Back to Journals » Drug Design, Development and Therapy » Volume 12
Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2– advanced breast cancer
Authors Corona SP , Generali D
Received 15 November 2017
Accepted for publication 11 January 2018
Published 16 February 2018 Volume 2018:12 Pages 321—330
DOI https://doi.org/10.2147/DDDT.S137783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Silvia Paola Corona,1 Daniele Generali2
1Radiation Oncology Department, Peter MacCallum Cancer Centre, Bentleigh East, VIC, Australia; 2Department of Medical, Surgery and Health Sciences, University of Trieste, Trieste, Italy
Abstract: Although early breast cancer (BC) is highly curable, advanced or metastatic disease poses numerous challenges in terms of medical management and treatment decisions and is associated with significantly worse prognosis. Among the new targeted agents, anticancer drugs exploiting the cell-cycle machinery have shown great potential in preclinical studies. CDK4/6 inhibitors target the cyclin D/CDK/retinoblastoma signaling pathway, inducing cell-cycle arrest, reduced cell viability and tumor shrinking. As the cyclin D/CDK complex is activated downstream of estrogen signaling, the combination of CDK4/6 inhibitors with standard endocrine therapies represents a rational approach to elicit synergic antitumor activity in hormone receptor-positive BC. The results of clinical trials have indeed confirmed the superiority of the combination of CDK4/6 inhibitors plus endocrine therapies over endocrine therapy alone. Currently approved are three compounds that exhibit similar structural characteristics as well as biological and clinical activities. Abemaciclib is the latest CDK4/6 inhibitor approved by the US Food and Drug Administration (FDA) in view of the results of the MONARCH 1 and 2 trials. Further trials are ongoing as other important questions await response. In this review, we focus on abemaciclib to examine preclinical and clinical results, describing current therapeutic indications, open questions and ongoing clinical trials.
Keywords: CDK4/6 inhibitor, abemaciclib, breast cancer, hormone receptor-positive BC, metastatic BC, mBC
Introduction
Breast cancer (BC) is the most common cancer in women worldwide,1 accounting for ~30% of all cancers, and the second cause of cancer-related death after lung cancer. The American Cancer Society estimated that 252,710 new cases will be diagnosed and 40,610 women will die of BC in the US in 2017.2
While the availability of new therapies and treatment combinations has drastically improved outcomes and early BC is highly curable, ~20% of women will experience local or distant recurrence at some point in time, even after 5–10 years from diagnosis,3,4 mainly due to acquired pharmacological resistance. Prognosis for these patients is worse, and new approaches are needed.
Among novel possible therapeutic targets, proteins involved in the control of the cell-cycle machinery have recently attracted a lot of interest, as dysregulation of cellular proliferation is recognized as one of the hallmarks of malignant transformation.5,6 Progression through the cell cycle is tightly regulated in mammalian cells by a group of proteins called cyclins, which in turn pair with and activate serine–threonine kinases (cyclin-dependent kinases [CDKs]). Specific cyclin/CDK heterodimeric complexes regulate transition through the stages of the cell cycle acting as checkpoints. One of their targets, the retinoblastoma (Rb) protein, also represents a critical checkpoint regulator in mammalian cells, directly controlling progression from Phase G1 to S,7 DNA synthesis and irreversible commitment to cellular division at the so-called “G1 restriction point”. When hypophosphorylated, Rb inhibits progression through the cell cycle by binding to and suppressing the activity of the E2F family of transcription factors.8 The G1–S cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase 6 (CDK6) in complexes with cyclin D first and the CDK2/cyclin E later promote phosphorylation of Rb, thus overriding Rb inhibition of proliferation and initiating cellular division (Figure 1).9
Alterations in the cyclin D/CDK/Rb pathway arise in 90% of cancers.10 In particular, multiple molecular aberrations result in disruption of this pathway in 50%–70% of BCs.11,12 Overexpression of cyclin D1 is the most frequent alteration, found in ~50%–60% of BCs, in particular in luminal B and HER2-positive BCs.11,13 Loss of expression of the Rb protein occurs in 20%–30% of BCs, mainly in the triple-negative BC (TNBC) molecular subtype. Finally, loss of p16, an endogenous CDK4 and CDK6 inhibitor, occurs in ~50% of invasive BCs.13–15
Considering the relative frequency of genetic aberrations of cell-cycle-regulating proteins found in BC, the therapeutic potential of targeting this pathway became increasingly clear in the past 10 years.
