Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI
Authors Li H, Dai X, Gong H, Nie X, Zhang W, Peng D
Received 4 September 2014
Accepted for publication 2 December 2014
Published 23 January 2015 Volume 2015:11 Pages 207—214
DOI https://doi.org/10.2147/NDT.S73730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Hai-Jun Li,1,* Xi-Jian Dai,1,2,* Hong-Han Gong,1 Xiao Nie,1 Wei Zhang,3 De-Chang Peng1
1Department of Radiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, People’s Republic of China; 2Department of Imaging and Interventional Radiology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong Special Administrative Region, People’s Republic of China; 3Department of Pneumology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Background: The majority of previous neuroimaging studies have demonstrated both structural and functional abnormalities in obstructive sleep apnea (OSA). However, few studies have focused on the regional intensity of spontaneous fluctuations during the resting state and the relationship between the abnormal properties and the behavioral performances. In the present study, we employed the amplitude of low-frequency fluctuation (ALFF) method to explore the local features of spontaneous brain activity in OSA patients (OSAs).
Methods: Twenty-five untreated male severe OSAs and 25 age-matched and years-of-education-matched male good sleepers (GSs) were included in this study. The ALFF method was used to assess the local features of spontaneous brain activity. The mean signal values of the altered ALFF areas were analyzed with receiver operating characteristic curve. Partial correlation analysis was used to explore the relationship between the observed mean ALFF values of the different areas and the behavioral performances.
Results: Compared with GSs, OSAs had significantly higher scores for body mass index, apnea–hypopnea index, arterial oxygen saturation <90%, arousal index, and Epworth Sleepiness Scale (ESS) score; furthermore, OSAs had significantly lower scores for rapid eye movement sleep and in the Montreal Cognitive Assessment (MoCA). Compared with GSs, OSAs showed significant lower-ALFF areas in the cluster of the right precuneus and bilateral posterior cingulate gyrus, as well as a higher-ALFF area in the left inferior frontal gyrus. The area under the curve values of the lower- and higher-ALFF areas were 0.90 and 0.93, respectively. Further diagnostic analysis exhibited that the sensibility and specificity of the two clusters were 80% and 92%, respectively. The mean signal value of the lower-ALFF cluster displayed significant positive correlations with lowest oxygen saturation (r=0.447, P=0.025) and MoCA score (r=0.405, P=0.045).
Conclusion: OSAs may involve in a dysfunction in the default mode network and an adaptive compensatory response in the frontal lobe, which reflect the underlying pathophysiology of cognitive impairment.
Keywords: obstructive sleep apnea, amplitude of low-frequency fluctuation, functional magnetic resonance imaging, resting state, spontaneous activity, blood oxygen-level-dependent
Introduction
Obstructive sleep apnea (OSA), characterized by repeated obstructions of upper airway with intermittent hypoxic exposure, is associated with multiple detrimental physiological and psychological consequences, in addition to being associated with cardiorespiratory diseases, including pulmonary hypertension,1 systemic hypertension,2 cardiac arrhythmias,3 cardiac ischemia4 and cerebral ischemia.5 OSA may also cause daytime sleepiness,6 increase the risk of a traffic accident,7 and diminish both quality of life8 and work performance,9 as well as being accompanied by impairment in several cognitive domains, including attention and vigilance decrements, memory gaps, psychomotor dysfunction, and abnormalities in executive functions.10–12 On the basis of available population-based studies, OSA affects 3%–7% of adult men, 2%–5% of adult women,13–15 and up to 4% of children.16,17 The main pathophysiologic mechanism of cognitive deficits from OSA, including intermittent hypoxia, intermittent hypercapnia, and sleep fragmentation,18 are still unclear.
