Back to Journals » Cancer Management and Research » Volume 11
Aberrant Expression Of PDCD4/eIF4A1 Signal Predicts Postoperative Recurrence For Early-Stage Oral Squamous Cell Carcinoma
Authors Zhao M, Ding L , Yang Y, Chen S, Zhu N, Fu Y, Ni Y, Wang Z
Received 15 July 2019
Accepted for publication 9 October 2019
Published 11 November 2019 Volume 2019:11 Pages 9553—9562
DOI https://doi.org/10.2147/CMAR.S223273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Mengxiang Zhao,1,* Liang Ding,1,* Yan Yang,2 Sheng Chen,2 Nisha Zhu,1 Yong Fu,1 Yanhong Ni,1 Zhiyong Wang2
1Central Laboratory of Stomatology, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing 210093, People’s Republic of China; 2Department of Oral and Maxillofacial Surgery, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing 210008, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiyong Wang
Department of Oral and Maxillofacial Surgery, Nanjing Stomatological Hospital, Medical School of Nanjing University, 30 Zhongyang Road, Nanjing 210008, Jiangsu, People’s Republic of China
Tel +86 137 709 2448
Email [email protected]
Yanhong Ni
Central Laboratory of Stomatology, Nanjing Stomatological Hospital, Medical School of Nanjing University, 22 Hankou Road, Nanjing, Jiangsu 210093, People’s Republic of China
Tel +86 138 1451 6225
Email [email protected]
Background: Programmed cell death 4 (PDCD4) as a tumor suppressor gene inhibits growth and metastasis of cancer cells, which involved with eIF4A1, the inhibitor of translation initiation. Although the prognosis of early-stage oral squamous cell carcinoma (OSCC) is generally better, but many patients occur recurrence after surgery. Understanding the clinical expression pattern of PDCD4/eIF4A1 signal would provide diagnostic biomarker and target therapy premise for early-stage OSCC patients.
Methods: Immunohistochemical analysis was performed on 69 early-stage (T1/2N0M0) OSCC samples to evaluate temporal expression and prognostic value of eIF4A1 and PDCD4 in early-stage OSCC according to cell types and microlocalization. The correlations between PDCD4/eIF4A1 signal and Ki-67, postoperative recurrence and metastasis were determined.
Results: We found that PDCD4 was presented in tumor cells (TCs) and tumor-infiltrating lymphocytes (TILs) but absent in fibroblast-like cells (FLCs). eIF4A1 was only presented in TCs. PDCD4TCs was negative associated with eIF4A1TCs in tumor center, and patients with low PDCD4TCs or high eIF4A1TCs had poorer differentiation. Moreover, aberrant PDCD4/eIF4A1 signal led to higher Ki-67 level. Interestingly, patients with low expressed PDCD4TILs had better prognosis, indicating the function heterogeneity of PDCD4 in different cell types. Furthermore, low PDCD4 TCs and high eIF4A1TCs predicted higher postoperative recurrence rate and are significant independent risk factors for early-stage OSCC.
Conclusion: Patients with low PDCD4TCs and high eIF4A1TCs have higher recurrence rate and poor clinical outcome. Of note, PDCD4TILs exerts contradictory function. Thus, PDCD4/eIF4A1 targeting therapeutics should consider the function heterogeneity of PDCD4.
Keywords: PDCD4, eIF4A1, early-stage OSCC, prognosis, diagnosis
Introduction
Oral squamous cell carcinoma (OSCC) is malignant oral tumor which accounts for 24% of head and neck cancers. Postoperative local recurrence is a main reason affecting 5-year survival rate of OSCC in early stage,1,2 therefore, discovery of effective biomarkers and their effects on therapeutic responses are awaited to improve the early-stage OSCC patient prognosis.
PDCD4 is a tumor suppressor gene that located at human chromosome 10q24. Compared with normal tissues, PDCD4 has a lower expression in many cancers, such as colorectal cancer, esophageal squamous cell carcinoma and medullary thyroid carcinoma.3–5 The deficiency of PDCD4 in colorectal cancer cells ultimately promoted cell survival, proliferation and metastasis.3 On the other hand, overexpression of PDCD4 in human prostate cancer cells induced a significant reduction in cell growth.6 The present research of PDCD4 are mainly conducted on cancer cells, but tumor is a heterogeneous cell population, it is necessary to study the expression pattern of PDCD4, including location and cell types.
