neuroimage.2007.01.052
5. Gundel H, Valet M, Sorg C, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137(2):413–421. doi:10.1016/j.pain.2007.10.003
6. Yoshino A, Okamoto Y, Yoshimura S, et al. Distinctive neural responses to pain stimuli during induced sadness in patients with somatoform pain disorder: an fMRI study. NeuroImage Clin. 2013;2:782–789. doi:10.1016/j.nicl.2013.06.001
7. Luo Y, Yan C, Huang T, et al. Altered neural correlates of emotion associated pain processing in persistent somatoform pain disorder: an fMRI study. Pain Pract. 2016;16(8):969–979. doi:10.1111/papr.12358
8. Yoshino A, Okamoto Y, Kunisato Y, et al. Distinctive spontaneous regional neural activity in patients with somatoform pain disorder: a preliminary resting-state fMRI study. Psychiatry Res. 2014;221(3):246–248. doi:10.1016/j.pscychresns.2013.12.006
9. Huang T, Zhao Z, Yan C, et al. Altered spontaneous activity in patients with persistent somatoform pain disorder revealed by regional homogeneity. PLoS One. 2016;11(3):e0151360. doi:10.1371/journal.pone.0151360
10. Zhao Z, Huang T, Tang C, et al. Altered resting-state intra- and inter- network functional connectivity in patients with persistent somatoform pain disorder. PLoS One. 2017;12(4):e0176494. doi:10.1371/journal.pone.0176494
11. Ye Q, Yan D, Yao M, Lou W, Peng W. Hyperexcitability of cortical oscillations in patients with somatoform pain disorder: a resting-state EEG study. Neural Plast. 2019;2019:2687150. doi:10.1155/2019/2687150
12. Zeng LL, Shen H, Liu L, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–1507. doi:10.1093/brain/aws059
13. Li T, Wang Q, Zhang J, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43(2):436–448. doi:10.1093/schbul/sbw099
14. Takagi Y, Sakai Y, Abe Y, et al. A common brain network among state, trait, and pathological anxiety from whole-brain functional connectivity. NeuroImage. 2018;172:506–516. doi:10.1016/j.neuroimage.2018.01.080
15. Tang Y, Liu BL, Yang Y, et al. Identifying mild-moderate Parkinson’s disease using whole-brain functional connectivity. Clin Neurophysiol. 2018;129(12):2507–2516. doi:10.1016/j.clinph.2018.09.006
16. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi:10.1006/nimg.2001.0978
17. Woods CA, Cumming B. The impact of test medium on use of visual analogue scales. Eye Contact Lens. 2009;35(1):6–10. doi:10.1097/ICL.0b013e3181909b03
18. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi:10.1016/S0033-3182(71)71479-0
19. Duan QQ. Beijing. Differential validity of SAS and SDS among psychiatric non-psychotic outpatients and their partners. Chin Ment Health J. 2012;26:676–679.
20. Zhang J, Yan C, Huang F. Applicability of Zung’s self-rating anxiety scale in evaluating inpatients in department of cardiovascular medicine. Pract Prev Med. 2017.
21. Zung WW. The measurement of affects: depression and anxiety. Mod Probl Pharmacopsychiatry. 1974;7:170–188.
22. Zhou F, Zhuang Y, Wu L, et al. Increased thalamic intrinsic oscillation amplitude in relapsing-remitting multiple sclerosis associated with the slowed cognitive processing. Clin Imaging. 2014;38(5):605–610. doi:10.1016/j.clinimag.2014.05.006
23. Liao W, Zhang Z, Pan Z, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5(1):e8525. doi:10.1371/journal.pone.0008525
24. Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The resting brain of alcoholics. Cereb Cortex. 2015;25(11):4155–4168.
25. Salvador R, Suckling J, Schwarzbauer C, Bullmore E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):937–946. doi:10.1098/rstb.2005.1645
26. Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26(1):63–72. doi:10.1523/JNEUROSCI.3874-05.2006
27. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi:10.1089/brain.2012.0073
28. Liu Q, Zeng XC, Jiang XM, Zhou ZH, Hu XF. Altered brain functional hubs and connectivity underlie persistent somatoform pain disorder. Front Neurosci. 2019;13. doi:10.3389/fnins.2019.00415
29. Saade NE, Al Amin H, Abdel Baki S, Safieh-Garabedian B, Atweh SF, Jabbur SJ. Transient attenuation of neuropathic manifestations in rats following lesion or reversible block of the lateral thalamic somatosensory nuclei. Exp Neurol. 2006;197(1):157–166. doi:10.1016/j.expneurol.2005.09.005
30. Saade NE, Al Amin H, Abdel Baki S, Chalouhi S, Jabbur SJ, Atweh SF. Reversible attenuation of neuropathic-like manifestations in rats by lesions or local blocks of the intralaminar or the medial thalamic nuclei. Exp Neurol. 2007;204(1):205–219. doi:10.1016/j.expneurol.2006.10.009
31. Anderson WS, O’Hara S, Lawson HC, Treede RD, Lenz FA. Plasticity of pain-related neuronal activity in the human thalamus. Prog Brain Res. 2006;157:353–364.