Previous neuroimaging studies have investigated 1) diminutions in gray matter concentration in the left hippocampus, left rectus gyrus, bilateral superior frontal gyrus, left precentral gyrus, bilateral frontomarginal gyrus, bilateral anterior cingulate gyrus, right insular gyrus, bilateral caudate nucleus, bilateral thalamus, bilateral amygdalo-hippocampus, bilateral inferior temporal gyrus, and cerebellum, and 2) gray matter volume deficits in bilateral hippocampus, bilateral lateral temporal areas, right cuneus, right middle temporal gyrus, left dorsolateral prefrontal cortex, right middle temporal gyrus, and left cerebellum using voxel-based morphometry techniques in OSA patients (OSAs).19–23 Moreover, diffusion tensor imaging studies showed a wide range of changes in white matter integrity within the corpus callosum, frontal cortex, temporal cortex, parietal cortexes, cingulate bundle, and cerebellum.24–26 A single-photon emission computed tomography study found that severe OSAs showed reduced cerebral blood flow in bilateral parahippocampal gyrus, right lingual gyrus, pericentral gyrus, and cuneus.27 Decreased neural activations associated with cognitive impairment have been found in multiple brain regions, including the cingulate gyrus, dorsolateral prefrontal gyrus, inferior frontal gyrus, left postcentral gyrus, inferior and posterior parietal lobes, insula, and right putamen using task-state functional magnetic resonance imaging (fMRI) in OSAs.28–30 Recently, use of resting-state fMRI (rs-fMRI) has been increasing to investigate the ongoing neuronal processes in OSA. Although the majority of previous neuroimaging studies have focused on the brain structural and task-state functional changes of OSAs, few studies have evaluated the changes in blood oxygen-level-dependent (BOLD) signals of regional spontaneous activity of OSAs during resting state and their relationships with behavioral performances.
Amplitude of low-frequency fluctuation (ALFF), a newly developed rs-fMRI approach, calculates the square root of the power spectrum in a low-frequency range (0.01–0.08 Hz), for detecting the regional intensity of spontaneous fluctuations in BOLD signals.31–33 Furthermore, the ALFF has been proven to have test–retest reliability34 and has already been applied to patient studies investigating attention deficit hyperactivity disorder,31 sleep deprivation,35 schizophrenia,36 and early Alzheimer’s disease.37 However, it has not yet been used to explore the pathophysiological changes in OSAs. This study is the first to utilize ALFF as an index to investigate the intrinsic brain activity traits of OSAs and its potential mechanisms.
Materials and methods
Subjects
Twenty-five untreated male severe OSAs and 25 age-matched and years-of-education-matched male good sleepers (GSs) were included in this study from the Sleep Monitoring Room of the Respiratory Department of The First Affiliated Hospital of Nanchang University. Each subject was assessed by a detailed clinical interview and physical examination; in addition, the subjects completed a sleep questionnaire and underwent overnight polysomnography. The inclusion and exclusion criteria for the OSAs and GSs were as the same as in our previous study.38,39 The inclusion criteria for the OSAs were as follows: male sex; age >22 years but <60 years; and an apnea–hypopnea index (AHI) >30 events per hour. The exclusion criteria for both OSAs and GSs were as follows: 1) other sleep disorders, such as insomnia and sleep-related eating disorders; 2) history of cardiovascular disease, hypertension, or diabetes mellitus; 3) central nervous system disorders (neurodegenerative diseases, epilepsy, head injury, psychosis, hypothyroidism, or current depression); 4) left-handedness; 5) alcohol or illicit drug abuse; 6) current intake of psychoactive medications; and 7) contraindications to MRI, such as claustrophobia, metallic implants, or devices in the body. This study was approved by The Human Research Ethics Committee at The First Affiliated Hospital of Nanchang University, and all participants provided written informed consent forms.
Polysomnography
The day before the sleep studies, all OSAs and GSs were asked to refrain from drinking alcohol or caffeinated beverages. Full nocturnal polysomnography monitoring was performed on OSAs and GSs using the Respironics LE-series physiological monitoring system (Alice 5 LE; Respironics, Orlando, FL, USA) in the Sleep Center of our hospital. Standard electroencephalogram, electrooculogram, chin electromyogram, electrocardiogram, thoracic and abdominal movements, and snoring were recorded. Arterial oxygen saturation (SaO2) was measured transcutaneously by fingertip pulse oximetry. In accordance with the American Academy of Sleep Medicine guidelines, apnea was defined as the continuous cessation of airflow for more than 10 seconds and hypopnea was defined as a decrease in airflow by >30% with arousal or oxygen desaturation >4%.40,41 The AHI was calculated as the average of the total number of apnea and hypopnea events experienced per hour of sleep. Subjects’ performances were recorded on videotape and were continuously observed by a polysomnography technician. Polysomnography was performed from 10 pm to 6 am next morning.