The first generation of CDK4 and CDK6 inhibitors (ie, flavopiridol) underwent clinical testing with generally disappointing results. In fact, due to the lack of selectivity for the target, these pan-CDK inhibitors showed an unfavorable safety profile, with a range of drug-mediated, dose-limiting side effects.16–18 Moreover, the intravenous route and the schedule of administration added complexity to the treatment regimens impacting on patient compliance. Flavopiridol, the most studied compound of this group, showed scarce activity as a single agent and only moderate activity in combination with chemotherapy.18
The second generation of CDK inhibitors, even though designed to be more selective, was limited by severe toxicities. Dinaciclib induced severe adverse events in 60% and 74% of patients in a Phase I and a Phase II clinical trial, respectively, while its effectiveness remained limited.19,20
The next generation of CDK inhibitors, besides the advantage of the oral route of administration, showed a much higher selectivity, specifically targeting CDK4 and CDK6.21,22 Three compounds, palbociclib (PD 0332991; Pfizer, Inc.), ribociclib (LEE011; Novartis International AG) and abemaciclib (LY2835219; Eli Lilly and Company), underwent preclinical and clinical testing and are currently approved for the treatment of advanced and/or metastatic hormone receptor-positive/HER2-neu-negative BC (HR+/HER2−).
This review focuses on abemaciclib in HR+/HER2− BC, examining preclinical and clinical results, current therapeutic indications and ongoing clinical trials.
Abemaciclib: mode of action and preclinical results
LY2835219 (abemaciclib) was identified via compound and biochemical screening by scientists at Eli Lilly and Company Research Laboratories and selected for its biological activity and highly selective inhibition of the complexes CDK4/cyclin D1 (IC50 =2 nmol/L) and CDK6/cyclin D1 (IC50 =10 nmol/L), with no activity against other CDK/cyclin complexes or cell-cycle-related kinases within the nanomolar ranges, except for inhibition of CDK9 at IC50 at least five times higher (Figure 2).23 The compound was shown to act as a competitive inhibitor of the ATP-binding domain of the CDK4 and CDK6 and to be 14 times more potent against CDK4 than against CDK6.24 In comparison to palbociclib and ribociclib, abemaciclib shows higher selectivity for the complex CDK4/cyclin D1, with IC50 values five times lower than those of the two other compounds (Table 1).
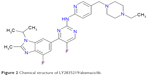  | Figure 2 Chemical structure of LY2835219/abemaciclib. |
  | Table 1 CDK4/6 inhibitors’ selectivity |
Inhibition of phosphorylation of Rb protein resulted in G1 cell-cycle arrest in both in vitro and in vivo experiments on multiple cancer cell lines from colorectal, lung, glioblastoma and blood cancers. When tested on tumor xenografts in nude mice, LY2835219 elicited potent dose-dependent antitumor activity, comparable to that of PD0332991 (palbociclib), inducing ~70% of tumor volume regression, and was well tolerated when administered on a continuous schedule. The authors also tested the compound in vivo in combination with gemcitabine, reporting a synergic effect of the combination on Calu-6 lung subcutaneous xenografts and a greater antitumor activity in comparison to the single treatments in the absence of enhanced toxicity.23
Besides the cell-cycle dependent activity, Goel et al26 recently showed that abemaciclib is able to boost antitumor immunity by potentiating tumor antigen presentation and selectively suppressing proliferation of regulatory T (Treg) cells at the same time. In this very elegant work, abemaciclib was shown to upregulate expression of type III interferons and interferon-stimulated genes/transcription factors, such as STAT1, STAT2, IRF2, IRF6, IRF9 and NLRC5, in the tumors of a transgenic mouse model of BC. At the same time, the CDK4/6 inhibitor reduced the number of Treg cells in the spleen and lymph nodes of both tumor-bearing and tumor-free wild-type mice (tumor-independent effect). When these cells were isolated and cultured in vitro, addition of abemaciclib slowed down their proliferation without affecting CD8+ or CD4+ T cells. The same effect was observed in vivo in abemaciclib-treated tumors.
Ultimately, all these effects induced cytotoxic T cell-mediated killing of tumor cells which, as suggested in the study, could be further increased with the addition of anti-immune checkpoint therapies. The authors were able to demonstrate that the antitumor activity of abemaciclib is dependent on the presence of intratumoral cytotoxic T lymphocytes.