The DEAD-box helicase eIF4A1 is needed to unwind structured RNA elements within the 5′ untranslated region (5′UTR) to enable ribosome binding and scanning. A number of known oncogenes such as CBC25B, SMAD2, c-myc, c-myb and TGFβ1 were confirmed as requiring eIF4A1 for their efficient translation.7 PDCD4 binds with eIF4A1 to inhibit its enzymatic activity, thereby leaving the mRNA methylated decapping process unfinished and inhibiting the proliferation of tumor cells. PDCD4/eIF4A1 signal influences breast cancer cell proliferation and cell cycle, decreased eIF4A1 activity slowed down cellular proliferation significantly.8 Degraded PDCD4 greatly enhanced eIF4A activity, then eIF4A-mediated enhancement of oncogene translation may be a critical component for lymphoma progression.9 However, the clinical significance of PDCD4/eIF4A1 signal axis is still unclear in OSCC, which limits its efficacy of targeting therapy.
In the present study, we focused on the expression pattern of PDCD4/eIF4A1 signal in OSCC, we analysed the temporal distribution of PDCD4/eIF4A1 signal in early-stage OSCC by IHC according to distinct cell components in tumor micro-environment, including tumor cells and tumor-infiltrating lymphocytes (TILs). Further, we determined correlations between the expression of PDCD4/eIF4A1 signal and clinic pathological parameters and postoperative local recurrence in this study. Our findings reveal this signal may promote OSCC progression with diagnostic and prognostic value, of which early-stage OSCC patients may have a worse prognosis.
Materials And Methods
Patients And Samples
The experimental study group randomly included 69 patients diagnosed from 2007 to 2014 with early-stage OSCC (T1N0M0-T2N0M0). The 5-year survival rate was 69.6% in the 69 samples. All the 69 cases of OSCC included 8 cases of gingival cancer, 8 cases of buccal cancer, 9 cases of palate cancer and 44 cases of tongue cancer. The patients with primary tumors were diagnosed by haematoxylin and eosin (H&E) staining by experienced pathologists, and this study was approved by the Research Ethics Committee of Nanjing Stomatology Hospital, Nanjing University. Written informed consent was obtained from all the patients. All these retrospective specimens were handled and anonymized according to ethical and legal standards. There were 21 patients died from OSCC (n=69) in our study until January 2019. None of the patients had received chemotherapy or radiotherapy prior to surgery and all 69 patients were followed-up until January 2019.
Immunohistochemistry
IHC was employed on 3 μm formalin-fixed paraffin-embedded sections using anti-PDCD4 (1:200; ab80590; Abcam, Cambridge, MA, USA), anti-Ki-67 (1:100; ab16667; Abcam) and anti-eIF4A1 (1:200; ab31217; Abcam). All sections were subsequently incubated with secondary antibody (Vector Laboratories, Burlingame, CA, USA) and developed in diaminobenzidine (DAB). All sections were then washed in PBS. Appropriate positive and negative controls were included for each relevant stain.
Quantification Of Immunohistochemistry
To evaluate the immune expression of PDCD4, eIF4A1 and Ki-67 in tumor cells, tumor-infiltrating lymphocytes (TILs) and stroma fibroblast-like cells (FLCs), slides were visualized by two senior pathologists who evaluated each expression quantitatively. The patterns of PDCD4 and eIF4A1 expression locations in OSCC specimens were defined and divided into two regions: tumor center and invasive front. Ki-67 staining was only scored in the tumor cells. We distinguished stroma FLCs from tumor cells according to the typical fibroblast shape, long spindle-shaped cells with small cytoplasmic protrusions located in the tumor stroma.
IHC staining was scored according to the percentage of positive cells and staining intensity.10 Staining intensity was graded as negative (=0), weak (=1), moderate (=2) or strong (=3), and reactivity was assessed by the percentage of positively staining cells (0–5% [=0], 5–25% [=1], 26–50% [=2], 51–75% [=3] or 76–100% [=4]). The IHC score was calculated by multiplying the grade of the staining intensity by that of the staining percentage, giving a minimum score of 0 and a maximum of 12. High and low expressions of PDCD4 and eIF4A1 were defined by the median of IHC scores.