Neuropsychological evaluation
All subjects filled in a sleep questionnaire to assess their daytime sleepiness by the Epworth Sleepiness Scale (ESS), with scores between 0 and 24.42 A score higher than 6 was considered somnolence. All subjects underwent a cognitive assessment using the Montreal Cognitive Assessment (MoCA)43 tool, administered by two independent neuropsychologists, to evaluate their executive function, naming, attention, calculation, language, abstraction, memory, and orientation. The total MoCA score is 30. A total MoCA score <26 indicates cognitive impairment, whereas a score ≥26 indicates normal cognitive function. If the length of schooling was <12 years, one point was added to the total score, so as to adjust for educational bias.44
MRI parameters
MRI scanning was performed on a 3-Tesla MRI scanner (Siemens, Erlangen, Germany). High-resolution T1-weighted images were acquired with a three-dimensional spoiled gradient-recalled echo sequence in a sagittal orientation: 176 images (repetition time=1,900 ms; echo time =2.26 ms; thickness =1.0 mm; gap =0.5 mm; acquisition matrix =256×256; field of view =250×250 mm2, flip angle =9°) were obtained. Finally, 240 functional images (repetition time =2,000 ms; echo time =30 ms; thickness =4.0 mm; gap =1.2 mm; acquisition matrix =64×64; flip angle =90°; field of view =230×230 mm2; 30 axial slices with gradient-recalled echo-planar imaging [EPI] pulse sequence) covering the whole brain were obtained.
fMRI data analysis
Functional data were checked using MRIcro software (www.MRIcro.com) to exclude defective data. The first ten time points of the functional images were discarded due to the possible instability of the initial MRI signals and the participants’ adaptation time to the scanning environment. On the basis of MATLAB2010a (Mathworks, Natick, MA, USA), the remainder of the data preprocessing was performed by DPARSFA (http://rfmri.org/DPARSF) software, including DICOM form transformation, slice timing, head motion correction, spatial normalization, smoothing with a Gaussian kernel of 6×6×6 mm3 full width at half maximum (FWHM). Motion time courses were obtained by estimating the values for translation (mm) and rotation (degrees) for each subject. Participants who had >1.5 mm maximum displacement in x, y, or z planes and 1.5° of angular motion during the whole fMRI scans were rejected. The Friston six head motion parameters were used to regress out head motion effects based on recent work showing that higher-order models were more effective in removing head motion effects.45,46 Linear regression was also applied to remove other sources of spurious covariates along with their temporal derivatives, including the signal from a ventricular region of interest (ROI) and the signal from a region centered in the white matter.47 Of note, the global signal was not regressed out in the present data, as in the study by Guo et al48 for the reason that there is still a controversy around the removal of the global signal in the preprocessing step of resting-state data.47,49 After head-motion correction, the fMRI images were spatially normalized to the Montreal Neurological Institute (MNI) space using the standard EPI template and resampling the images at a resolution of 3×3×3 mm3. After preprocessing, the time series for each voxel were linearly detrended to reduce low-frequency drift, physiological high-frequency respiratory and cardiac noise, and time series linear detrending. The time series for each voxel were transformed to the frequency domain, and the power spectrum was then obtained. Because the power of a given frequency is proportional to the square of the amplitude of this frequency component, the square root was calculated at each frequency of the power spectrum, and the averaged square root was obtained across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF. The details of ALFF calculation are as described in a previous study.31 To reduce the global effects of variability across the participants, the ALFF of each voxel was divided by the global mean ALFF value for each participant.
Statistical analysis
Subject characteristics, including age, body mass index (BMI), education, ESS score, MoCA score, and sleep-disordered breathing parameters, were tested using independent sample t-tests to compare OSAs with GSs using IBM Statistical Package for the Social Sciences version 19.0 (SPSS 19.0), and a P-value <0.05 was deemed significant. For functional data, two-sample Student’s t-test was used to analyze the difference between the two groups, with age and years of education as nuisance covariates of no interest. A corrected significance level of individual voxel P<0.001 and a cluster volume (V) ≥270 mm3 (a minimum continuous V of 270 mm3), using a false discovery rate (FDR)-corrected cluster threshold of P<0.05, was used to determine statistical significance.