In addition, the authors confirmed previous reports’ finding that LY2835219/abemaciclib acts by promoting cellular senescence phenotypes in BC cells, as shown by the presence of marked hypermethylation and accumulation of endogenous beta-galactosidase.24,26
More specific to LY2835219 in comparison to other CDK4 and CDK6 inhibitors is the ability to cross the blood–brain barrier, with concentrations of the drug in the cerebrospinal fluid comparable to the ones in plasma.27–31
Experiments in vitro and in vivo on mouse xenografts models of glioblastoma showed that palbociclib can also cross the blood–brain barrier,32 but subsequent clinical studies have provided inconsistent results.33
In view of these findings, abemaciclib is being tested in the clinic and holds promise in primary brain tumors (NCT03220646, NCT02981940) and in brain metastases from breast or other cancers (Bachelot et al. Poster presentation at 2017 San Antonio Breast Cancer Symposium; December 6–9, 2017; San Antonio, TX. Abstract P1-17-03).30,31
Abemaciclib in clinical trials
Phase I
Based on the very promising results obtained in preclinical studies, abemaciclib entered clinical development. In Phase I studies, abemaciclib, alone and in combination with fulvestrant or other antihormone therapies, showed favorable pharmacokinetic and toxicity profiles in patients with hormone-positive metastatic breast cancer (mBC), with most common grade 3 treatment-related side effects being diarrhea, neutropenia, nausea and fatigue. No febrile neutropenia or grade 4 events were reported.34–36 Single-agent abemaciclib was well tolerated when given on a continuous schedule to patients with different cancers, and fatigue was the dose-limiting side effect in a more recent Phase I study.30 In all the trials, the drug showed antitumor activity in multiple tumor types, including BC, and in often heavily pretreated patients, with an objective response rate (ORR) of 26% in hormone-refractory estrogen receptor positive (ER+) mBC when given as single therapy30 and disease control rates ranging from 70% in all tumor types to 81% in HR+ patients.34 The most encouraging results were obtained in the group of HR+ mBC patients treated with the combination of abemaciclib and fulvestrant, which elicited 62% of confirmed partial responses (PRs) in patients who had received on average four prior systemic therapies.35
Phase II
These results prompted the launch of a Phase II trial, MONARCH 1, to evaluate the antitumor activity of abemaciclib as a single agent in patients with refractory HR+/HER2– mBC who received prior chemotherapy after progression on endocrine therapies.37 This single-arm study enrolled 132 hormone receptor-positive mBC patients who had progressed on endocrine therapy and already received multiple systemic therapies (average of three prior systemic regimens). Abemaciclib was orally administered, at a dose of 200 mg twice daily, on a continuous schedule, until disease progression or unacceptable toxicity. The primary end point of the study was ORR, calculated as the total number of complete response (CR) or PR divided by the total number of patients; secondary end points were clinical benefit rate, progression-free survival (PFS) and overall survival (OS).
Worth noting was that 90.2% of patients had visceral disease and 50.8% had more than three sites of metastases.
Single-agent abemaciclib induced PRs (measured by RECIST criteria v 1.1) in 26 (19.7%) of the total 132 patients enrolled. No CRs were detected, with an ORR of 19.7% (95% CI: 13.3–27.5). The clinical benefit rate was 42.4%. Median PFS was 6 months (95% CI: 4.2–7.5), and median OS was 17.7 months (95% CI: 16–not reached). At the final analysis, at 18 months, median OS was 22.3 months (95% CI: 17.7–not reached).
Serious adverse events (SAEs) were reported in 32 (24.2%) patients, and grade 5 events, all deemed not related to the drug, occurred in three patients. Diarrhea was the most frequent adverse event, reported by 119 (90.2%) patients, most often grade 1 (41.7%) or grade 2 (28.8%) and most frequently during the first cycle of treatment. Grade 3 diarrhea was observed in 26 (19.7%) patients. Duration of episodes was generally limited, and loperamide was administered in 60.6% of patients. The majority of patients did not need abemaciclib dose reduction or discontinuation (72.3%), and only one patient permanently ceased treatment because of this adverse event. Other common adverse events were fatigue, nausea and decreased appetite, in accordance with previous reports.
All grades neutropenia was observed in 87.7% of patients. Grade 3 neutropenia occurred in 22.3% of patients, whereas 4.6% of patients had a grade 4 event. One patient had febrile neutropenia in the follow-up period and after discontinuation of the drug, during successive chemotherapy.
A dose reduction of abemaciclib due to adverse events other than diarrhea was necessary in 65 (49.2%) patients.
Phase III
The favorable pharmacokinetics and the strong evidence of an antitumor effect prompted the initiation of Phase III clinical trials.
MONARCH 2 compared the combination of abemaciclib and fulvestrant to fulvestrant alone in patients with HR+/HER2− advanced BC, which had progressed on endocrine therapy.38 In this international double-blind Phase III trial, 669 women were randomized to receive fulvestrant plus placebo or fulvestrant plus abemaciclib. Patients received abemaciclib or placebo twice daily on a continuous schedule of 28-day cycles. When the study began, patients in the abemaciclib arm were receiving 200 mg twice daily. After a safety data review and the analysis of dose-reduction rates among patients, the original dose was reduced to 150 mg for all patients, new and already enrolled. The trial’s primary end point was investigator-assessed PFS, and the secondary end points were OS, ORR and duration, clinical benefit rate, quality of life and safety.