Statistical Analysis
The SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) and Prism statistical software packages (version 8.0, GraphPad Software Inc., San Diego, CA, USA) were used for statistical analyses. Subgroups of each immunostaining parameter were divided by their median values. Pearson’s chi-square test and Fisher’s exact test were used to compare clinicopathological parameters of the patients. Kolmogorov–Smirnov and Shapiro–Wilk tests showed that the expression scores of PDCD4, eIF4A1 and Ki-67 did not follow a normal distribution. The Mann–Whitney U-test was used to compare the two groups (e.g. PDCD4 in TCs with recurrence, with recurrence versus without recurrence). OS and relapse-free survival (RFS) comparing high expression groups and low expression groups were estimated using Kaplan–Meier curves. Survival time was defined as the interval between the date of surgery and the last date when the patient was known to be relapse-free or alive (censored). The Cox regression model was used to examine interactions between different prognostic factors in a multivariate analysis. Differences were considered statistically significant with P<0.05.
Results
PDCD4 Is Expressed In Both TCs And TILs, But eIF4A1 Is Only Expressed In TCs
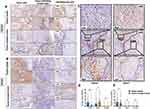
We assessed the PDCD4/eIF4A1 signal in different cell types including TCs, TILs and FLCs from tumor center to invasive front. A wide distribution of PDCD4 can be observed in TCs as well as in TILs, but it was absent in FLCs (Figure 1A and D). The expression of eIF4A1 was observed in TCs but absent in TILs and FLCs (Figure 1B and E). Considering the compartment heterogeneity of the tumor, we also assessed the expression of PDCD4/eIF4A1 signal in tumor center and invasive front, respectively. Interestingly, the expression of PDCD4 in IF is significantly lower than tumor center. Since the interaction between PDCD4 and eIF4A1 has been proved, we analysed the correlation of them and found that patients with high expression of PDCD4 attend to have lower expression of eIF4A1 in TCs (Tables 1 and 2). The staining results of 69 consecutive sections showed that the intensity of PDCD4 and eIF4A1 was conversed in the same region of the same specimen (Figure 1C).
 |
Table 1 Correlations Between PDCD4 And eIF4A1 Expression On Tumor Cell In Tumor Center |
 |
Table 2 Correlations Between PDCD4 And eIF4A1 Expression On Tumor Cell In Tumor Invasive Front |
Associations Of PDCD4/Eif4a1 Signal With Clinicopathological Characteristics
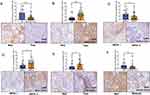
Considering that PDCD4/eIF4A1 signal was correlated with OSCC progression, we further analysed correlations between the distinct signal expression patterns and the clinicopathological characteristics of early-stage OSCC patients (Table 3 and S1). Here, we focus on PDCD4 in TCs and TILs and eIF4A1 in TCs. In tumor center, both PDCD4 and eIF4A1 expression had no association with age, smoking habits and T-stage, but we found that low expressed PDCD4 in TCs had poorer differentiation (Figure 2A) and a worse WPOI (Figure 2C). On the contrary, low expressed PDCD4 in TILs and eIF4A1 in TCs were found to have a better differentiation (Figure 2B and E). High expressed eIF4A1 in TCs was found to have a worse WPOI (Figure 2D). In invasive front, only the low expressed PDCD4 in TCs was detected to associate with a poorer differentiation (Figure 2F). Based on the conversed correlation between PDCD4 expression pattern in TCs and TILs with differentiation, the expression of PDCD4 seemed to exert distinct functions between TCs and TILs. According to these results, tumor cell-derived PDCD4 may negatively regulate the function of eIF4A1 to promote OSCC malignant phenotype.
 |
Table 3 Relationships Between PDCD4 With eIF4A1 Expression On Different Cell Types In Tumor Center And Clinicopathological Characteristics In OSCC Patients |
Low PDCD4 And High eIF4A1 Were Accompanied With Higher Ki-67 Staining
Since PDCD4 was found to inhibit cell proliferation in intraductal papillary mucinous neoplasm,11 we proposed the hypothesis that cell proliferation is attributed to the aberrant PDCD4/eIF4A1 signal expression. Herein, we assessed the expression of proliferation index Ki-67 in OSCC according to the PDCD4/eIF4A1 level. Results indicated that patients with low PDCD4 expression or high eIF4A1 expression were accompanied with higher Ki-67 staining (Figure 3A and B), suggesting PDCD4 and eIF4A1 may regulate OSCC cell proliferation.