On the basis of the ALFF findings, the brain regions that demonstrated significant level of difference between groups were indentified. These regions were classified as ROIs and saved as masks using the REST Version1.8 software (http://www.resting-fmri.Sourceforge.net). For each ROI, the mean ALFF value was extracted by averaging the ALFF values over all voxels for each OSA. Finally, the mean ALFF values were entered into IBM SPSS 19.0 to calculate their correlations with the behavioral performances.
Results
Demographic and clinical results
Demographic and clinical characteristics of each group are summarized in Table 1. The OSAs had significantly higher scores for BMI (t=6.25, P<0.001), AHI (t=15.51, P<0.001), SaO2 <90% (t =6.66, P<0.001), arousal index (t =6.85, P<0.001), and ESS score (t =7.64, P<0.001) and had significantly lower scores for rapid eye movement (REM) sleep (t =−5.4, P<0.001) and MoCA (t =−2.16, P=0.036) than the GSs.
ALFF results
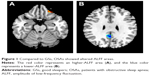
Compared with GSs, OSAs showed significant lower-ALFF areas in the cluster of right precuneus and bilateral posterior cingulate gyrus and a higher-ALFF area in the left inferior frontal gyrus. The details are presented in Table 2 and Figure 1. The mean ALFF values of these altered areas were extracted (Figure 2).
Correlation results
In the OSAs, the AHI score displayed a significant positive correlation with the arousal index (r=0.642, P=0.001) and negative correlations with REM% (r=−0.429, P=0.032) and MoCA score (r=−0.405, P=0.045). N2% (percentage of total sleep time at diagnostic polysomnography spent in the relevant stage) displayed negative correlations with REM% (r=−0.584, P=0.002) and ESS score (r=−0.531, P=0.006). BMI displayed significant positive correlation with arousal index (r=0.582, P=0.002) and negative correlation with the lowest oxygen saturation (r=−0.647, P<0.001).
The mean signal value of the observed lower-ALFF area displayed significant positive correlations with the lowest oxygen saturation (r=0.447, P=0.025) and MoCA score (r=0.405, P=0.045).
Receiver operating characteristic curve
Because different ALFF areas were found between OSAs and GSs, they might be utilized as markers to separate the OSAs from the GSs. To test this possibility, the mean ALFF values of the different brain regions were extracted and used for analysis of the receiver operating characteristic curves. In the present study, the values for the areas under the curves of the left inferior frontal gyrus and the cluster of right precuneus and bilateral posterior cingulate gyrus were 0.93 and 0.90, respectively. Further diagnostic analysis showed that the sensibility and specificity of the two clusters were 92% and 80%, respectively. The details are presented in Figure 3.
Discussion
Our previous study50 had demonstrated that many brain areas have obvious sex differences after normal sleep status and during sleep loss status. In this study, so as to avoid the influence of the sex differences or a lopsided sex ratio, only male OSAs were recruited. Our study is the first to investigate the effect of OSA on resting-state brain activity using the ALFF method. In our current study, we found that OSAs showed higher ALFF in the left inferior frontal lobe and lower ALFF in the cluster of right precuneus and bilateral posterior cingulate gyrus, compared with GSs, and showed lower MoCA score than GSs. Moreover, the observed lower-ALFF area displayed significant positive correlations with the lowest oxygen saturation and MoCA score.
As known, the lower-ALFF area in the cluster of right precuneus and bilateral posterior cingulate gyrus is largely included in the default mode networks (DMNs).51 Similarly, Prilipko et al52 found that OSAs showed abnormal deactivation in the DMN during working-memory tasks and a significantly positive correlation between the deactivation of DMN regions and behavioral performance,52 suggesting that suppression of activity in the DMN plays a role in cognitive impairment. Beebe et al53 also found, using sophisticated meta-analytic models, that OSA had a substantial impact on vigilance and executive functions but a negligible impact on intellectual and verbal function. Yaouhi et al54 found that the glucose metabolic activity of precuneus and cingulate gyrus, which were not atrophic, was reduced in OSAs, compared with that in GSs, using a 18F-fluoro-2-deoxy-D-glucose positron emission tomography method,54 and that this functional impairment may have been caused partly by remote effects originating from morphologically impaired areas with decreased connectivity.55 Furthermore, our study found a positive correlation between cerebral deactivation in parts of regions of the DMN and the lowest oxygen saturation, suggesting that intermittent hypoxia may be an important factor for the DMN dysfunction in OSAs.