The combination of abemaciclib and fulvestrant demonstrated superiority to treatment with fulvestrant alone in this group of patients: median PFS in the combination group was 16.4 months in comparison to 9.3 months of the fulvestrant-alone group (hazard ratio [HR] 0.553; 95% CI: 0449–0.681; p<0.001). ORR in patients with measurable disease was 48.1% (95% CI: 42.6–53.6) in the abemaciclib plus fulvestrant group versus 21.3% (95% CI: 15.1–27.6) in the fulvestrant-alone group (p<0.001). The median duration of response was not reached in the abemaciclib plus fulvestrant group at the time of the analysis. CR occurred in 11 (3.5%) patients with measurable disease. No CR was achieved in the fulvestrant-alone group.
The most common adverse events of any grade were diarrhea, neutropenia, nausea, fatigue and abdominal pain. Only 1.4% of the patients on abemaciclib experienced febrile neutropenia. Of these, one febrile neutropenia occurred 53 days after discontinuation of investigational drug, when the patient had already started chemotherapy. Grade 1 or 2 diarrhea occurred in 73% of patients on abemaciclib plus fulvestrant versus 24.2% of the patients on fulvestrant alone. Grade 3 diarrhea occurred in 13.4% of patients on the combination therapy versus 0.4% of those on fulvestrant alone. The majority of patients who experienced diarrhea did not require dose adjustments or discontinuation (70.1%), and only 2.9% of patients discontinued abemaciclib due to this side effect.
Thromboembolic events were the most frequently reported SAEs, occurring in 2% of the abemaciclib group patients versus 0.4% of the fulvestrant-alone group patients. None of these episodes resulted in death.38
In view of these results, on the September 28, 2017, the US Food and Drug Administration (FDA) granted approval for abemaciclib in combination with fulvestrant in patients with HR+/HER2− advanced BC or mBC who progressed following endocrine therapy. On the same date, and on the basis of the MONARCH-1 trial results,39 abemaciclib also gained FDA approval as a single-agent therapy in patients with HR+/HER2− mBC who experienced disease progression following endocrine therapies and prior chemotherapy.
Furthermore, in September 2017, results of the MONARCH 3 trial of abemaciclib in combination with a nonsteroidal aromatase inhibitor (letrozole or anastrozole) were presented at the European Society for Medical Oncology (ESMO) 2017 Congress in Madrid40 and published immediately after.41
This randomized Phase III study compared abemaciclib or placebo in combination with a nonsteroidal aromatase inhibitor in postmenopausal patients with advanced HR+/HER2− BC who had no previous systemic therapies. The trial enrolled 493 patients. Abemaciclib was given at the standard dose of 150 mg twice daily (on a continuous schedule), while anastrozole and letrozole were given at 1 and 2.5 mg daily, respectively. The primary objective of the trial was investigator-assessed PFS, and secondary objectives were evaluation of response and safety assessment.
Addition of abemaciclib to nonsteroidal aromatase inhibitors significantly prolonged PFS with an observed HR of 0.54 (95% CI: 0.41–0.72; p<0.000021), while the median PFS was not reached in the abemaciclib arm at interim analysis against 14.7 months in the placebo arm. Independent central review reached similar results (HR 0.51; 95% CI: 0.36–0.72).
ORR was 48.2% (95% CI: 42.8%–53.6%) in the patients receiving abemaciclib versus 34.5% (95% CI: 27.3%–41.8%) in the placebo-assigned patients (p<0.002). In patients with measurable disease, ORR was 59.2% (95% CI: 53.3%–65.1%) with abemaciclib and 43.8% (95% CI: 35.3%–52.4%) with placebo (p<0.004). Clinical benefit was achieved by 78% (95% CI: 73.6%–82.5%) of the patients on abemaciclib and 71.5% (95% CI: 64.6%–78.4%) of the ones on placebo. Although all patients’ subgroups benefited from the addition of abemaciclib to endocrine therapy, it is worth noting that patients with better prognostic factors, such as prolonged treatment-free interval, bone-only secondary disease and no liver metastases, responded well to endocrine therapy alone. On the other hand, patients with shorter treatment-free interval or who presented liver metastases at baseline recorded higher benefit rate with abemaciclib.
The safety assessment demonstrated consistent results with previous abemaciclib trials: the most frequent all-grade adverse events in the investigational arm were diarrhea (81.3%), neutropenia (41.3%), fatigue (40.1%) and nausea (38.5%). The vast majority of diarrhea events were grade 1 and 2 and occurred during the first cycle of therapy. Grade 3 diarrhea was reported in 9.5% of cases. SAEs occurred in 27.5% of patients treated with abemaciclib and in 14.9% of patients treated with placebo. The most common SAE was lung infection, occurring in 2.8% of patients on abemaciclib versus none of the patients on placebo.