Recurrent Patients Have A Low PDCD4TCs But A High eIF4A1TCs And PDCD4TILs Expression
In this study, all the 69 patients underwent complete surgical excision with negative margin, but there are still 14 patients occurred postoperative recurrence. Patients with recurrence have a lower expression of PDCD4 and a higher eIF4A1 expression in TCs (Figure 3C). PDCD4 overexpressed in TILs correlated with high recurrence rate, which also indicated the conversed function of PDCD4 in TILs and TCs. We also evaluated the correlation between metastasis and the signal expression, but the results demonstrated no significance (Figure 3D). Thus, we could speculate that the recurrence potential of early-stage OSCC is susceptive to low expressed PDCD4TCs, high expressed eIF4A1TCs and PDCD4TILs.
Low Expressed PDCD4TCs And High Expressed eIF4A1TCs Correlated With Shorter Survival Time Both In OS And RFS
The potential relationship between PDCD4/eIF4A1 signal expression and OS and RFS were evaluated in the 69 patients included in this study. All the 69 OSCC patients were divided into PDCD4high/low and eIF4A1high/low in TCs or TILs groups. The results indicated that high expression of PDCD4 in TCs correlated with shorter overall survival time (P=0.0004) (Figure 4A), but high expression of eIF4A1 in TCs correlated with longer overall survival time than the low expression group (P=0.0119) (Figure 4B). The contrasting correlation between PDCD4 and eIF4A1 expression and clinical outcome suggested that their impact on prognosis is completely opposite. Thus, our further analysis found that the patients with PDCD4low eIF4A1high expression in TCs had a poorer prognosis than those who with low expression of PDCD4 or high expression of eIF4A1 in TCs (Figure 4C). The same result also presented in the analysis of RFS in tumor center (Figure 4G–L). However, in invasive front, we found no significant difference between OS or RFS and PDCD4/eIF4A1 signal expression in this study (Figure 4D–F).
Low Expressed PDCD4TCs And High Expressed PDCD4TILs In Tumor Center Are Significant Independent Risk Factors For Early-Stage OSCC
The prognostic significance of clinicopathologic features were analyzed by univariate and multivariate analysis (Table 4 and S2). Results revealed that age, smoking, T-stage, differentiation, WPOI and eIF4A1 expression in TCs were not significant prognostic indicators for OS or RFS (P>0.05). However, low expression of PDCD4 in TCs or high expression in TILs were significant independent risk factors for OSCC with conversed function. However, eIF4A1 is not an independent risk factor for early-stage OSCC.
 |
Table 4 Prognostic Factors In The Cox Proportional Hazards Model For OS |
Discussion
Although one study has investigated the role of PDCD4 in OSCC,12 little is known about the co-expression pattern and prognostic value of PDCD4 and eIF4A1 in the same tumor. Ovarian cancer patients with lower PDCD4 expression were found to have shorter disease-free survival.13 Patricia et al found that loss of PDCD4 expression in OSCC is correlated with poorer survival and poorer disease-free survival of OSCC patients.10 eIF4A1 was also found to predict a poor prognosis in breast cancer.8 In our survival analysis, patients with low expression of PDCD4 and high expression of eIF4A1 in tumor cells had a greater risk of OSCC recurrence. These findings suggest that the PDCD4/eIF4A1 signal could be a potential indicator with an increased risk of early-stage OSCC recurrence.
PDCD4 is downregulated or lost in certain aggressive human carcinomas including the breast cancer, lung and colon cancer,14–16 and it is speculated to shuttle between nucleus and cytoplasm of tumor cells.17 eIF4A1 has not been widely investigated in tumors, while its upregulation in cytoplasm of ER-negative breast cancer cells was associated with a poor prognosis.8 We found that the expression pattern of PDCD4/eIF4A1 signal in TCs of OSCC is consistent with the results of previous studies on other cancer cells. However, due to the limited expression of eIF4A1 in TILs, the effection by PDCD4 on tumor progression may through other factors in TILs. Studies on PDCD4 mainly focused on tumor center, but was deficient on invasive front. Interestingly, we detected a significant decrease expression of PDCD4 in the invasive front. Thus, PDCD4 expression exhibited a highly heterogeneous pattern within the tumors depending on the different area of tumor, which could certainly have a contribution on the functional heterogeneity of these tumor regions.