The cingulate area is activated by dyspnea,56 breathlessness,57 and emotion related to the need for air58 and is involved in autonomic functions, including maintenance of blood pressure and salivary secretion, which suggests that the cingulate cortex has a complex and indirect relationship with the central networks that control respiration.59,60 Previous structural neuroimaging studies have found gray matter loss and white matter integrity reduction in the cingulate in OSAs.25,26 Joo et al20 also found that OSAs had decreased gray matter concentrations in the cingulate cortex, which may explain the clinical manifestations such as respiratory, affective, and cardiovascular disturbances. Ayalon et al61 found that OSAs showed decreased brain activation in left precentral gyrus, left anterior, and posterior cingulate, compared with control subjects, during an attention task. Similar results have been reported during valsalva maneuvers.62 These studies suggest that the cingulate gyrus may be particularly vulnerable to OSA-related impairment, independent of the specific cognitive challenge measured.In support of these functional and structural findings, our study found lower-ALFF areas in the posterior cingulate in OSAs compared with GSs. Moreover, a significant positive correlation between the lower-ALFF area and the MoCA score was observed in the OSAs. This brain–behavior relationship demonstrated that the abnormal properties of the posterior cingulate were associated with impaired cognitive function in OSAs.
The precuneus plays an important role in fundamental cognitive functioning, including episodic memory retrieval, visual–spatial imagery, self-processing, and consciousness.63 Our previous study38 found lower regional homogeneity area in the precuneus, which showed a significant negative correlation with sleep time, suggesting that decreased sleep time may be an important factor for dysfunction in the precuneus. In the current study, the lower-ALFF area in the right precuneus displayed significant positive correlation with MoCA score, suggesting that the abnormalities of the precuneus may be associated with a cognitive dysfunction.
Ayalon et al64 found that compared with GSs, OSAs showed increased activity in the bilateral inferior frontal gyrus, and a significant relationship between increased activation in the left inferior frontal gyrus and better immediate recall during a verbal learning task was found, suggesting an adaptive compensatory response. In support of this finding, in our study, OSAs showed higher ALFF in the left inferior frontal lobe, compared with GSs, which was consistent with previous studies on sleep deprivation65 and healthy aging.66
Conclusion
In conclusion, our findings suggest that the ALFF method may be a useful noninvasive imaging tool and a symbolic early biomarker for the detection of cerebral changes and for indexing of the changes of cognitive function, which may be helpful in the development of imaging biomarkers for the detection of cerebral changes. Furthermore, the abnormal spontaneous activity of the DMN region and the frontal lobe may be involved in the underlying pathophysiology of OSAs. However, there are several limitations that should be paid attention to. First, a larger sample size, as well as female and children populations, should be studied. Second, another group comparison, both before and after the treatment, will yield significant insight.
Acknowledgments
This work was supported by the Jiangxi Provincial Department of Science and Technology Support Program (grant numbers 20132BBG70061, 20121BBG70056, and 20141BBG70026), Jiangxi Provincial Department of Natural Science Foundation Project (grant 20132BAB205100), Jiangxi Provincial Department of Graduate Innovation Foundation (grant YC2013-S007), Chinese Department of National Innovation Experiment Program for University Students (grants 201210403052 and 2012181).
Disclosure
The authors report no conflicts of interest in this work. This was not an industry-supported study. None of the authors have any personal or financial involvement with organizations that hold financial interest in the subject matter.