Other ongoing Phase III studies are currently looking at the combination of abemaciclib and standard endocrine therapies in patients with early stage, high risk, node positive HR+/HER2− BC (NCT03155997, MONARCH E), as well as in postmenopausal patients with locoregionally recurrent or metastatic HR+/HER2− BC (NCT02763566, MONARCH plus; Table 2).
Abemaciclib is also being tested in other BC molecular subtypes, including HER2-positive BC and TNBC (NCT02675231, NCT03130439; Table 2).
Discussion
Numerous studies have highlighted the strong direct link existing between estrogen signaling pathway and the cell cycle.
Estrogen stimulation induces cell-cycle progression from G1 to S phase42 and increases cellular proliferation via upregulation of downstream cyclin D1 and CDK4/6, as well as C-MYC and cyclin E/CDK2.43 On the other hand, estrogen inhibition therapy, with aromatase inhibitors, tamoxifen and fulvestrant, induces cell-cycle arrest and consequently decreases cell viability.44,45
Moreover, Rb gene-negative breast tumors are resistant to tamoxifen in xenograft models and in the clinical setting,46,47 proving that disruption of the Rb signaling pathway is indeed implicated in the cancer-inducing effects of estrogens.
Selective CDK4/6 inhibitors have shown impressive results in combination with antihormone therapies in advanced BC, leading to FDA approval of three compounds, palbociclib, ribociclib and most recently abemaciclib. The main representative and the first of the anti-CDK4/6 family obtaining approval for first-line therapy in ER-positive advanced BC and mBC was palbociclib, in combination with letrozole and fulvestrant, respectively. In the PALOMA-1/TRIO-18 trial, the combination of palbociclib plus letrozole significantly increased the PFS from 7.5 months to 26.1 months (HR 0.37; p<0.001) in post-menopausal women with advanced BC.48 Subsequently, palbociclib was tested in combination with fulvestrant in comparison to fulvestrant alone in patients with HR+/HER2− BC who progressed or relapsed after endocrine therapy (PALOMA 3 trial).49,50 The combination of palbociclib with fulvestrant granted longer PFS and a better quality of life in comparison to fulvestrant alone in these patients, irrespective of menopausal status. Median PFS was 9.2 months with palbociclib–fulvestrant versus 3.8 months with placebo–fulvestrant (HR 0.42; p<0.001).
Ribociclib’s approval by the FDA for first-line treatment of postmenopausal women with HR+/HER2− advanced BC or mBC is based on the results from the Phase III MONALEESA-2 trial.51 The trial enrolled 668 postmenopausal women with HR+/HER2− advanced BC or mBC who received no prior systemic therapy for their disease. The combination of ribociclib plus letrozole reduced the risk of progression (or death) by 44% over letrozole alone (with median PFS not reached in the combination arm [95% CI: 19.3 months–not reached] versus 14.7 months [95% CI: 13.0–16.5 months] in the letrozole alone arm; HR 0.556 [95% CI: 0.429–0.720]; p<0.0001). The 24-month second interim analysis confirmed the superiority of the combination, with PFS rates of 54.7% for ribociclib plus letrozole versus 35.9% for letrozole alone.52
Abemaciclib was the last CDK4/6 inhibitor approved by the FDA in combination with fulvestrant for the treatment of HR+/HER2− advanced BC or mBC, which has progressed with endocrine therapy. Notably, on the basis of the results of the MONARCH 1 trial,39 abemaciclib also obtained approval as a single-agent therapy in patients with HR+/HER2− mBC who experienced disease progression following endocrine therapy and prior chemotherapy.
While all three CDK4/6 inhibitors exhibit the same mechanism of action, inhibiting the ATP-binding domain of the kinases, there are some structural and biochemical differences between the compounds (reviewed in Chen et al53), which account for differences in clinical activity.
While single-agent palbociclib and ribociclib showed only minimal antitumor activity,54–56 abemaciclib showed clinical benefit in heavily pretreated patient populations with metastatic HR+/HER2− BC who experienced progression with previous treatments37,39 and has thus been approved with this indication. Even though MONARCH 1 was a small Phase II study, the results indicate that abemaciclib can be used in endocrine-resistant metastatic tumors. In this patient group, the only remaining choice is chemotherapy, with very low response rates and median PFS of 3–4 months. On this trial, patients on abemaciclib, 90% of whom had visceral metastases and >50% had three or more sites of metastasis, showed a longer PFS (6 months) and a clinical benefit rate of 42.4%.