32. Yen CT, Lu PL. Thalamus and pain. Acta Anaesthesiol Taiwan. 2013;51(2):73–80. doi:10.1016/j.aat.2013.06.011
33. Nakata H, Sakamoto K, Kakigi R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol. 2014;5:1489. doi:10.3389/fpsyg.2014.01489
34. Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav R. 2015;54:89–107. doi:10.1016/j.neubiorev.2015.01.014
35. Lenz FA, Tasker RR, Dostrovsky JO, et al. Abnormal single-unit activity recorded in the somatosensory thalamus of a quadriplegic patient with central pain. Pain. 1987;31(2):225–236. doi:10.1016/0304-3959(87)90038-8
36. Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res. 1989;496(1–2):357–360. doi:10.1016/0006-8993(89)91088-3
37. Lenz FA, Garonzik IM, Zirh TA, Dougherty PM. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience. 1998;86(4):1065–1081. doi:10.1016/S0306-4522(98)00099-2
38. Vos BP, Benoist JM, Gautron M, Guilbaud G. Changes in neuronal activities in the two ventral posterior medial thalamic nuclei in an experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve. Somatosens Mot Res. 2000;17(2):109–122. doi:10.1080/08990220050020535
39. Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009;1268:154–161. doi:10.1016/j.brainres.2009.02.063
40. Flodin P, Martinsen S, Altawil R, et al. Intrinsic brain connectivity in chronic pain: a resting-state fMRI study in patients with rheumatoid arthritis. Front Hum Neurosci. 2016;10:107. doi:10.3389/fnhum.2016.00107
41. Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain. 2014;155(8):1472–1480. doi:10.1016/j.pain.2014.04.022
42. Boissoneault J, Letzen J, Lai S, Robinson ME, Staud R. Static and dynamic functional connectivity in patients with chronic fatigue syndrome: use of arterial spin labelling fMRI. Clin Physiol Funct Imaging. 2016;38(1).
43. Fashler SR, Katz J. More than meets the eye: visual attention biases in individuals reporting chronic pain. J Pain Res. 2014;7:557–570. doi:10.2147/JPR
44. Fashler SR, Katz J. Keeping an eye on pain: investigating visual attention biases in individuals with chronic pain using eye-tracking methodology. J Pain Res. 2016;9:551–561. doi:10.2147/JPR
45. Martucci KT, Shirer WR, Bagarinao E, et al. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP research network. Pain. 2015;156(9):1755–1764. doi:10.1097/j.pain.0000000000000238
46. Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 2012;8:32. doi:10.1186/1744-8069-8-32
47. Ichesco E, Puiu T, Hampson JP, et al. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain. 2016;20(7):1079–1089. doi:10.1002/ejp.832
48. Cifre I, Sitges C, Fraiman D, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74(1):55–62. doi:10.1097/PSY.0b013e3182408f04
49. Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2(3):289–293. doi:10.1038/6404
50. Richter-Levin G. The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist. 2004;10(1):31–39. doi:10.1177/1073858403259955
51. Kimura M, Aosaki T, Ishida A. Neurophysiological aspects of the differential roles of the putamen and caudate nucleus in voluntary movement. Adv Neurol. 1993;60:62–70.
52. Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi:10.1146/annurev.neuro.25.112701.142937
53. Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: a coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav R. 2016;68:120–133. doi:10.1016/j.neubiorev.2016.04.001
54. Kiviniemi VJ, Haanpaa H, Kantola JH, et al. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23(4):531–537. doi:10.1016/j.mri.2005.02.009
55. Licata SC, Nickerson LD, Lowen SB, Trksak GH, Maclean RR, Lukas SE. The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. NeuroImage. 2013;70:211–222. doi:10.1016/j.neuroimage.2012.12.055
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