References
Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis. 2009;51(5):363–370. | ||
Stradling JR. Sleep apnoea and systemic hypertension. Thorax. 1989;44(12):984–989. | ||
Flemons WW, Remmers JE, Gillis AM. Sleep apnea and cardiac arrhythmias: Is there a relationship? Am Rev Respir Dis. 1993;148(3):618–621. | ||
Koskenvuo M, Kaprio J, Telakivi T, et al. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J. 1987;294:16–19. | ||
Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. | ||
Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. | ||
George CFP. Sleep. 5: driving and automobile crashes in patients with obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(9):804–807. | ||
Engleman HM, Douglas NJ. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. | ||
Carotenuto M, Esposito M, Parisi L, et al. Depressive symptoms and childhood sleep apnea syndrome. Neuropsychiatr Dis Treat. 2012;8:369–373. | ||
Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits: a population-based study. Am J Respir Crit Care Med. 1997;156(6):1813–1819. | ||
Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull. 2003;61(1):87–92. | ||
Saunamäki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115(1):1–11. | ||
Feng J, Chen BY. Prevalence and incidence of hypertension in obstructive sleep apnea patients and the relationship between obstructive sleep apnea and its confounders. Chin Med J (Engl). 2009;122(12):1464–1468. | ||
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. | ||
[No authors listed]. Sleep apnea: is your patient at risk?. National heart, lung, and blood institute working group on sleep apnea. Am Fam Physician. 1996;53(1):247–253. | ||
Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. | ||
Feng J, Wu Q, Zhang D, Chen BY. Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J (Engl). 2012;125(4):696–701. | ||
Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40(10):1683–1692. | ||
Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4(5):451–454. | ||
Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. | ||
Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. | ||
Zhang Q, Wang D, Qin W, et al. Altered resting-state brain activity in obstructive sleep apnea. Sleep. 2013;36(5):651–659. | ||
Morrell MJ, Jackson ML, Twigg GL, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65(10):908–914. | ||
Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90(10):2043–2052. | ||
Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(10):1382–1387. | ||
Macey PM, Kumar R, Woo MA, et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. | ||
Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30(11):1515–1520. | ||
Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98(6):2226–2234. | ||
Ayalon L, Ancoli-Israel S, Drummond SP. Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res. 2009;18(2):204–208. | ||
Archbold KH, Borghesani PR, Mahurin RK, Kapur VK, Landis CA. Neural activation patterns during working memory tasks and OSA disease severity: preliminary findings. J Clin Sleep Med. 2009;5(1):21–27. | ||
Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. | ||
Lu H, Zuo Y, Gu H. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A. 2007;104:18265–18269. | ||
Kiviniemi V, Jauhiainen J, Tervonen O, et al. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44(3):373–378. | ||
Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. | ||
Dai XJ, Min YJ, Gong HH, et al. Evaluation of the post-effect of acupuncture at Sanyinjiao (SP 6) under sleep deprivation by resting-state amplitude of low-frequency fluctuation:a fMRI study. Zhongguo Zhen Jiu. 2012;32(1):47–52. | ||
Hoptman MJ, Zuo XN, Butler PD, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117(1):13–20. | ||
He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35(2):488–500. | ||
Peng DC, Dai XJ, Gong HH, Li HJ, Nie X, Zhang W. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2014;10:1819–1826. | ||
Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–2175. | ||
Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3(2):169–200. | ||
[No authors listed]. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. | ||
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. | ||
Wang WH, He GP, Xiao XP, Gu C, Chen HY. Relationship between brain-derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac J Trop Med. 2012;5(11):906–910. | ||
Zhang X. Modern Sleep Medicine. Beijing: People’s Military Medical Press; 2007:315. | ||
Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. | ||
Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. | ||
Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. | ||
Guo W, Jiang J, Xiao C, et al. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res. 2014;152(1):170–175. | ||
Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. | ||
Dai XJ, Gong HH, Wang YX, et al. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13(6):720–727. | ||
Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. | ||
Prilipko O, Huynh N, Schwartz S, et al. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. Sleep. 2011;34:293–301. | ||
Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. | ||
Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18(1):36–48. | ||
Chetelat G, Desgranges B, de laSayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. | ||
Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. 2001;163(4):951–957. | ||
Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11(10):2117–2120. | ||
Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500–1511. | ||
Brannan S, Liotti M, Egan G, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98(4):2029–2034. | ||
Frysinger RC, Harper RM. Cardiac and respiratory relationships with neural discharge in the anterior cingulate cortex during sleep-walking states. Exp Neurol. 1986;94(2):247–263. | ||
Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32(3):373–381. | ||
Henderson LA, Woo MA, Macey PM, et al. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94(3):1063–1074. | ||
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. | ||
Ayalon L, Ancoli-Israel S, Klemfuss Z, Shalauta MD, Drummond SP. Increased brain activation during verbal learning in obstructive sleep apnea. Neuroimage. 2006;31(4):1817–1825. | ||
Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140(3):211–223. | ||
Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–219. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.