The reason behind the antitumor activity of abemaciclib in monotherapy is not completely understood. The administration schedule of abemaciclib is continuous, in contrast with that of both palbociclib and ribociclib, which is given consecutively for 21 days, followed by a 7 days break to control the dose-limiting myelosuppressive effect of these compounds. Preclinical studies in vitro and in vivo have shown that short-term inhibition of CDK4/6 in tumor cells causes a rebound effect with increased cellular proliferation when the inhibition is withdrawn.23,57 This effect could explain the efficacy of continuously dosed abemaciclib as monotherapy in comparison to intermittently dosed palbociclib and ribociclib.
In addition, the safety profile of the three drugs is quite different. The dose-limiting toxicity (DLT) with palbociclib and ribociclib is neutropenia, while that with abemaciclib is fatigue. Grade 3 neutropenia is generally a relatively rare event with abemaciclib but occurs in up to 65% of the patients treated with palbociclib58 and 59.3% of the patients treated with ribociclib.51 On the other hand, gastrointestinal on-target effects, such as diarrhea, nausea and abdominal pain, are the most frequent toxicities reported with abemaciclib.
Abemaciclib is a 14 times more potent inhibitor of the CDK4/cyclin D1 complex24 than CDK6/cyclin D1/2/3. While activation of CDK4 is involved in breast tumorigenesis via cyclin D1 overexpression or genetic aberrations, CDK6 is important for hematopoiesis and differentiation of blood cells.59,60 Inhibition of CDK6 by palbociclib and ribociclib is responsible for the myelosuppression reported in the majority of patients using these drugs, which ultimately explains the intermittent regimen of administration.
Scientists are actively trying to elucidate the mechanisms behind non-hematological toxicities of abemaciclib. Besides CDK4 and CDK6, the ability, exclusive to abemaciclib, to target CDK9, a broadly expressed CDK regulating gene transcription, embryogenesis and cellular proliferation, may account for the specific toxicity profile and gastrointestinal adverse events.61
Furthermore, abemaciclib has shown a regular distribution across multiple systems, including the cerebrospinal fluid, with concentrations of drug comparable to those found in plasma.62 This finding has sparked interest for its use in treating primary and secondary brain tumors, and these indications are currently being investigated in clinical trials (NCT02308020, NCT02981940, NCT03220646, NCT02644460) with encouraging preliminary results.31 Palbociclib, on the other hand, has provided contrasting results in this setting,33 and further clinical studies are ongoing (NCT02774681, NCT02255461, NCT02255461).
Given the importance of PI3K signaling pathway’s genetic alterations in the pathogenesis of ER+/HER2− BC, abemaciclib is currently being investigated in doublet and triplet combinations with dual PI3K/mTOR inhibitors plus or minus hormonal therapies (NCT02057133, NCT01655225).
Furthermore, in view of the recent pivotal report on the immune-boosting antitumor activity of abemaciclib,26 randomized trials testing the combination of the anti-CDK4/6 inhibitor with approved immunotherapies such as pembrolizumab (NCT02779751) and the novel anti PD-L1 antibody LY3300054 (PACT/NCT02791334) hold great promise.
Finally, following evidence that HER2 positive breast tumors resistant to trastuzumab are driven by cyclin D1 overexpression,63 abemaciclib is currently being investigated in ER+/HER2+ advanced BC in combination with trastuzumab and fulvestrant in patients who progressed on previous anti-HER2 therapies (at least two lines) (monarcHER/NCT02675231).
A parallel comparison of the three CDK4/6 inhibitors currently approved for therapy in advanced and metastatic HR+/HER2− BC could potentially help defining better therapeutic indications but is still lacking.
In addition, as acquired resistance to CDK4/6 inhibitors occurs, the question of whether a CDK4/6 can be substituted with another after progression is important and open. In fact, given the biological and clinical differences between the compounds, it may be hypothesized that the mechanisms of pharmacological resistance to one compound differ from the mechanisms of resistance to others. If this is the case, sequential therapy with CDK4/6 inhibitors could still be of benefit, perhaps in combination with other targeted therapies in selected patient subsets.
The identification of specific biomarkers could also help selecting subgroups of patients who are more likely to respond to one CDK4/6 inhibitor rather than the others.
HR+/HER2− advanced BC unmet needs
In the last 20 years, enormous progress in translational research has resulted in the development of numerous targeted therapies for the treatment of HR+/HER2− BC. Even in the presence of such armamentarium of new drugs, clinical results have been somehow disappointing, specifically in the metastatic setting, major setbacks being occurrence of pharmacological resistance and lack of reliable biomarkers of response to treatment.
Before the advent of CDK4/6 inhibitors, the only remaining option for patients who progressed on endocrine therapies was chemotherapy, with response rates in the order of 10%–20% only.