Previous studies revealed that PDCD4 inhibits eIF4A1 to suppress translation of mRNAs with highly structured 5′UTR.17 However, the widespread clinical significance of this signal is impeded by the current lack of studies on the PDCD4/eIF4A1 in tumors. Limited evidence showed that the expression of PDCD4 and eIF4A1 was negatively related in breast cancer,8 similarly, we found a conversed expression pattern of these two proteins in OSCC, which was consistent with the known inhibitory effect on eIF4A1 by PDCD4.
This study demonstrated that the PDCD4/eIF4A1 signal expression is associated with the differentiation and WPOI of tumors. It is well known that epithelial differentiation is a determining factor in the prognoses of cancers, poorly differentiated cancers are highly proliferative compared with highly differentiated counterparts. In addition, differentiation is important in maintaining the tumorigenic and treatment-resistant of cancer stem cell in head and neck cancers.18 Valproic acid and all-trans retinoic acid were demonstrated to inhibit the growth of head and neck cancers by inducing terminal differentiation,19 another study has shown that the expression of PDCD4 was induced in acute myelocytic leukemia (AML) cell lines while in the presence of all-trans retinoic acid.20 Hence, PDCD4 restoration may be a novel approach for differentiation cancer therapy in OSCC, leading to effective suppression of tumor development and improving the prognosis.
We observed an opposite effect on prognosis exerted by the expression of PDCD4 between TCs and TILs,it demonstrated the functional heterogeneity of PDCD4 in different cell types. Lingel et al found that deficiency of PDCD4 in CD8+T cell has been proved to attenuate effector functions, leading to abrogated anti-tumor responses.21 Shankaran et al also observed that increased IFN-γ levels in PDCD4-deficient CTLs could be responsible for an enhanced anti-tumor activity.22 Although we have not classified lymphocyte subsets, the significance of PDCD4 expression in OSCC TILs is still consistent with anti-tumor role of low expressed PDCD4. So it is tempting to speculate that PDCD4 as a promising target in tumor immune microenvironment for therapeutic interventions.
In conclusion, the PDCD4/eIF4A1 signal expression level in early-stage OSCC was related to parameters reflecting primary tumor burden, including differentiation and WPOI at initial diagnosis. High expressed eIF4A1 or low expressed PDCD4 in TCs were correlated with a greater risk of recurrence, and also predict a poor outcome in early-stage OSCC. However, compared with TCs, the prognostic value of PDCD4 expression in TILs presents a converse phenomenon, suggesting that we should take the distinct expression pattern of PDCD4 into consideration when the PDCD4/eIF4A1 targeting therapeutics is administered to OSCC.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81772880 to YN), Natural Science Foundation of Jiangsu Province (No. BK20190304 to LD), China Postdoctoral Science Foundation (No. 2019M651789 to LD), Nanjing Medical Science and Technology Development Foundation, Nanjing Department of Health (No. ZKX17032 to ZW), Jiangsu Key Research & Development Program (No. BE2018618 to ZW), Fundamental Research Funds for the Central Universities (No. 021014380117 to LD).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang TY, Hsu LP, Wen YH, et al. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010;46:49–55. doi:10.1016/j.oraloncology.2009.10.011
2. Kernohan MD, Clark JR, Gao K, Ebrahimi A, Milross CG. Predicting the prognosis of oral squamous cell carcinoma after first recurrence. Arch Otolaryngol Head Neck Surg. 2010;136:1235–1239. doi:10.1001/archoto.2010.214