Development of acquired resistance to endocrine and targeted therapies represents a major issue in the management of advanced and metastatic ER+ BC. Personalized medicine implies knowledge of the single tumor mutational landscape. While the advent of liquid biopsies and the analysis of secondary lesions via solid tumor biopsy will certainly facilitate identification of newly occurring mutations, subclonal heterogeneity within both primary and secondary lesions may limit efficacy of targeted therapies.
To tackle this intrinsic heterogeneity, next-generation sequencing offers the possibility to characterize the whole genotype of the tumor, identifying the mutations cells are addicted to.
In this context, multiple reports have highlighted the importance of a combinatorial therapeutic approach to overcome occurrence of pharmacological resistance by targeting multiple signaling pathways at the same time,64–66 and the design of clinical trials is, in fact, moving in this direction. On the other hand, even when sequential therapies are used, liquid biopsies would allow a “real-time” analysis of the tumor’s mutational landscape, with the possibility of adjusting therapies accordingly, as resistance occurs.67
Besides the phenomenon of acquired resistance, the lack of reliable predictive biomarkers for selection of the patients most likely to respond to targeted therapies represents another important limitation to applying precision medicine.
For instance, while amplification of cyclin D1 was initially evaluated as a biomarker for stratification of patients treated with CDK4/6 inhibitors, it failed to show any meaningful correlation with response in clinical trials.48
CDK4/6 inhibitors require functional Rb protein to exert their antitumor activity. Loss of Rb is a marker of resistance to treatment, but the majority of HR+ BCs are Rb proficient.11 Acquired pharmacological resistance to palbociclib occurs via loss of Rb and amplification of cyclin E1,68 and the combination of a CDK4/6 inhibitor and a CDK2 inhibitor may represent a strategy to overcome resistance to CDK4/6 inhibitors.
Conclusion
The approval of abemaciclib adds another option to the armamentarium of effective CDK4/6 inhibitors currently available. The latest agent, such as palbociclib and ribociclib, responds to a pressing unmet need of patients with hormone receptor positive, HER2-negative mBC patients who have progressed on endocrine therapies, offering a more effective option than chemotherapy. More specifically than the other CDK4/6 inhibitors, abemaciclib seems to obtain the best results in heavily pretreated patients with visceral disease and worse prognosis.
Furthermore, the ability to cross the blood–brain barrier and the potential synergism between abemaciclib and targeted immunotherapies represent interesting aspects and offer possibilities for more effective multiple combination therapies.
Important questions about the correct use of CDK4/6 inhibitors, potential biomarkers of response and mechanisms of acquired resistance are still open and may be addressed in the future by specifically designed randomized clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. | ||
Dieci MV, Arnedos M, Delaloge S, Andre F. Quantification of residual risk of relapse in breast cancer patients optimally treated. Breast. 2012;22(suppl 2):S92–S95. | ||
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71(4):1286–1290. | ||
Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12(15):2245–2262. | ||
Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18(2):753–761. | ||
Altenburg JD, Farag SS. The potential role of PD0332991 (Palbociclib) in the treatment of multiple myeloma. Expert Opin Investig Drugs. 2015;24(2):261–271. | ||
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. | ||
Peurala E, Koivunen P, Haapasaari K-M, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013;15(1):R5. | ||
Ehab M, Elbaz M. Profile of palbociclib in the treatment of metastatic breast cancer. Breast Cancer. 2016;8:83–91. | ||
Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2(12):910–917. | ||
Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14(5):297–306. | ||
Gallorini M, Cataldi A, di Giacomo V. Cyclin-dependent kinase modulators and cancer therapy. BioDrugs. 2012;26(6):377–391. | ||
Jessen BA, Lee L, Koudriakova T, et al. Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. J Appl Toxicol. 2007;27(2):133–142. | ||
Fornier MN, Rathkopf D, Shah M, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13(19):5841–5846. | ||
Nemunaitis JJ, Small KA, Kirschmeier P, et al. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med. 2013;11:259. | ||
Mita MM, Joy AA, Mita A, et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14(3):169–176. | ||
Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. | ||
Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. | ||
Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825–837. | ||
Lallena MJ, Boehnke K, Torres R, et al. Abstract 3101: In-vitro characterization of Abemaciclib pharmacology in ER+ breast cancer cell lines. Cancer Res. 2015;75(15 suppl):3101. | ||
Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res. 2017;23(13):3251–3262. | ||
Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. | ||
Sanchez-Martinez C, Gelbert LM, Shannon H, et al. Abstract B234: LY2835219, a potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts. Mol Cancer Ther. 2011;10(11 suppl):B234. | ||
Raub TJ, Wishart GN, Kulanthaivel P, et al. Brain Exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43(9):1360–1371. | ||
Raub TJ, Gelbert LM, Wishart GN, et al. Abemaciclib (LY2835219) is an oral inhibitor of the cyclin-dependent kinases 4/6 that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts. Drug Metab Disposition. 2015;43(9):1360–1371. | ||
Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–753. | ||
Tolaney SM, Lin NU, Thornton D, et al. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer. J Clin Oncol. 2017;35(15_Suppl):1019. | ||
Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cdk4/6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70(8):3228–3238. | ||
Parrish KE, Pokorny J, Mittapalli RK, Bakken K, Sarkaria JN, Elmquist WF. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther. 2015;355(2):264–271. | ||
Patnaik A, Rosen LS, Tolaney SM, et al. Abstract CT232: clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with metastatic breast cancer. Cancer Res. 2014;74(19 suppl):CT232. | ||
Patnaik A, Rosen LS, Tolaney SM, et al. LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol. 2014;32(15_Suppl):534. | ||
Tolaney SM, Beeram M, Beck JT, et al. A phase Ib study of abemaciclib with therapies for metastatic breast cancer. J Clin Oncol. 2015;33(15_Suppl):522. | ||
Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224. | ||
George W, Sledge J, Toi M, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. | ||
Rugo H, Tolaney S, Cortés J, et al. Abstract CT044: MONARCH 1: Final Overall Survival Analysis of a Phase 2 Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as Monotherapy, in Patients with HR+/HER2− Breast Cancer, after Chemotherapy for Advanced Disease. Vol. 77. Washington, DC: AACR Annual Meeting 2017; 2017. | ||
Di Leo A, Toi M, Campone M, et al. 236O_PRMONARCH 3: abemaciclib as initial therapy for patients with HR+/HER2− advanced breast cancer. Ann Oncol. 2017;28(Suppl_5):mdx440.008. | ||
Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. | ||
Nair BC, Vadlamudi RK. Regulation of hormonal therapy resistance by cell cycle machinery. Gene Ther Mol Biol. 2008;12:395. | ||
Foster JS, Henley DC, Ahamed S, Wimalasena J. Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab. 2001;12(7):320–327. | ||
Carroll JS, Prall OW, Musgrove EA, Sutherland RL. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. J Biol Chem. 2000;275(49):38221–38229. | ||
Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18(3):333–345. | ||
Bosco EE, Wang Y, Xu H, et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest. 2007;117(1):218–228. | ||
Lehn S, Ferno M, Jirstrom K, Ryden L, Landberg G. A non-functional retinoblastoma tumor suppressor (RB) pathway in premenopausal breast cancer is associated with resistance to tamoxifen. Cell Cycle. 2011;10(6):956–962. | ||
Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. | ||
Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21(10):1165–1175. | ||
Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. | ||
Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. | ||
Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2−), advanced breast cancer (ABC). J Clin Oncol. 2017;35(15_Suppl):1038. | ||
Chen P, Lee NV, Hu W, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15(10):2273–2281. | ||
DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995–1001. | ||
Infante JR, Cassier PA, Gerecitano JF, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2016;22(23):5696–5705. | ||
Yamada Y, Ishikawa N, Kakizume T, Tajima T, Hewes B, Doi T. Abstract B31: a phase I study of single-agent ribociclib in Japanese patients with advanced solid tumors. Mol Cancer Ther. 2015;14(12 suppl 2):B31. | ||
O’Brien NA, Conklin D, Luo T, et al. Abstract 2828: preclinical activity of abemaciclib as a single agent or in combination with anti-mitotic or targeted therapies for breast cancer. Cancer Res. 2016;76(14 suppl):2828. | ||
Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. | ||
Laurenti E, Frelin C, Xie S, et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16(3):302–313. | ||
Scheicher R, Hoelbl-Kovacic A, Bellutti F, et al. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125(1):90–101. | ||
Matrone G, Mullins JJ, Tucker CS, Denvir MA. Effects of cyclin dependent kinase 9 inhibition on zebrafish larvae. Cell Cycle. 2016;15(22):3060–3069. | ||
Shapiro G, Rosen LS, Tolcher AW, et al. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol. 2013;31(15_Suppl):2500. | ||
Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 2016;29(3):255–269. | ||
Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. | ||
Misale S, Bozic I, Tong J, et al. Vertical suppression of the EGFR pathway prevents onset of resistance in colorectal cancers. Nat Commun. 2015;6:8305. | ||
Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21(9):1038–1047. | ||
Piotrowska Z, Niederst MJ, Karlovich CA, et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov. 2015;5(7):713–722. | ||
Herrera-Abreu MT, Asghar US, Elliot R, et al. 86OPI3 kinase/mTOR inhibition increases sensitivity of ER positive breast cancers to CDK4/6 inhibition by blocking cell cycle re-entry driven by cyclinD1 and inducing apoptosis. Ann Oncol. 2015;26(suppl_3):iii29–iii29. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