3. Wang L, Zhao M, Guo C, et al. Pdcd4 deficiency aggravated colitis and colitis-associated colorectal cancer via promoting il-6/stat3 pathway in mice. Inflamm Bowel Dis. 2016;22:1107–1118. doi:10.1097/MIB.0000000000000729
4. Wu Y, Hu L, Liang Y, et al. Up-regulation of lncRNA casc9 promotes esophageal squamous cell carcinoma growth by negatively regulating pdcd4 expression through ezh2. Mol Cancer. 2017;16:150. doi:10.1186/s12943-017-0715-7
5. Pennelli G, Galuppini F, Barollo S, et al. The pdcd4/mir-21 pathway in medullary thyroid carcinoma. Hum Pathol. 2015;46:50–57. doi:10.1016/j.humpath.2014.09.006
6. Shiota M, Izumi H, Tanimoto A, et al. Programmed cell death protein 4 down-regulates y-box binding protein-1 expression via a direct interaction with twist1 to suppress cancer cell growth. Cancer Res. 2009;69:3148–3156. doi:10.1158/0008-5472.CAN-08-2334
7. Stoneley M, Willis AE. Eif4a1 is a promising new therapeutic target in er-negative breast cancer. Cell Death Differ. 2015;22:524–525. doi:10.1038/cdd.2014.210
8. Modelska A, Turro E, Russell R, et al. The malignant phenotype in breast cancer is driven by eif4a1-mediated changes in the translational landscape. Cell Death Dis. 2015;6:e1603. doi:10.1038/cddis.2014.542
9. Steinhardt JJ, Peroutka RJ, Mazan-Mamczarz K, et al. Inhibiting card11 translation during BCR activation by targeting the eif4a RNA helicase. Blood. 2014;124:3758–3767. doi:10.1182/blood-2014-07-589689
10. Reis PP, Tomenson M, Cervigne NK, et al. Programmed cell death 4 loss increases tumor cell invasion and is regulated by mir-21 in oral squamous cell carcinoma. Mol Cancer. 2010;9:238. doi:10.1186/1476-4598-9-238
11. Hayashi A, Aishima S, Miyasaka Y, et al. Pdcd4 expression in intraductal papillary mucinous neoplasm of the pancreas: its association with tumor progression and proliferation. Hum Pathol. 2010;41:1507–1515. doi:10.1016/j.humpath.2010.02.019
12. Desai KM, Kale AD. Immunoexpression of programmed cell death 4 protein in normal oral mucosa, oral epithelial dysplasia and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2017;21:462. doi:10.4103/jomfp.JOMFP_115_17
13. Wei NA, Liu SS, Leung TH, et al. Loss of programmed cell death 4 (pdcd4) associates with the progression of ovarian cancer. Mol Cancer. 2009;8:70. doi:10.1186/1476-4598-8-70
14. Wang Y, Liu Z, Shen J. MicroRNA-421-targeted pdcd4 regulates breast cancer cell proliferation. Int J Mol Med. 2018. doi:10.3892/ijmm
15. Zhen Y, Li D, Li W, et al. Reduced pdcd4 expression promotes cell growth through pi3k/akt signaling in non-small cell lung cancer. Oncol Res. 2016;23:61–68. doi:10.3727/096504015X14478843952861
16. Lu Y, Han B, Yu H, Cui Z, Li Z, Wang J. Berberine regulates the microRNA-21-itgbeta4-pdcd4 axis and inhibits colon cancer viability. Oncol Lett. 2018;15:5971–5976. doi:10.3892/ol.2018.7997
17. Wang Q, Yang HS. The role of pdcd4 in tumour suppression and protein translation. Biol Cell. 2018. doi:10.1111/boc.201800014
18. Lim YC, Kang HJ, Kim YS, Choi EC. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of wnt/beta-catenin pathway. Eur J Cancer. 2012;48:3310–3318. doi:10.1016/j.ejca.2012.04.013
19. Gan CP, Hamid S, Hor SY, et al. Valproic acid: growth inhibition of head and neck cancer by induction of terminal differentiation and senescence. Head Neck. 2012;34:344–353. doi:10.1002/hed.v34.3
20. Ozpolat B, Akar U, Steiner M, et al. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid-induced terminal granulocytic differentiation of human myeloid leukemia cells. Mol Cancer Res. 2007;5:95–108. doi:10.1158/1541-7786.MCR-06-0125
21. Lingel H, Wissing J, Arra A, et al. CTLA-4-mediated posttranslational modifications direct cytotoxic t-lymphocyte differentiation. Cell Death Differ. 2017;24:1739–1749. doi:10.1038/cdd.2017.102
22. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi:10.1038/35074122
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.